PLANT WATER RELATIONS Sheokand S 2013 Importance of











- Slides: 47

PLANT WATER RELATIONS Sheokand S 2013

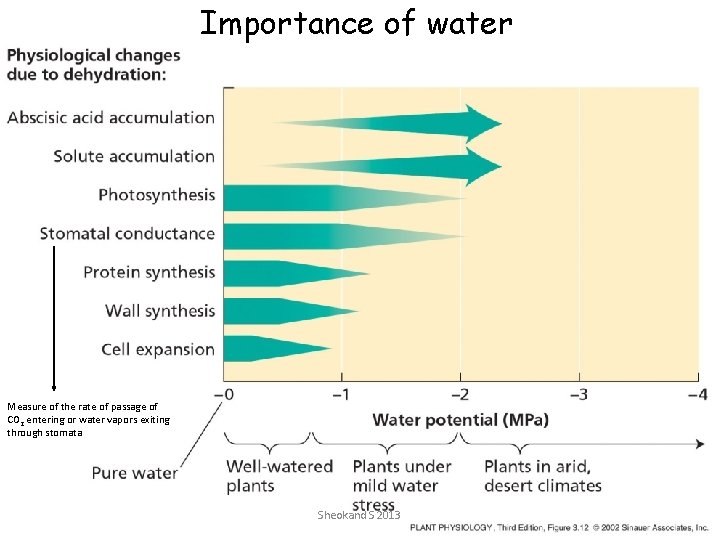
Importance of water Measure of the rate of passage of CO 2 entering or water vapors exiting through stomata Sheokand S 2013

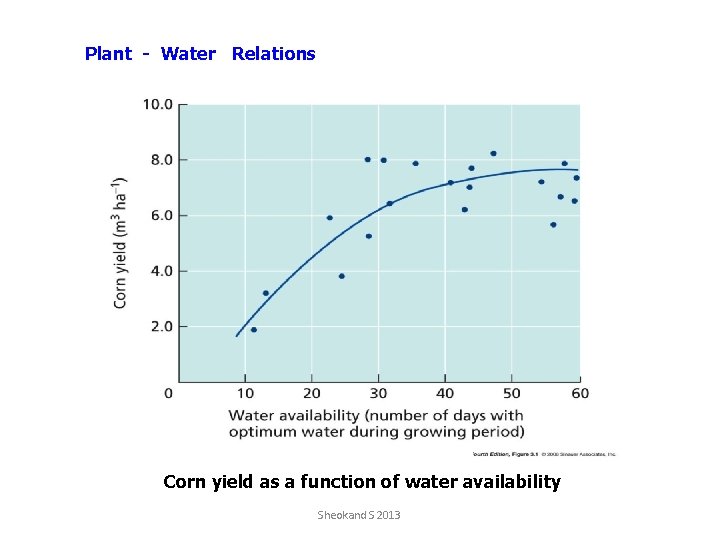
Plant - Water Relations Corn yield as a function of water availability Sheokand S 2013

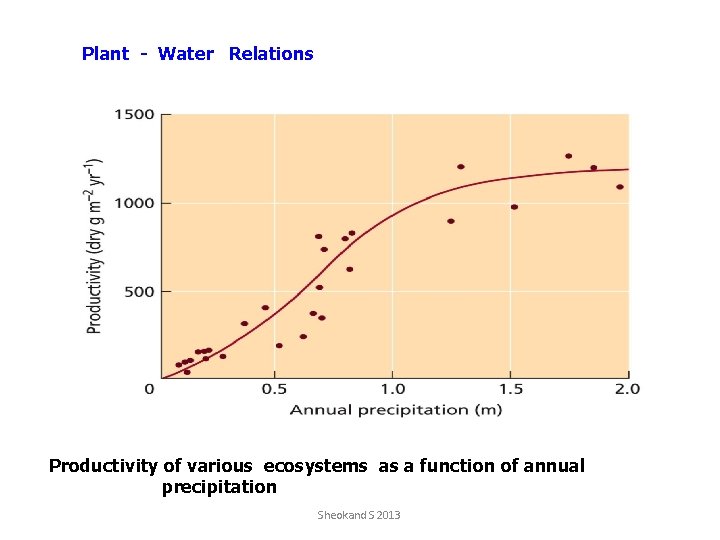
Plant - Water Relations Productivity of various ecosystems as a function of annual precipitation Sheokand S 2013

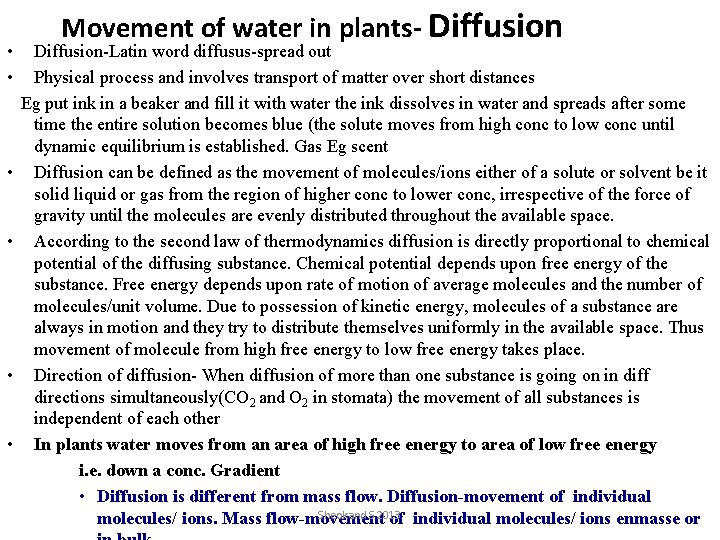
• • • Movement of water in plants- Diffusion-Latin word diffusus-spread out Physical process and involves transport of matter over short distances Eg put ink in a beaker and fill it with water the ink dissolves in water and spreads after some time the entire solution becomes blue (the solute moves from high conc to low conc until dynamic equilibrium is established. Gas Eg scent Diffusion can be defined as the movement of molecules/ions either of a solute or solvent be it solid liquid or gas from the region of higher conc to lower conc, irrespective of the force of gravity until the molecules are evenly distributed throughout the available space. According to the second law of thermodynamics diffusion is directly proportional to chemical potential of the diffusing substance. Chemical potential depends upon free energy of the substance. Free energy depends upon rate of motion of average molecules and the number of molecules/unit volume. Due to possession of kinetic energy, molecules of a substance are always in motion and they try to distribute themselves uniformly in the available space. Thus movement of molecule from high free energy to low free energy takes place. Direction of diffusion- When diffusion of more than one substance is going on in diff directions simultaneously(CO 2 and O 2 in stomata) the movement of all substances is independent of each other In plants water moves from an area of high free energy to area of low free energy i. e. down a conc. Gradient • Diffusion is different from mass flow. Diffusion-movement of individual Sheokand S 2013 molecules/ ions. Mass flow-movement of individual molecules/ ions enmasse or

