Water Properties of Water Polar molecule Cohesion and







- Slides: 11

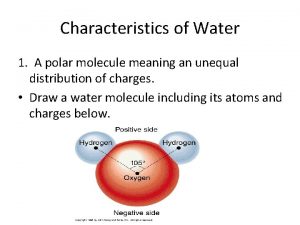
Water

Properties of Water • Polar molecule • Cohesion and adhesion • High specific heat and evaporative cooling • Density – greatest at 4 o. C • Universal solvent of life

Organisms Depend on Cohesion and Adhesion Cohesion – water sticking to itself Adhesion – water sticking to other molecules • Cohesion among water molecules plays a key role in the transport of water against gravity in plants • Adhesion, clinging of one substance to another, contributes too, as water adheres to the wall of the vessels.

• Surface tension, a measure of the force necessary to stretch or break the surface of a liquid, is related to cohesion. – Water has a greater surface tension than most other liquids of cohesion. – Water behaves as if covered by an invisible film. – Some animals can stand, walk, or run on water without breaking the surface. Fig. 3. 3

Water stabilizes air temperatures by absorbing heat from warmer air and releasing heat to cooler air. Three-fourths of the earth is covered by water. The water serves as a large heat sink responsible for: 1. Prevention of temperature fluctuations that are outside the range suitable for life. 2. Coastal areas having a mild climate 3. A stable marine environment

Evaporative Cooling • The cooling of a surface occurs when the liquid evaporates • This is responsible for: – Moderating earth’s climate – Stabilizes temperature in aquatic ecosystems – Preventing organisms from overheating

Density of Water • Most dense at 4 o. C • Contracts until 4 o. C • Expands from 4 o. C to 0 o. C The density of water: 1. Prevents water from freezing from the bottom up. 2. Ice forms on the surface first—the freezing of the water releases heat to the water below creating insulation. 3. Makes transition between season less abrupt.

Solvent for Life • Solution – when all of the solvent is evenly distributed in the solvent. (evenly mixed) – Solute –the item being dissolved or mixed in. Usually in lower concentration – Solvent – the substance that the solute is dissolved in. • Suspension – the materials mixed on the solute are not dissolved completely and are instead suspended in the solution.

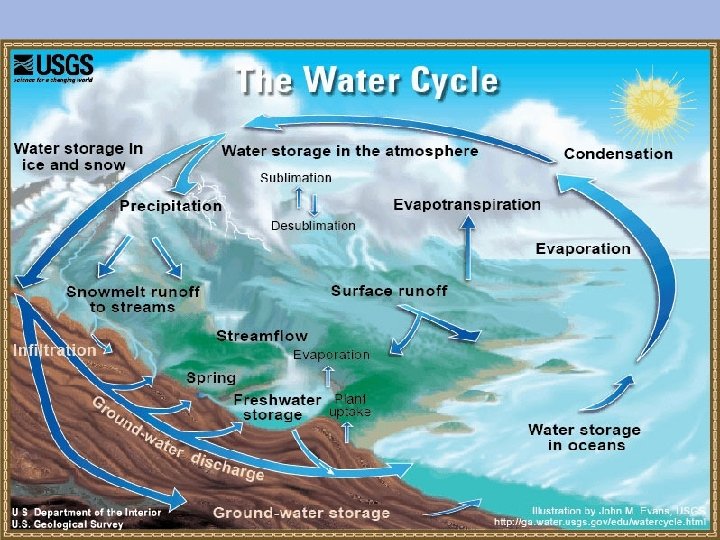
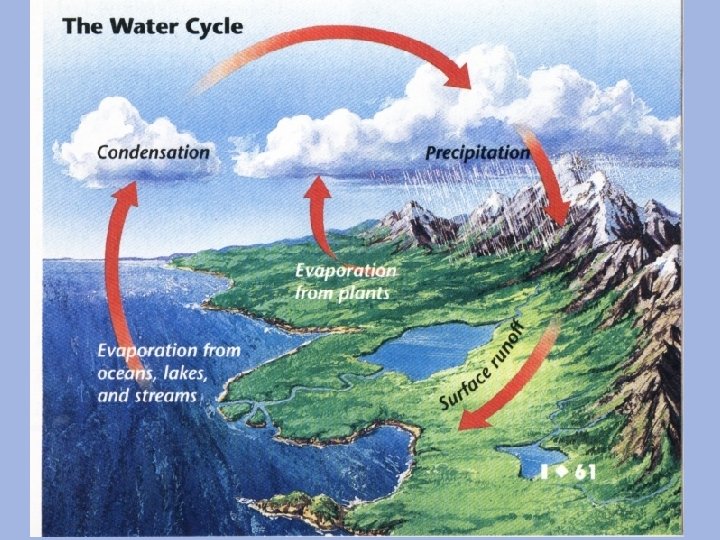
Water Cycle


The Water Cycle All living things need water to survive. Water is recycled. Evaporation: liquid gas Transpiration: evaporation from plant leaves Condensation: gas liquid (forms clouds) Precipitation: rain (droplets fall from the sky) Runoff: rain runs along the surface of the ground until entering a stream or river • Seepage: seeps into the soil and becomes ground water • •