The Amazing Water Molecule PROPERTIES Water Water is








- Slides: 17

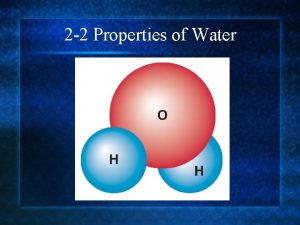
The Amazing Water Molecule PROPERTIES

Water • Water is a colorless, clear, odorless, and tasteless liquid. (Liquid at room temperature) • Water is everywhere! • It makes up over 78% of our planet. It makes up our oceans, lakes, rivers, seas, and even over 96% of our bodies. Water is necessary to keep us alive.


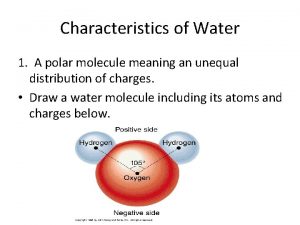
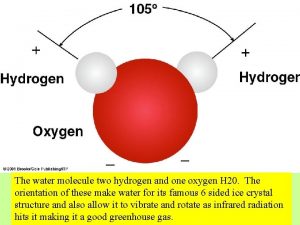
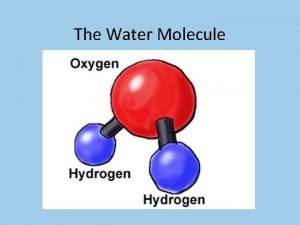
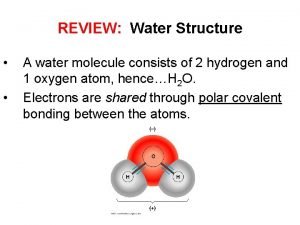
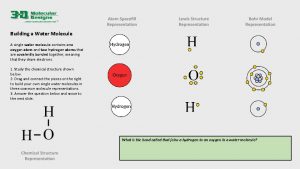
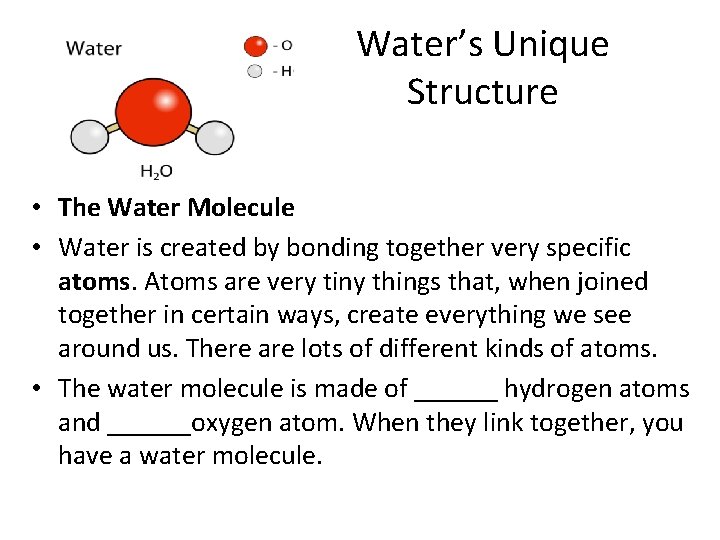
Water’s Unique Structure • The Water Molecule • Water is created by bonding together very specific atoms. Atoms are very tiny things that, when joined together in certain ways, create everything we see around us. There are lots of different kinds of atoms. • The water molecule is made of ______ hydrogen atoms and ______oxygen atom. When they link together, you have a water molecule.

The Water Molecule is “Polar” • has positive and negative “poles” or ends • This means that one end of the water molecule is positively charged, and the other end is ________ charged. • If you've ever looked at a battery, this is kind of what it's like: one end is positive and the other is negative.