SIGNIFICANCE OF DIFFUSION • Diffusion is an effective means of transport over short distances. • phloem translocation • absorption of water and minerals • transpiration-diffusion of water from intercellular spaces to outside the atmosphere • Diffusion of gases through stomata • Bulk flow Movement of water in response to a pressure gradient Analogous to water flowing in a pipe Affected by: Radius of pipe Viscosity of liquid Pressure gradient Sheokand S 2013

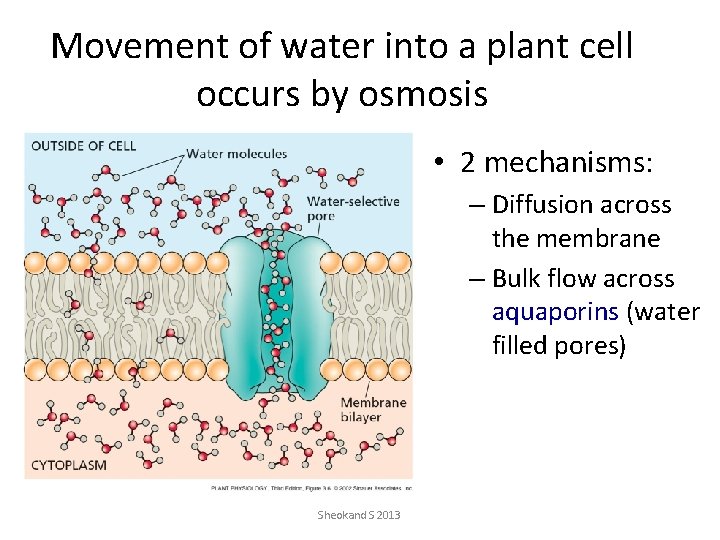
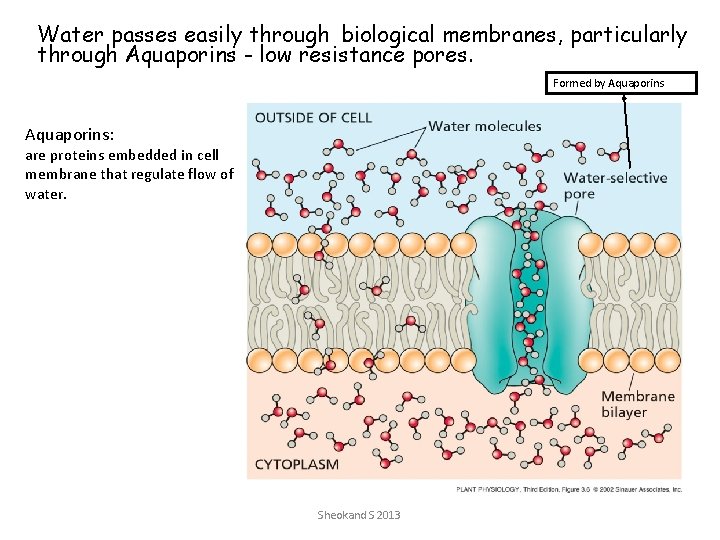
Movement of water into a plant cell occurs by osmosis • 2 mechanisms: – Diffusion across the membrane – Bulk flow across aquaporins (water filled pores) Sheokand S 2013

Movement of water into a plant cell occurs by osmosis • Osmosis is a greek word which literally means pushing. Osmosis refers to the movement of water (solvent) from a region of higher water conc to the region of lower water conc when the two solutions are separated by a semipermeable membrane. Thus osmosis may be regarded as a special type of diffusion through a semipermeable membrane where diffusion of only solvent molecules takes place. • In the light of free energy concept, osmosis may be defined as the movement of water (solvent) from a region of high free energy to a region of low free energy through a semipermeable membrane. Rate of osmosis is proportional to the steepness of free energy gradient Higher the diff in free energy, faster the rate of osmosis. Pure water has more free energy than that of a solution. Pure water>dilute solution>concentrated solution Endosmosis Sheokand S 2013 Exosmosis

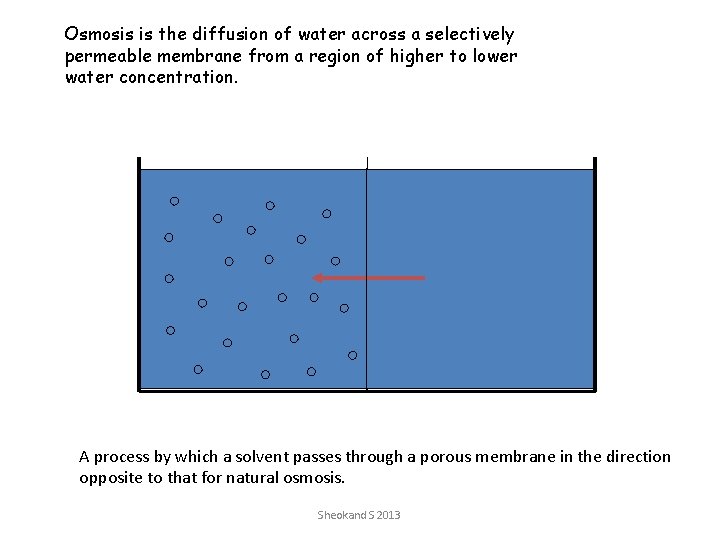
Osmosis is the diffusion of water across a selectively permeable membrane from a region of higher to lower water concentration. A process by which a solvent passes through a porous membrane in the direction opposite to that for natural osmosis. Sheokand S 2013

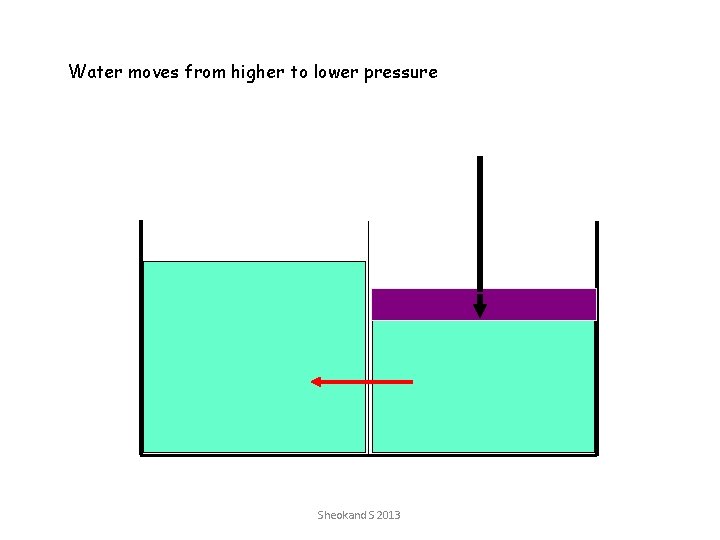
Water moves from higher to lower pressure Sheokand S 2013

WATER POTENTIAL Water Potential (Ψ) • The potential of water to do work • The more free water molecules there are in a solution, the more work can be done by water • Adding solute to a solution decreases the amount of free water so it decreases the water potential • Hypotonic solutions have greater water potential than hypertonic solutions • Water flows from a solution with high water potential to a solution with low water potential • Water potential is the difference between the free energy of water molecules in pure water and that of water in any solution. • Water potential is the chemical potential of water. Chemical potential is free energy per molecule or per gram at constant temp and pressure • Water uptake is driven by a free energy gradient composed of: – Concentration gradient – Pressure gradient Free energy gradient for water movement is referred to as a Water Sheokand S 2013 Potential Gradient

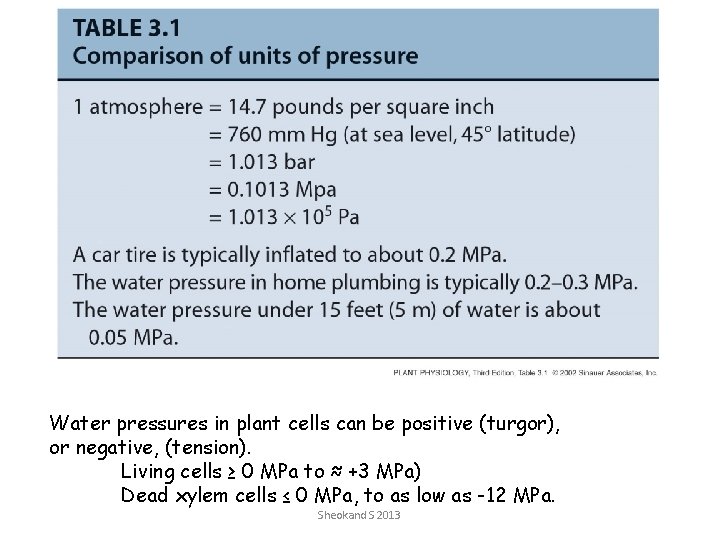
• Water potential (Yw) is equivalent to the free energy of water & influenced by: – Concentration (or activity) – Pressure – Gravity • Yw is the free energy of water per unit volume (J m-3) – Units equivalent to pressure (Pa) Measured in megapascals (MPa) Unit of pressure-1 MPa = 10 bar = 10 atmosphere Sheokand S 2013

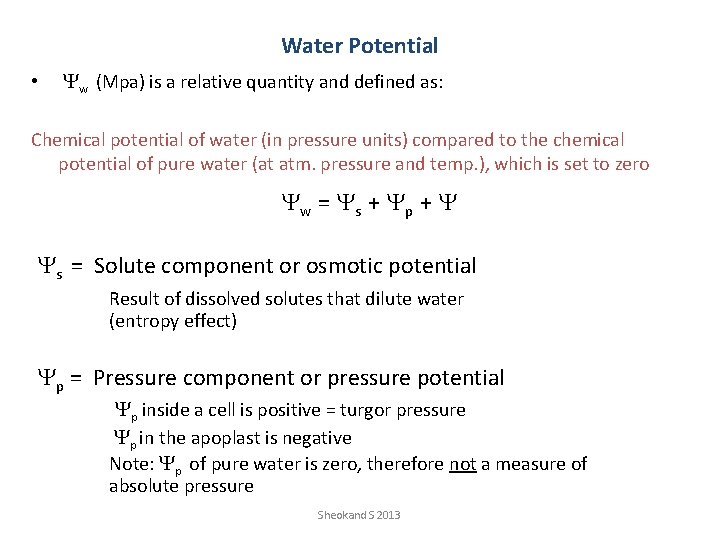
Water Potential • Yw (Mpa) is a relative quantity and defined as: Chemical potential of water (in pressure units) compared to the chemical potential of pure water (at atm. pressure and temp. ), which is set to zero Yw = Ys + Yp + Y Ys = Solute component or osmotic potential Result of dissolved solutes that dilute water (entropy effect) Yp = Pressure component or pressure potential Yp inside a cell is positive = turgor pressure Yp in the apoplast is negative Note: Yp of pure water is zero, therefore not a measure of absolute pressure Sheokand S 2013

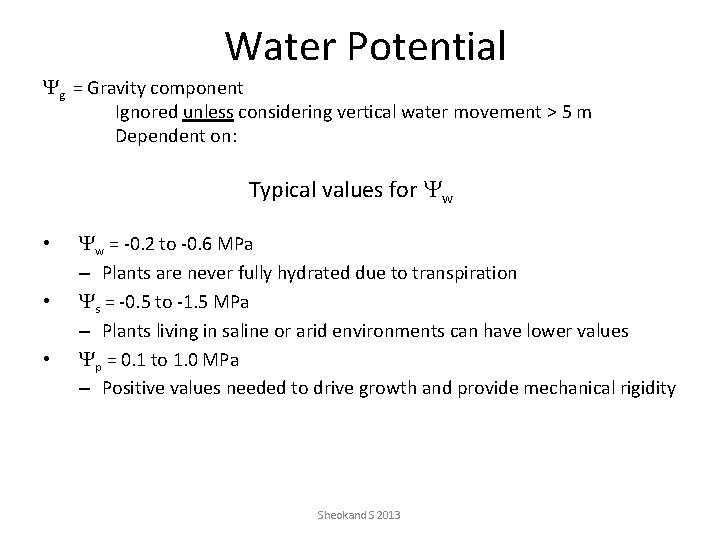
Water Potential Yg = Gravity component Ignored unless considering vertical water movement > 5 m Dependent on: Typical values for Yw • • • Yw = -0. 2 to -0. 6 MPa – Plants are never fully hydrated due to transpiration Ys = -0. 5 to -1. 5 MPa – Plants living in saline or arid environments can have lower values Yp = 0. 1 to 1. 0 MPa – Positive values needed to drive growth and provide mechanical rigidity Sheokand S 2013

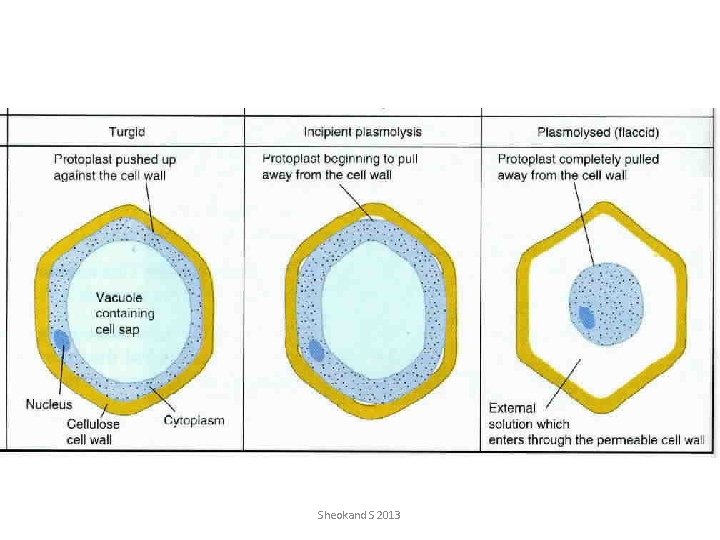
• Osmotic potential is the component of water potential that is due to the presence of solutes. • Pressure potential is the component of water potential that is due to the hydrostatic pressure. • Incipient plasmolysis is the point at which the protoplast of the cell just lost contact with the cell wall. • Plasmolysis is a condition of the cell when the protoplast shrinks away from the cell wall due to osmosis. Sheokand S 2013