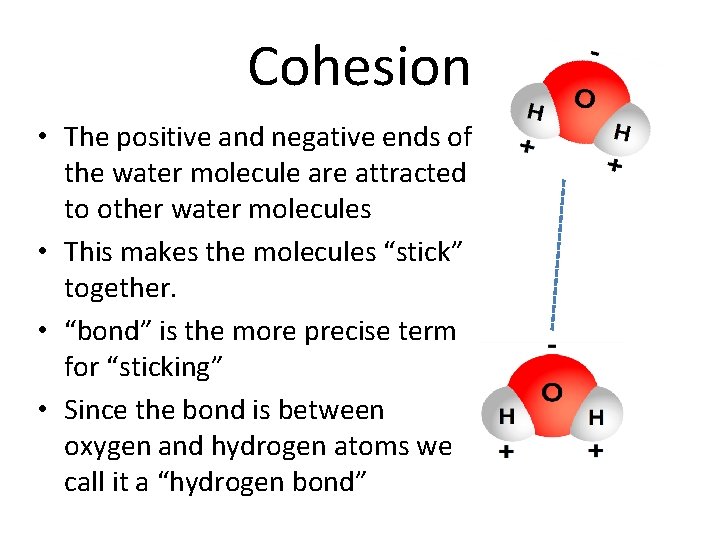
Cohesion • The positive and negative ends of the water molecule are attracted to other water molecules • This makes the molecules “stick” together. • “bond” is the more precise term for “sticking” • Since the bond is between oxygen and hydrogen atoms we call it a “hydrogen bond”

Adhesion • Water sticks to ? molecules (other than water)

Cohesion & Adhesion Together Make Capillary Action of Water Possible This is why water can defy gravity and move upward in very tall trees to bring water from the roots all the way up to the top.

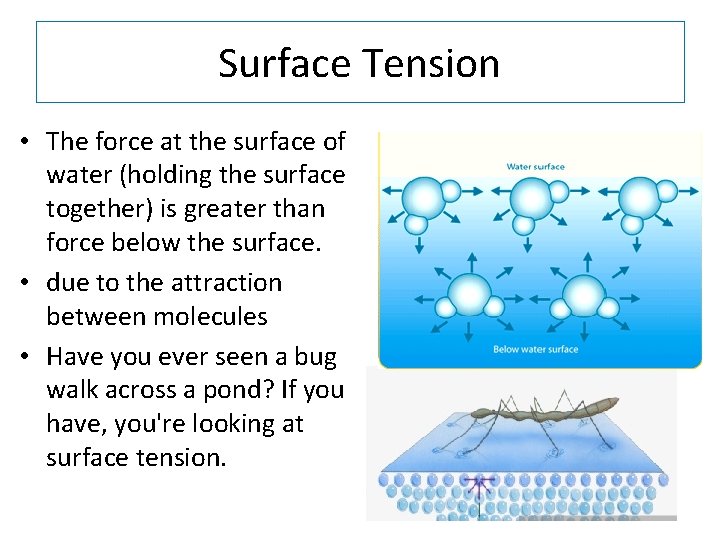
Surface Tension • The force at the surface of water (holding the surface together) is greater than force below the surface. • due to the attraction between molecules • Have you ever seen a bug walk across a pond? If you have, you're looking at surface tension.

Surface Tension of Water • Why a belly flop hurts!

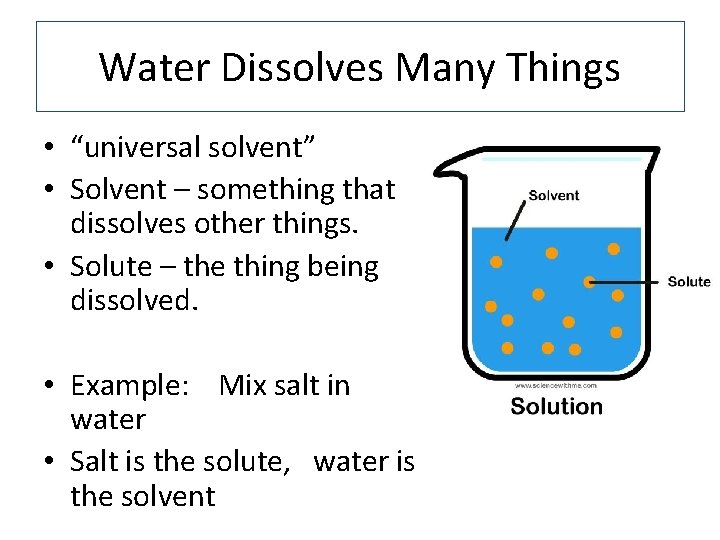
Water Dissolves Many Things • “universal solvent” • Solvent – something that dissolves other things. • Solute – the thing being dissolved. • Example: Mix salt in water • Salt is the solute, water is the solvent