Sheokand S 2013

Cell and Leaf Water Relations 1. The osmotic potential at plasmolysis can be a variety of values, depending on the amount of solutes in the vacuole and cytoplasm. There is no set value. However, the turgor potential is defined as being zero at plasmolysis. 2. Water potential is simply the sum of the osmotic and turgor potentials, so if the turgor potential = 1. 0 MPa and the osmotic potential is -2. 5 MPa, then: Yw = 1. 0 + (-2. 5) = -1. 5 MPa 3. If a cell loses water by transpiring, the water volume of the cell will decrease. Osmotic potential is determined by the number of solutes per Kg of water. Thus if the amount of water decreases, this in effect raises the ratio of solutes to water, which in turn, is equivalent to lowering (making more negative) the osmotic potential. As for turgor potential, this will decrease rapidly as the cell loses water, and the force exerted by intracellular water on the cell walls will be eliminated. Eventually, the turgor pressure will drop to zero (plasmolysis). After turgor loss, water potential is determined solely by the osmotic potential. 4. If the osmotic potential of a cell is -12 bars and its pressure potential is 7 bars what would its water potential be? 5. You measure the total water potential of a cell and find it to be -0. 24 MPa. If the pressure potential of the same cell is 0. 46 MPa, what is the solute potential of that cell 6. If a cell having a solute potential of -0. 35 Mpa is placed in a solution of pure water (Ψ = 0), what will be its pressure potential at equilibrium 7. A hypertonic solution has a HIGH / LOW water potential compared to the cell. Why? According to water potential rules, which. Sheokand way will water move in this system? S 2013

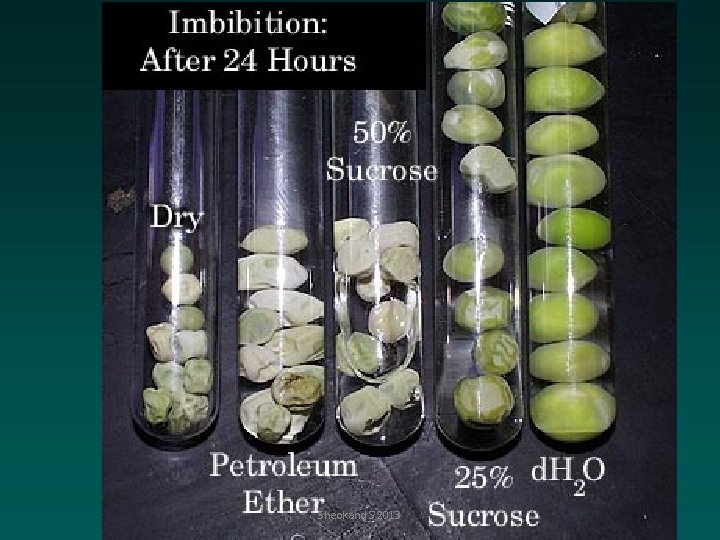
IMBIBITION § Certain substances if placed in a particular liquid absorb it and swell up, For example, a piece of dry wood or dry seeds are placed in water they absorb water quickly and swell up considerably and increase their volume. § Substances called imbibants, the phenomenon is imbibition. § In plants there are certain forces of attraction between a large number of hydrophilic colloids in living and dead cells in the form of proteins, carbohydrates such as starch and cellulose, pectic substances and between water. Sheokand S 2013

Role of Imbibition in Plants Imbibition plays a very important role in the life of plants: 1. The first step in the absorption of water by the roots of higher plants is the imbibition of water by cell walls of the root hairs. 2. Imbibition of water is very essential for dry seeds before starting germination. Sheokand S 2013

Sheokand S 2013

Water passes easily through biological membranes, particularly through Aquaporins - low resistance pores. Formed by Aquaporins: are proteins embedded in cell membrane that regulate flow of water. Sheokand S 2013

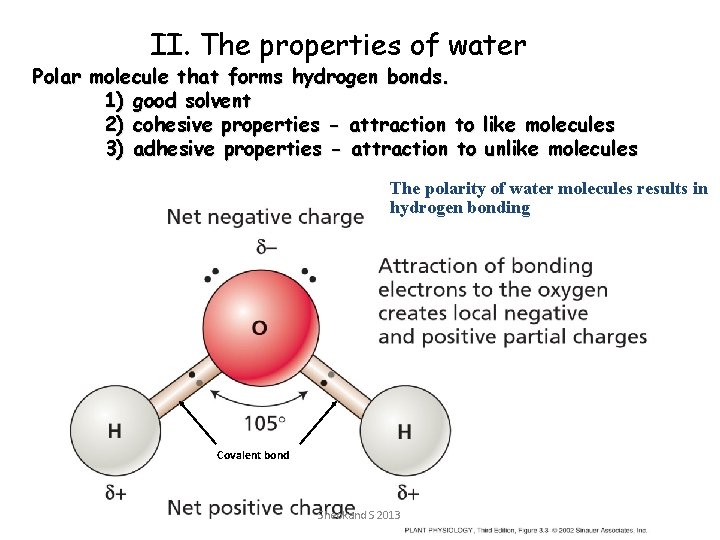
II. The properties of water Polar molecule that forms hydrogen bonds. 1) good solvent 2) cohesive properties - attraction to like molecules 3) adhesive properties - attraction to unlike molecules The polarity of water molecules results in hydrogen bonding Covalent bond Sheokand S 2013

Properties of water, continued • Cohesion is the attraction of like molecules (H 2 O here) that gives water its tensile strength. • Adhesion is the attraction of unlike molecules. Water adheres to cell walls, soil particles, glass tubes, etc. Adhesion explains capillarity & surface tension. • Surface Tension is caused by cohesive forces within liquid molecules. All of above forces give rise to a phenomenon called Capillarity (the movement of water along a capillary tube) What factors determine the direction of water movement (through the soil, between cells, from roots to leaves, from leaves into air)? 1. Gravity 2. Pressure 3. Concentration Sheokand S 2013

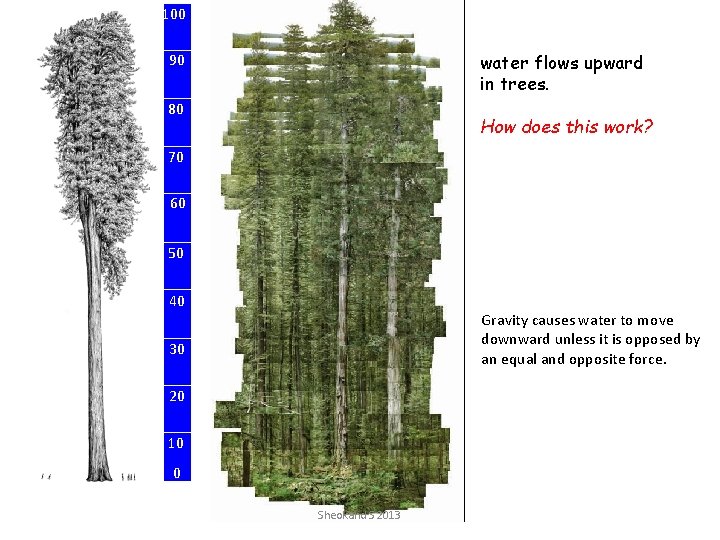
100 water flows upward in trees. 90 80 How does this work? 70 60 Height, meters 50 40 Gravity causes water to move downward unless it is opposed by an equal and opposite force. 30 20 10 0 Sheokand S 2013

Water pressures in plant cells can be positive (turgor), or negative, (tension). Living cells ≥ 0 MPa to ≈ +3 MPa) Dead xylem cells ≤ 0 MPa, to as low as -12 MPa. Sheokand S 2013

Sheokand S 2013

Water Relations • How water and minerals flow through the plant. • Why does the plant need water? – Turgidity – growth – photosynthesis – cooling (evaporative) – solvent/metabolic medium Sheokand S 2013

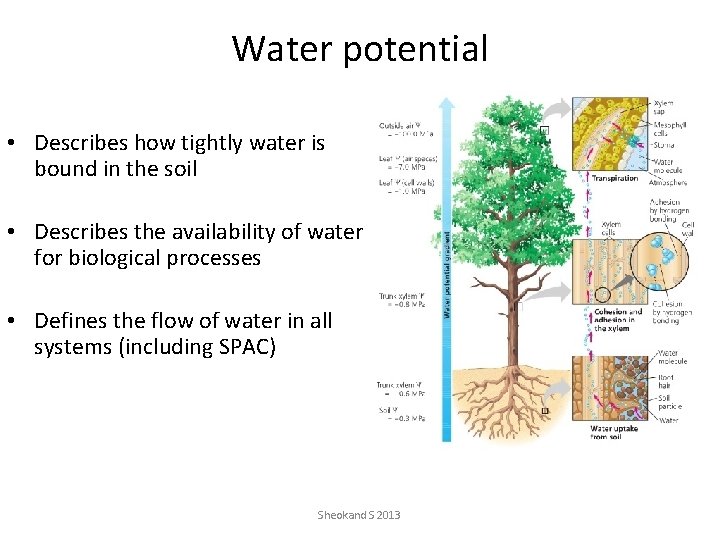
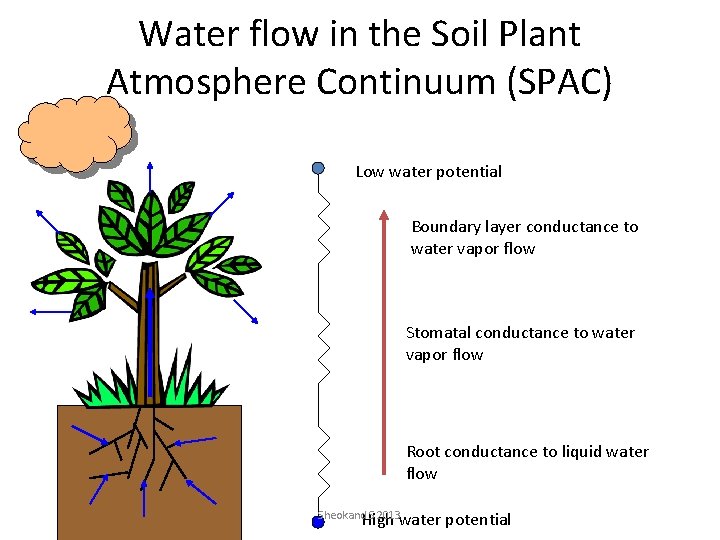
Water potential • Describes how tightly water is bound in the soil • Describes the availability of water for biological processes • Defines the flow of water in all systems (including SPAC) Sheokand S 2013

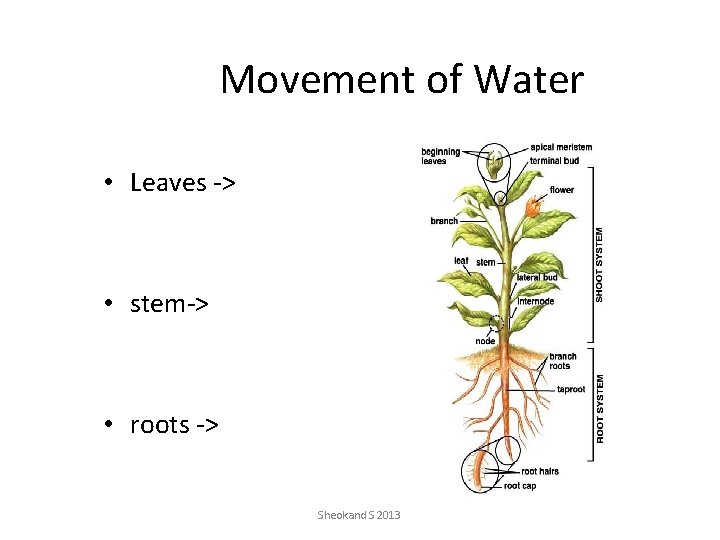
Movement of Water • Leaves -> • stem-> • roots -> Sheokand S 2013

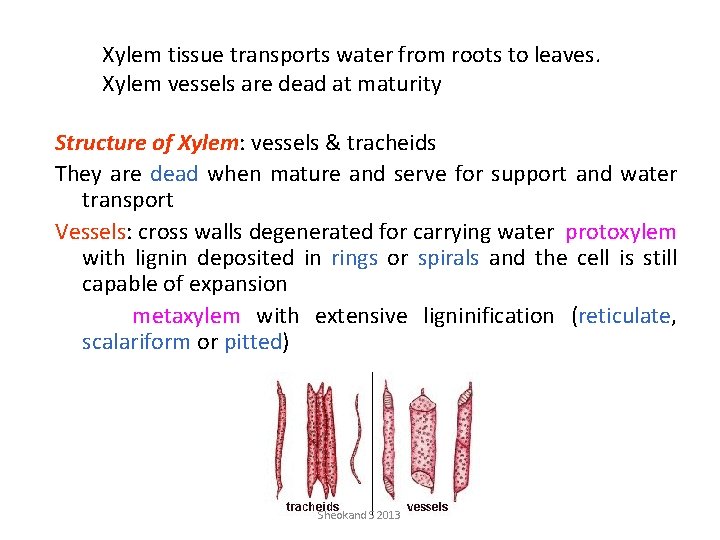
Xylem tissue transports water from roots to leaves. Xylem vessels are dead at maturity Structure of Xylem: vessels & tracheids They are dead when mature and serve for support and water transport Vessels: cross walls degenerated for carrying water protoxylem with lignin deposited in rings or spirals and the cell is still capable of expansion metaxylem with extensive ligninification (reticulate, scalariform or pitted) Sheokand S 2013

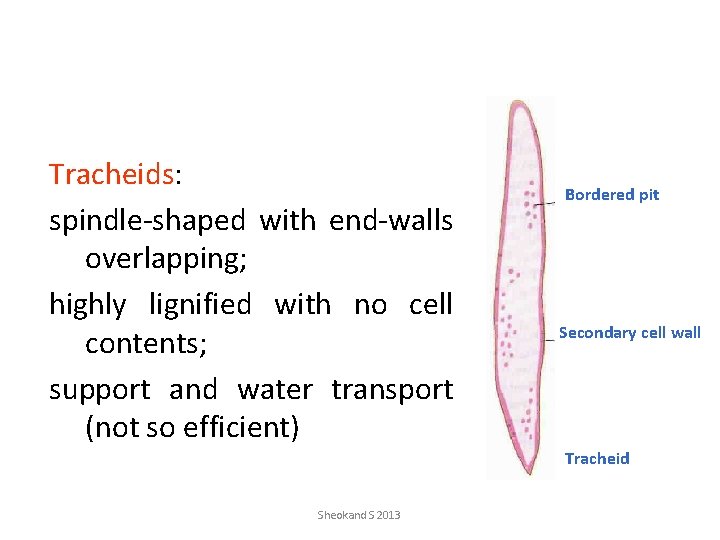
Tracheids: spindle-shaped with end-walls overlapping; highly lignified with no cell contents; support and water transport (not so efficient) Bordered pit Secondary cell wall Tracheid Sheokand S 2013

Xylem structure related to its role of water transport 1. Both vessels and tracheids consist of long cells joined end to allow water to flow in a continuous column. 2. End walls of xylem vessels broken to give uninterrupted flow of water. Tracheids have large bordered pits to reduce the resistance to flow. 3. Lateral flow of water by pits in the lignified walls. 4. Lignin enables xylem walls very rigid to prevent them collapsing under the large tension forces set up by transpiration pull. 5. Cellulose in lignin increases the adhesion of water molecules & creates capillarity. 6. Very narrow lumen of vessels & tracheids increases the capillarity forces. Sheokand S 2013

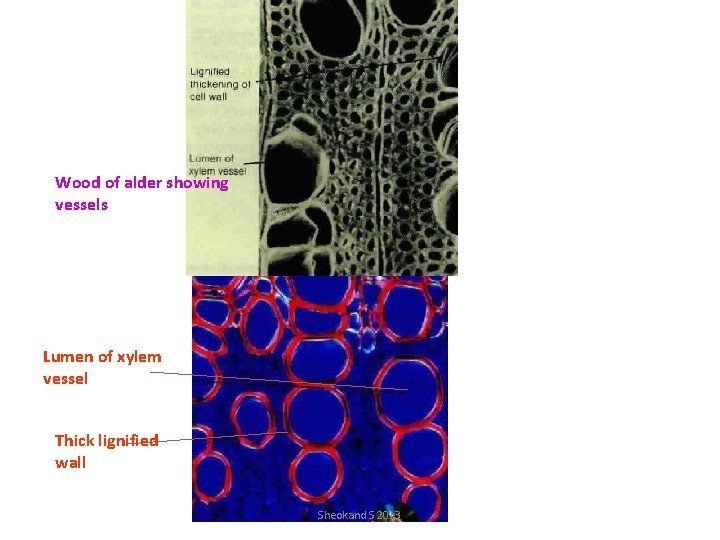
Wood of alder showing vessels Lumen of xylem vessel Thick lignified wall Sheokand S 2013

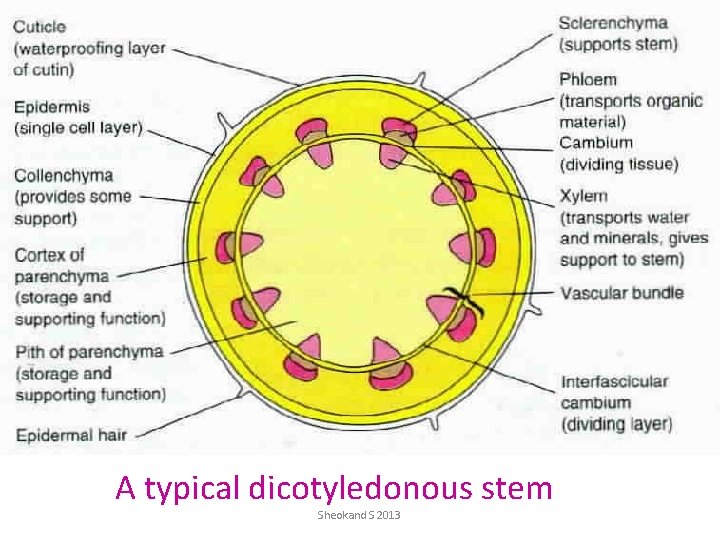
A typical dicotyledonous stem Sheokand S 2013

Movement of water up the stem • Water moves up the stem and into the leaves through xylem vessels and tracheids. Evidences that xylem carries water up the stem: • 1. Experiment using a dye, e. g. eosin • 2. Removal of xylem causes leaf wilting • 3. Metabolic poison has no effect on the uptake of water by xylem • 4. Wilting of plants by drawing up fatty substances + microscopic examination Sheokand S 2013

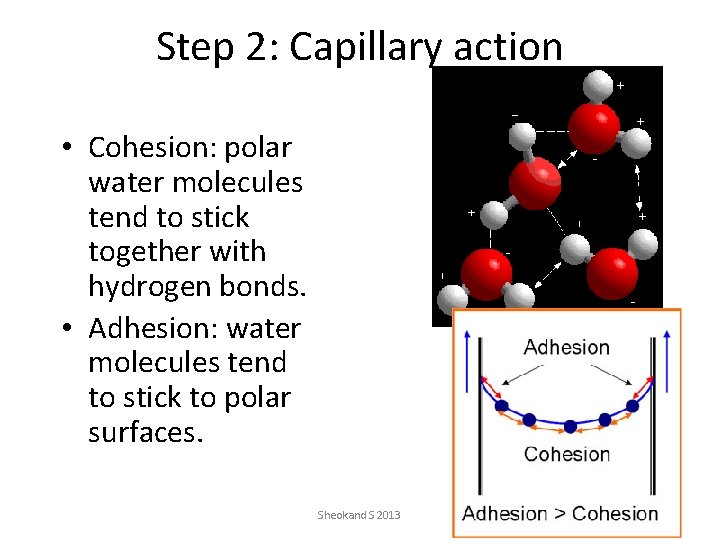
Step 2: Capillary action • Cohesion: polar water molecules tend to stick together with hydrogen bonds. • Adhesion: water molecules tend to stick to polar surfaces. Sheokand S 2013

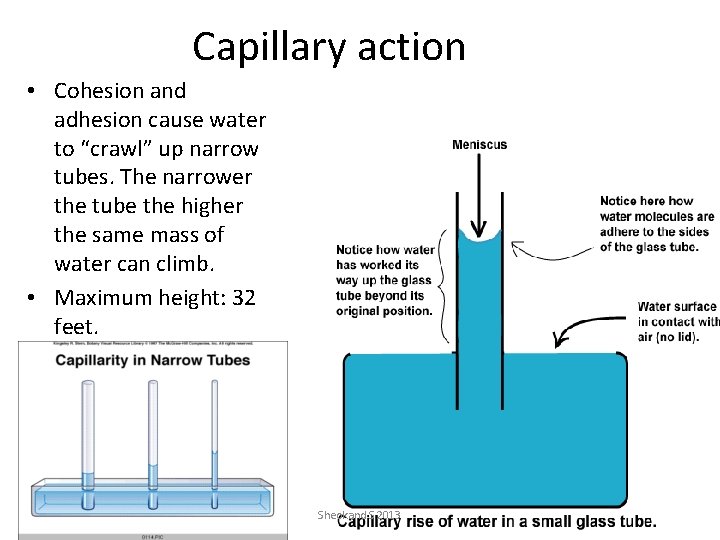
Capillary action • Cohesion and adhesion cause water to “crawl” up narrow tubes. The narrower the tube the higher the same mass of water can climb. • Maximum height: 32 feet. Sheokand S 2013

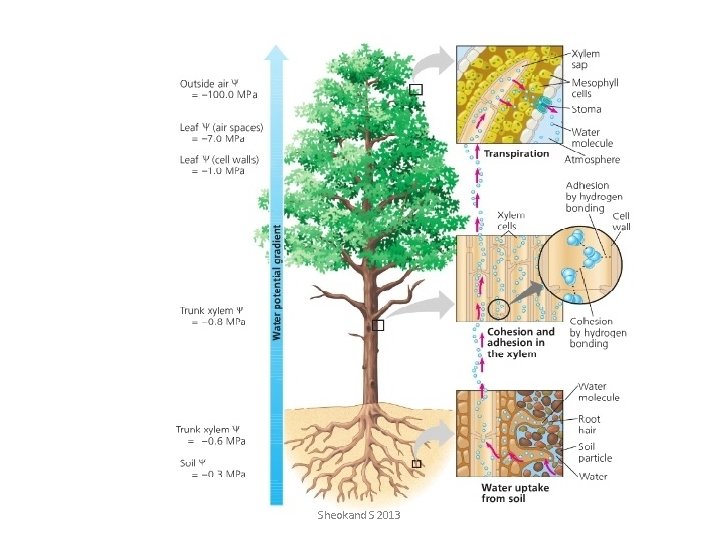
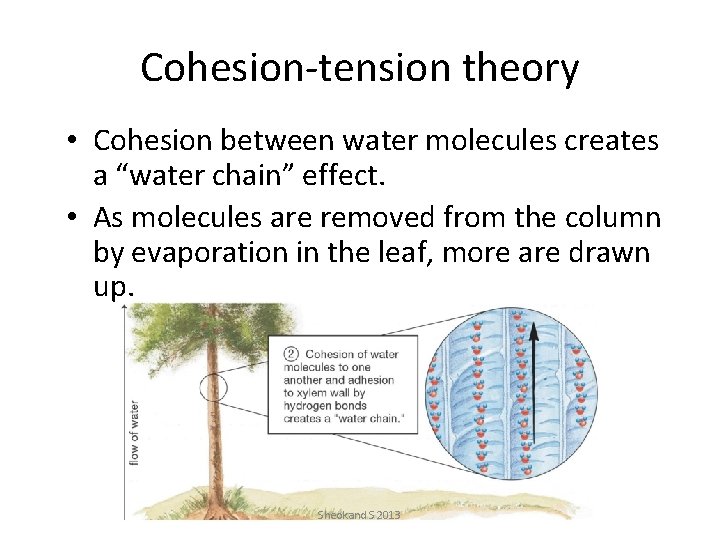
Cohesion-tension theory • Cohesion between water molecules creates a “water chain” effect. • As molecules are removed from the column by evaporation in the leaf, more are drawn up. Sheokand S 2013

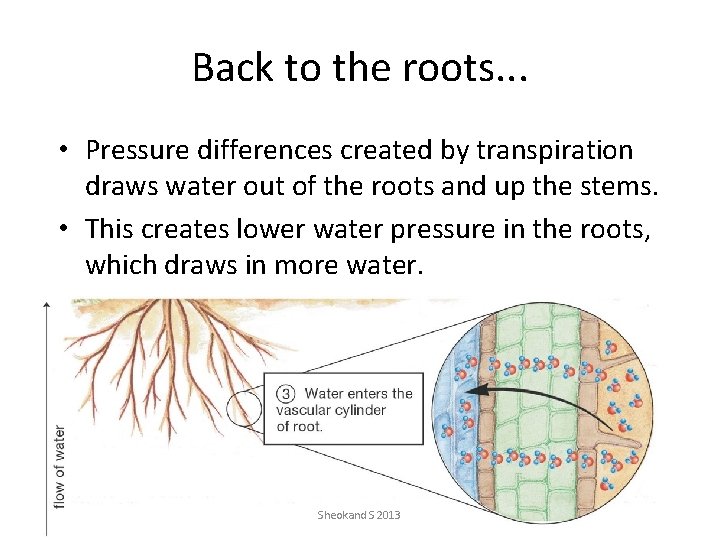
Back to the roots. . . • Pressure differences created by transpiration draws water out of the roots and up the stems. • This creates lower water pressure in the roots, which draws in more water. Sheokand S 2013

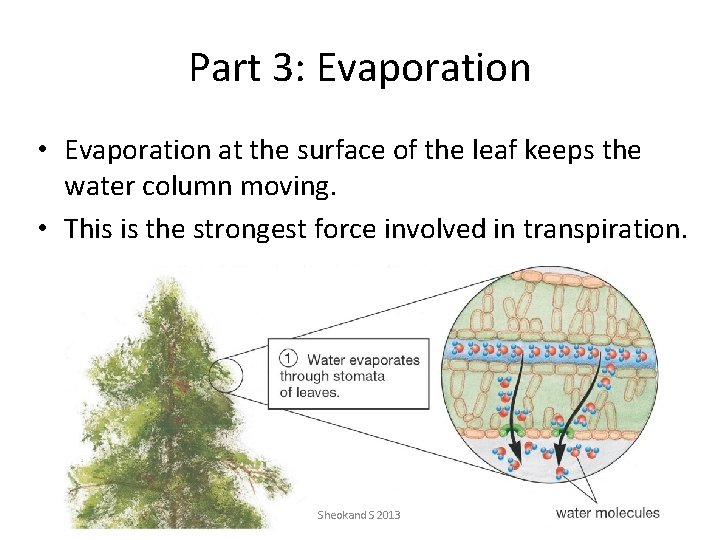
Part 3: Evaporation • Evaporation at the surface of the leaf keeps the water column moving. • This is the strongest force involved in transpiration. Sheokand S 2013