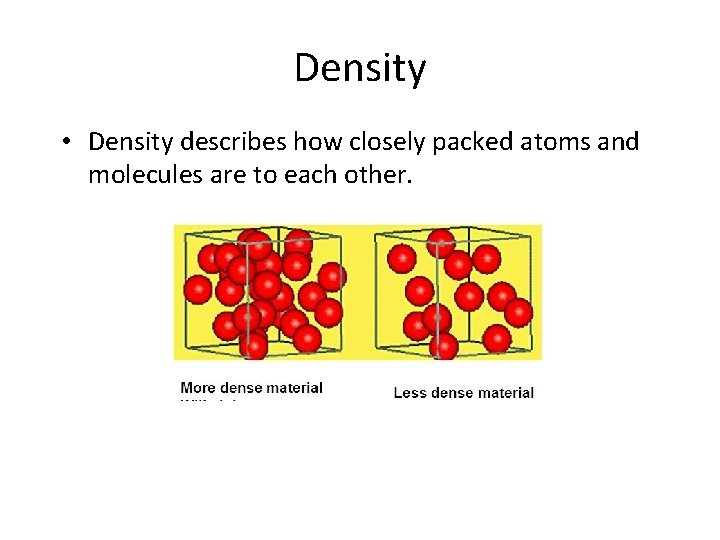
Density • Density describes how closely packed atoms and molecules are to each other.

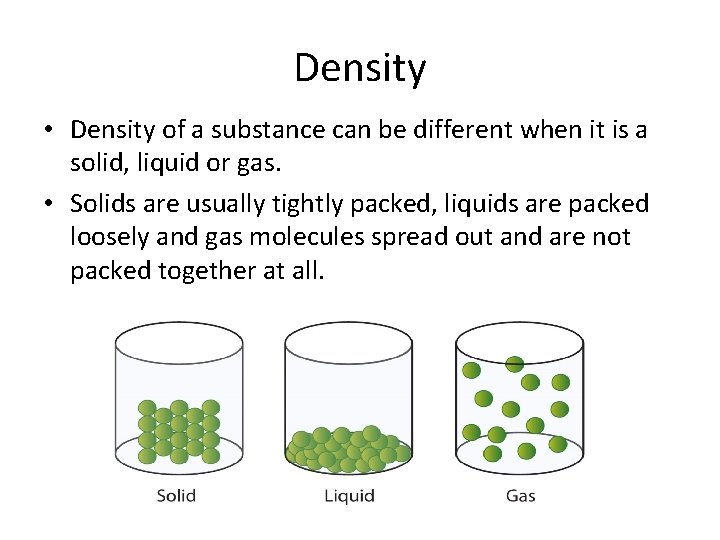
Density • Density of a substance can be different when it is a solid, liquid or gas. • Solids are usually tightly packed, liquids are packed loosely and gas molecules spread out and are not packed together at all.

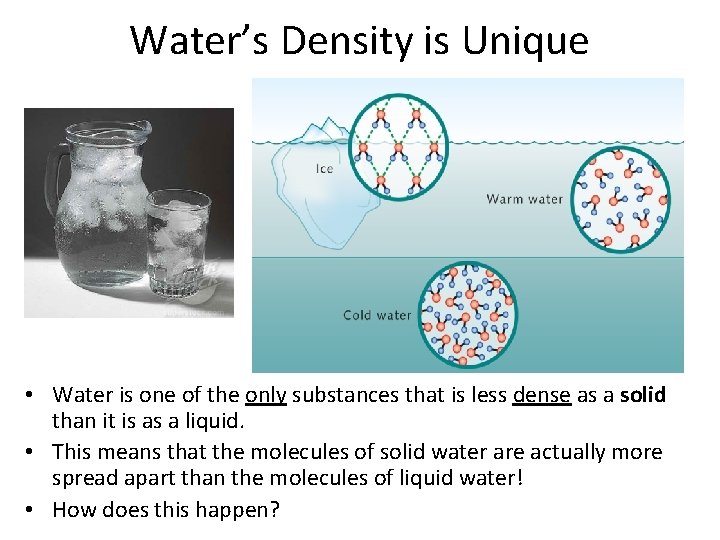
Water’s Density is Unique • Water is one of the only substances that is less dense as a solid than it is as a liquid. • This means that the molecules of solid water are actually more spread apart than the molecules of liquid water! • How does this happen?