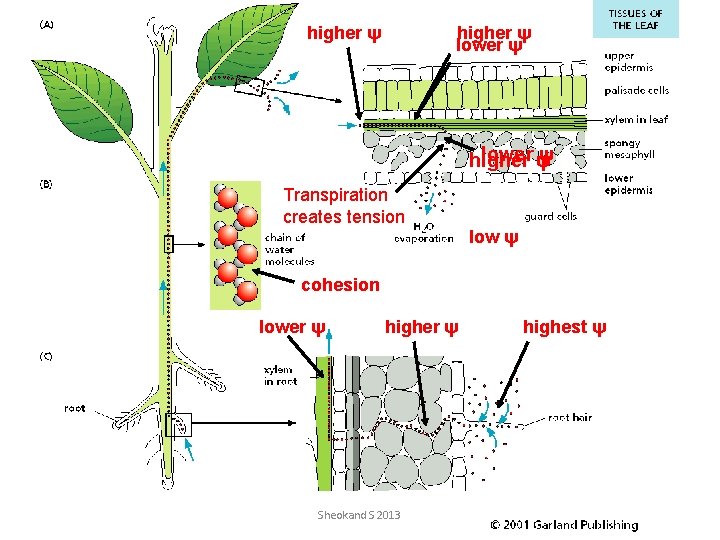
Cohesion-Tension Theory: • The transpiration of water from the leaves draws water across the leaf. • This water is replaced by that entering the mesophyll cells from the xylem by osmosis. • As water molecules leave xylem cells in the leaf, they pull up other water molecules. • This pulling effect (Transpiration Pull) is possible because of the large cohesive forces between water molecules. • The pull creates a tension in the xylem cells which, if cut, exude water. • Adhesive forces between the water molecules and the walls of xylem vessels help water to rise upwards in xylem - capillarity. • Root pressure also contributes to the uprise of water. Sheokand S 2013

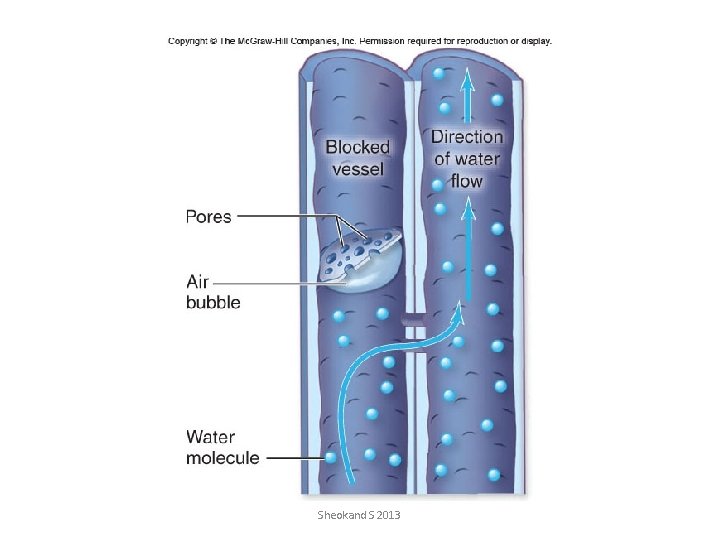
Xylem transport of water in trees faces physical challenges • large tensions that develop in xylem of trees, water under tension transmits an inward force to the walls of xylem, if walls were weak they would collapse. The secondary wall thickenings and lignification of tracheids and vessles are adaptations that offset this tendency to collapse • • Cavitation-phenom of bubble expansion Embolism-resulting gas filled void – An air bubble can break the tensile strength of a water column – A gas-filled bubble can expand block the tracheid or vessel – Damage can be minimized by anatomical adaptations • Presence of alternative pathways • Pores smaller than air bubbles The most permeable regions of xylem wall are pit membranes themselves the site where water flows between conduits. Normally these prevent the spread of gas between conduits Sheokand S 2013

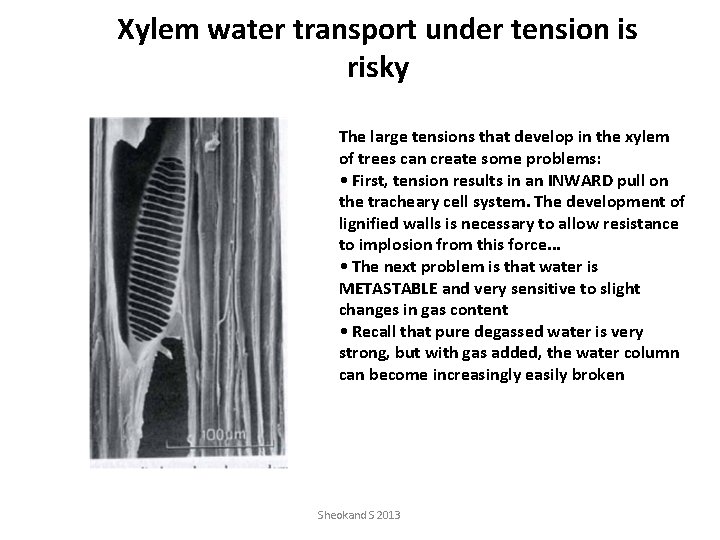
Xylem water transport under tension is risky The large tensions that develop in the xylem of trees can create some problems: • First, tension results in an INWARD pull on the tracheary cell system. The development of lignified walls is necessary to allow resistance to implosion from this force. . . • The next problem is that water is METASTABLE and very sensitive to slight changes in gas content • Recall that pure degassed water is very strong, but with gas added, the water column can become increasingly easily broken Sheokand S 2013

Metastable state of water under tension • The other problem is that water is METASTABLE and very sensitive to slight changes in gas content • • Recall that pure degassed water is very strong, but with gas added, the water column can become increasingly easily broken • • As tensions increase, there is an increased tendency for air to be pulled in from microscopic pores in the xylem wall that contain air (from respiring living cells or just close to lenticels, etc. ) • • This is called air-seeding. Sheokand S 2013

Sheokand S 2013

higher ψ lower ψ ψ higher Transpiration creates tension low ψ cohesion lower ψ higher ψ Sheokand S 2013 highest ψ

Water flow in the Soil Plant Atmosphere Continuum (SPAC) Low water potential Boundary layer conductance to water vapor flow Stomatal conductance to water vapor flow Root conductance to liquid water flow Sheokand. High S 2013 water potential