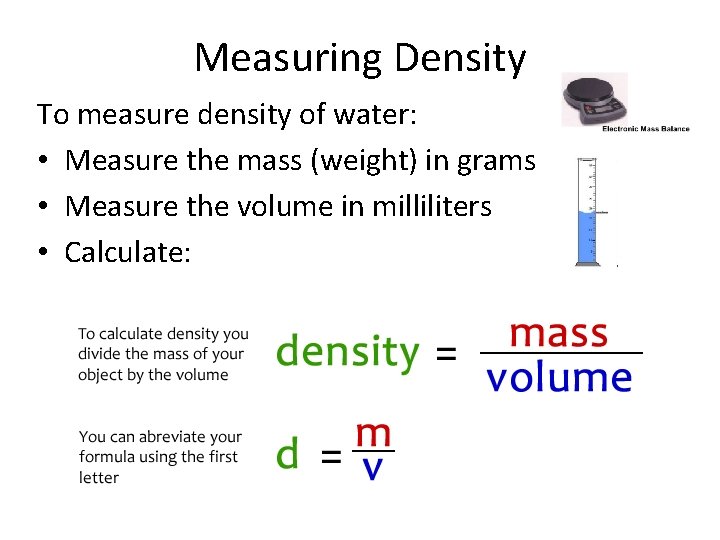
Measuring Density To measure density of water: • Measure the mass (weight) in grams • Measure the volume in milliliters • Calculate:

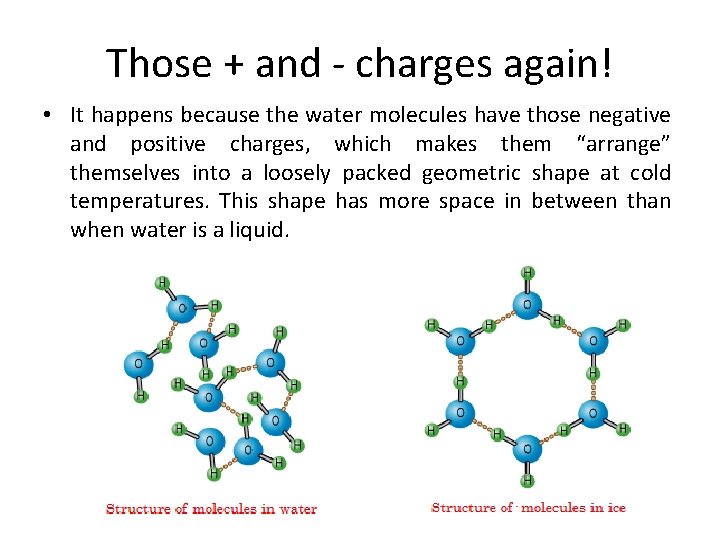
Those + and - charges again! • It happens because the water molecules have those negative and positive charges, which makes them “arrange” themselves into a loosely packed geometric shape at cold temperatures. This shape has more space in between than when water is a liquid.

Why do you think snowflakes have 6 sides?
 Water
Water Describe the polar characteristics of a water molecule.
Describe the polar characteristics of a water molecule. Water molecule
Water molecule Is water covalent
Is water covalent Polar covalent bond in water molecule
Polar covalent bond in water molecule Water molecule
Water molecule Water molecule
Water molecule Water molecule diagram
Water molecule diagram Polar molecules
Polar molecules Polar covalent bond
Polar covalent bond Water and water and water water
Water and water and water water Extensive and intensive properties
Extensive and intensive properties Chemical property of matter
Chemical property of matter Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói