VSEPR Theory Each of the following counts as


- Slides: 16

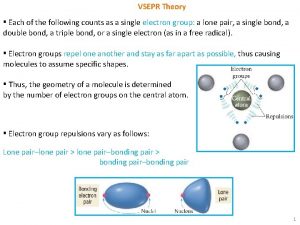
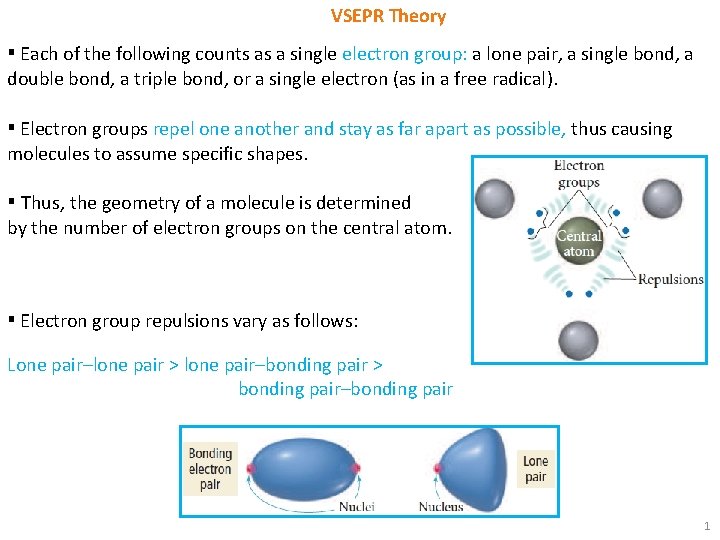
VSEPR Theory ▪ Each of the following counts as a single electron group: a lone pair, a single bond, a double bond, a triple bond, or a single electron (as in a free radical). ▪ Electron groups repel one another and stay as far apart as possible, thus causing molecules to assume specific shapes. ▪ Thus, the geometry of a molecule is determined by the number of electron groups on the central atom. ▪ Electron group repulsions vary as follows: Lone pair–lone pair > lone pair–bonding pair > bonding pair–bonding pair 1

VSEPR Theory ▪ Explanation: A lone electron pair is more spread out in space than a bonding electron pair because a lone pair is attracted to only one nucleus while a bonding pair is attracted to two nuclei. ▪ The lone pair occupies more of the angular space around a nucleus than does a bond pair, exerting a greater repulsive force on the neighboring electrons. ▪ Steps for determining the geometry using VSEPR theory: 1) Determine the no. of electron groups from the Lewis structure of the molecule. • If the Lewis structure contains resonance structures, use any one of the resonance structures to determine the number of electron groups. 2) Predict the arrangement of electron groups around each atom by assuming that the groups are oriented in space as far away from one another as possible. • How the electron groups achieve this orientation depends on their number. 2

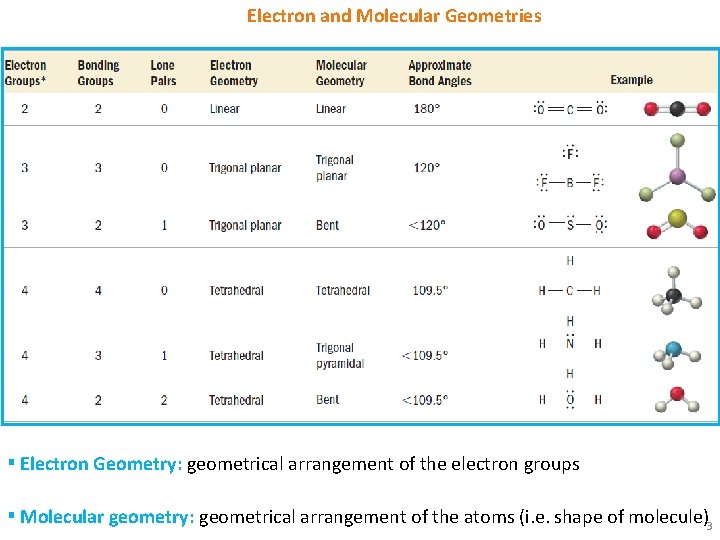
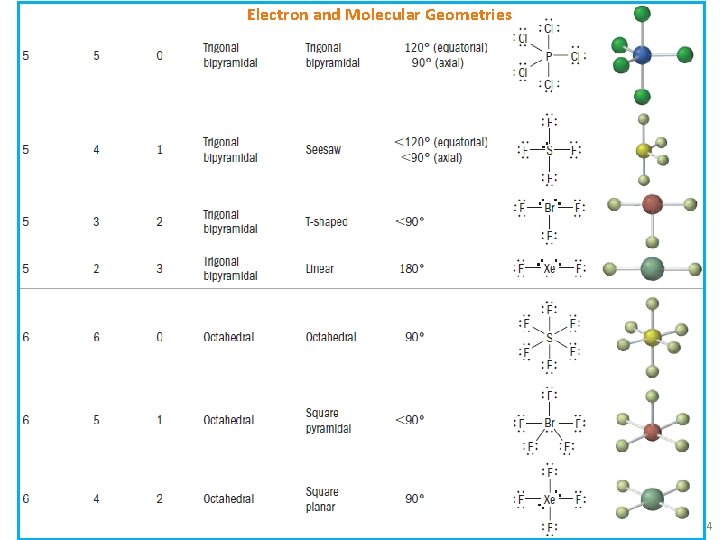
Electron and Molecular Geometries ▪ Electron Geometry: geometrical arrangement of the electron groups ▪ Molecular geometry: geometrical arrangement of the atoms (i. e. shape of molecule)3

Electron and Molecular Geometries 4

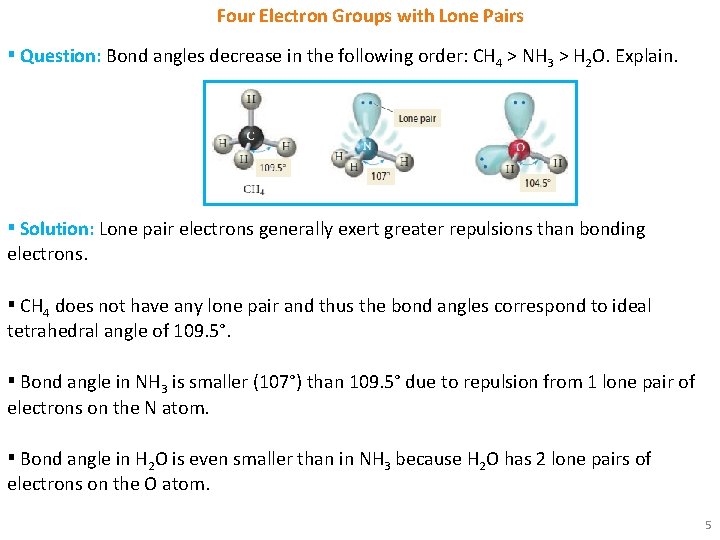
Four Electron Groups with Lone Pairs ▪ Question: Bond angles decrease in the following order: CH 4 > NH 3 > H 2 O. Explain. ▪ Solution: Lone pair electrons generally exert greater repulsions than bonding electrons. ▪ CH 4 does not have any lone pair and thus the bond angles correspond to ideal tetrahedral angle of 109. 5°. ▪ Bond angle in NH 3 is smaller (107°) than 109. 5° due to repulsion from 1 lone pair of electrons on the N atom. ▪ Bond angle in H 2 O is even smaller than in NH 3 because H 2 O has 2 lone pairs of electrons on the O atom. 5

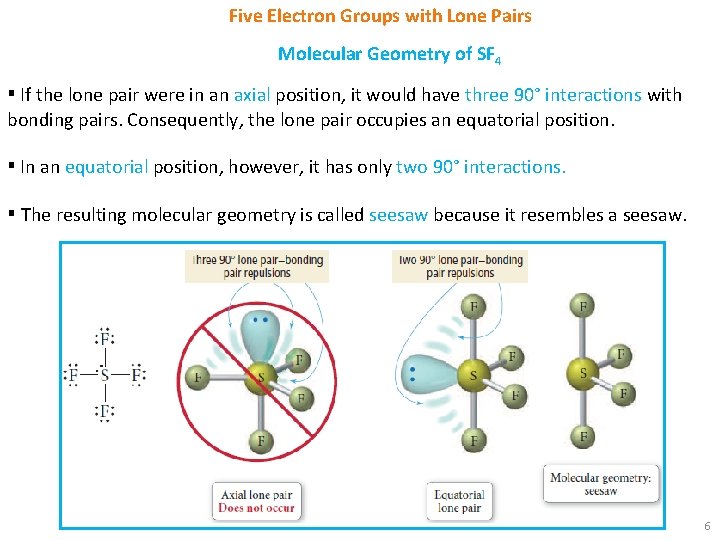
Five Electron Groups with Lone Pairs Molecular Geometry of SF 4 ▪ If the lone pair were in an axial position, it would have three 90° interactions with bonding pairs. Consequently, the lone pair occupies an equatorial position. ▪ In an equatorial position, however, it has only two 90° interactions. ▪ The resulting molecular geometry is called seesaw because it resembles a seesaw. 6

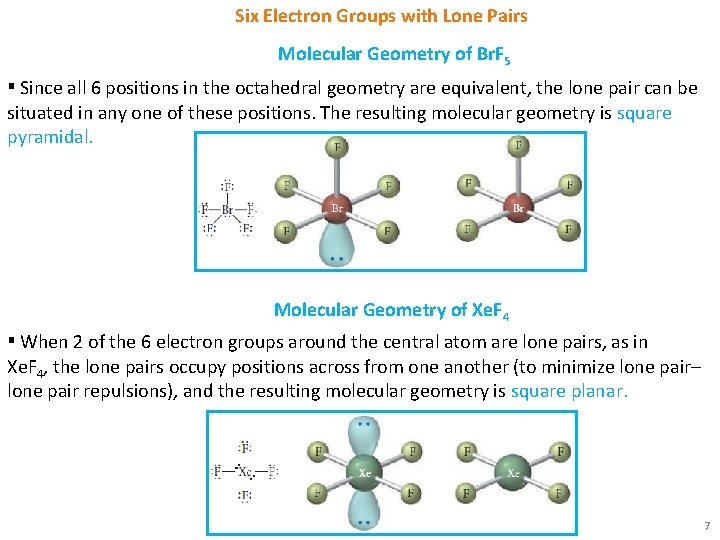
Six Electron Groups with Lone Pairs Molecular Geometry of Br. F 5 ▪ Since all 6 positions in the octahedral geometry are equivalent, the lone pair can be situated in any one of these positions. The resulting molecular geometry is square pyramidal. Molecular Geometry of Xe. F 4 ▪ When 2 of the 6 electron groups around the central atom are lone pairs, as in Xe. F 4, the lone pairs occupy positions across from one another (to minimize lone pair– lone pair repulsions), and the resulting molecular geometry is square planar. 7

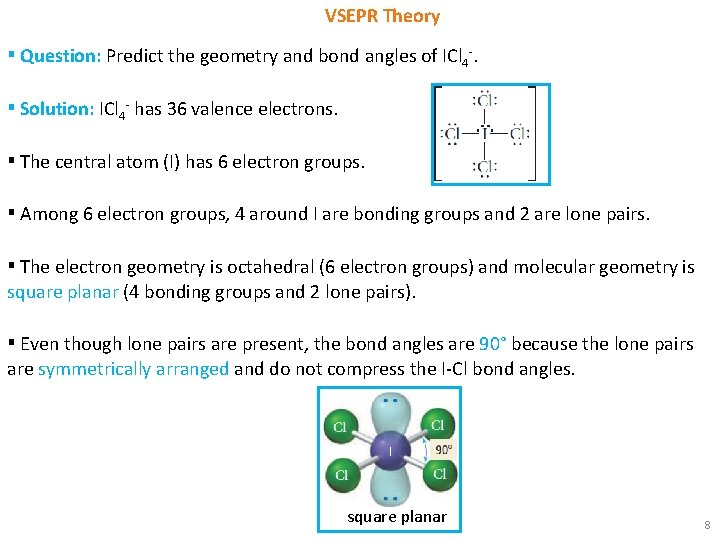
VSEPR Theory ▪ Question: Predict the geometry and bond angles of ICl 4 -. ▪ Solution: ICl 4 - has 36 valence electrons. ▪ The central atom (I) has 6 electron groups. ▪ Among 6 electron groups, 4 around I are bonding groups and 2 are lone pairs. ▪ The electron geometry is octahedral (6 electron groups) and molecular geometry is square planar (4 bonding groups and 2 lone pairs). ▪ Even though lone pairs are present, the bond angles are 90° because the lone pairs are symmetrically arranged and do not compress the I-Cl bond angles. square planar 8

Determination of the Geometry of Molecules Having Single Bonds 1) Write down the number of electrons in the outer level of the central atom. That will be the same as the group number. 2) Add one electron for each bond being formed. (This allows for the electrons coming from the other atoms. ) 3) Allow for any ion charge. For example, if the ion has a 1 - charge, add one more electron. For a 1+ charge, deduct an electron. 4) Divide by 2 to find the total number of electron pairs around the central atom. 5) Work out how many of these are bonding pairs, and how many are lone pairs. You know how many bonding pairs there are because you know how many other atoms are joined to the central atom (assuming that only single bonds are formed). • For example, if you have 4 pairs of electrons but only 3 bonds, there must be 1 lone pair as well as the 3 bonding pairs. 6) Arrange these electron pairs in space to minimize repulsions. 9

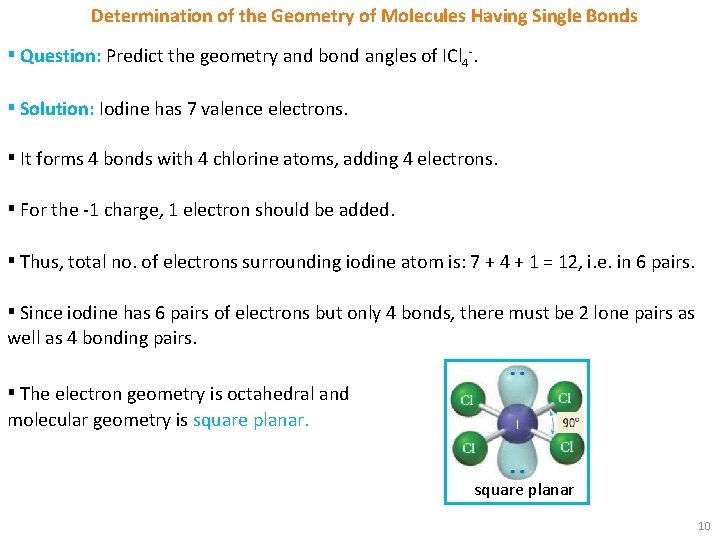
Determination of the Geometry of Molecules Having Single Bonds ▪ Question: Predict the geometry and bond angles of ICl 4 -. ▪ Solution: Iodine has 7 valence electrons. ▪ It forms 4 bonds with 4 chlorine atoms, adding 4 electrons. ▪ For the -1 charge, 1 electron should be added. ▪ Thus, total no. of electrons surrounding iodine atom is: 7 + 4 + 1 = 12, i. e. in 6 pairs. ▪ Since iodine has 6 pairs of electrons but only 4 bonds, there must be 2 lone pairs as well as 4 bonding pairs. ▪ The electron geometry is octahedral and molecular geometry is square planar 10

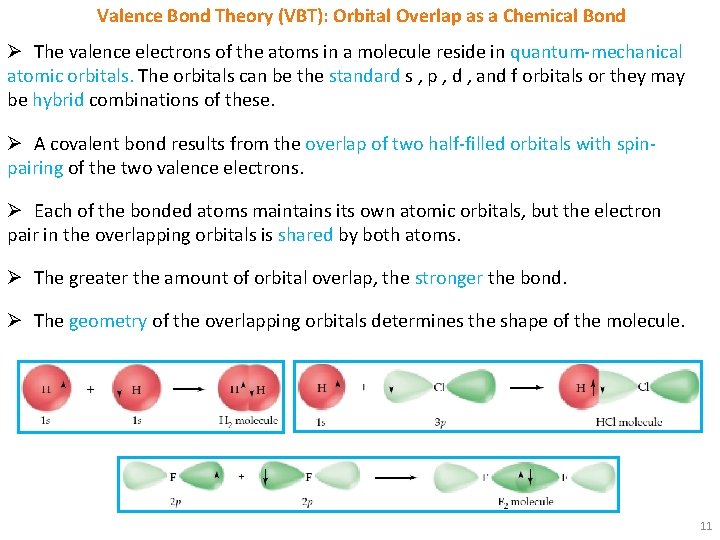
Valence Bond Theory (VBT): Orbital Overlap as a Chemical Bond Ø The valence electrons of the atoms in a molecule reside in quantum-mechanical atomic orbitals. The orbitals can be the standard s , p , d , and f orbitals or they may be hybrid combinations of these. Ø A covalent bond results from the overlap of two half-filled orbitals with spinpairing of the two valence electrons. Ø Each of the bonded atoms maintains its own atomic orbitals, but the electron pair in the overlapping orbitals is shared by both atoms. Ø The greater the amount of orbital overlap, the stronger the bond. Ø The geometry of the overlapping orbitals determines the shape of the molecule. 11

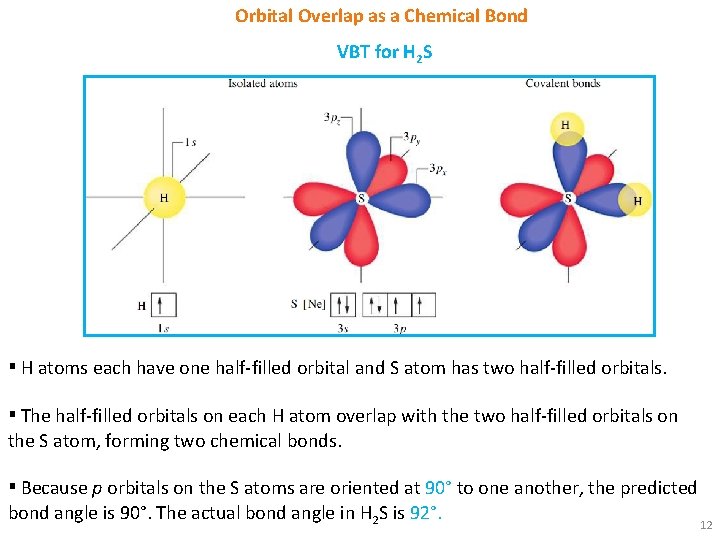
Orbital Overlap as a Chemical Bond VBT for H 2 S ▪ H atoms each have one half-filled orbital and S atom has two half-filled orbitals. ▪ The half-filled orbitals on each H atom overlap with the two half-filled orbitals on the S atom, forming two chemical bonds. ▪ Because p orbitals on the S atoms are oriented at 90° to one another, the predicted bond angle is 90°. The actual bond angle in H 2 S is 92°. 12

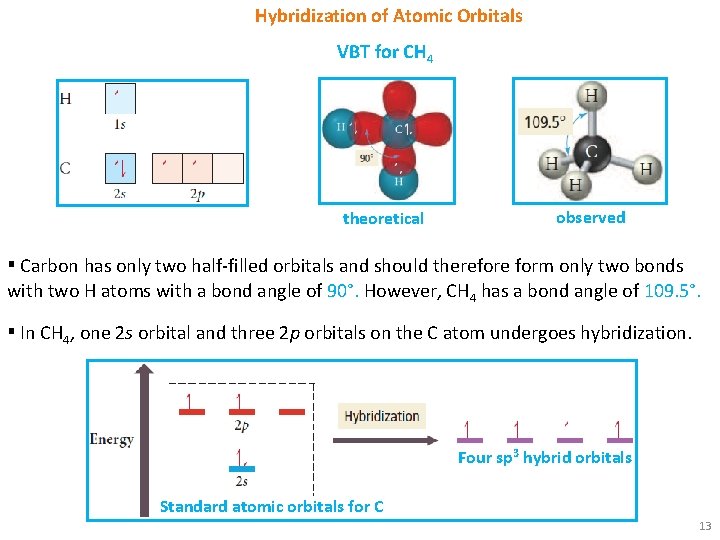
Hybridization of Atomic Orbitals VBT for CH 4 theoretical observed ▪ Carbon has only two half-filled orbitals and should therefore form only two bonds with two H atoms with a bond angle of 90°. However, CH 4 has a bond angle of 109. 5°. ▪ In CH 4, one 2 s orbital and three 2 p orbitals on the C atom undergoes hybridization. Four sp 3 hybrid orbitals Standard atomic orbitals for C 13

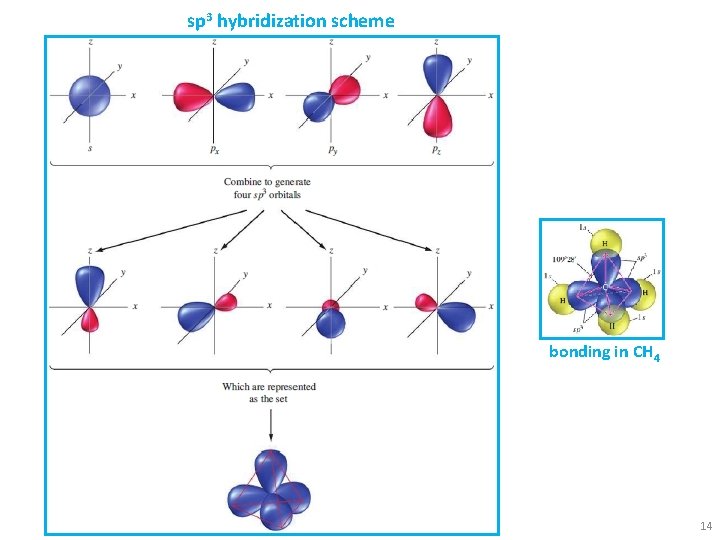
sp 3 hybridization scheme bonding in CH 4 14

Hybridization of Atomic Orbitals ▪ Hybridization: It is a mathematical procedure in which the standard atomic orbitals are combined to form new atomic orbitals called hybrid orbitals. ▪ Hybrid orbitals are still localized on individual atoms, but they have different shapes and energies from those of standard atomic orbitals. ▪ Number of hybrid orbitals equals total number of atomic orbitals that are combined. ▪ Why do we hypothesize that electrons in some molecules occupy hybrid orbitals? ▪ The energy required for promotion and hybridization is recovered by: 1) Greater Strength of Bond: Greater the overlap, stronger the bond and the lower the energy. In hybrid orbitals, the electron probability density is more concentrated in a single directional lobe, allowing greater overlap with the orbitals of other atoms. 2) Greater Number of Bonds: Often more number of bonds are formed after promotion and hybridization. 15

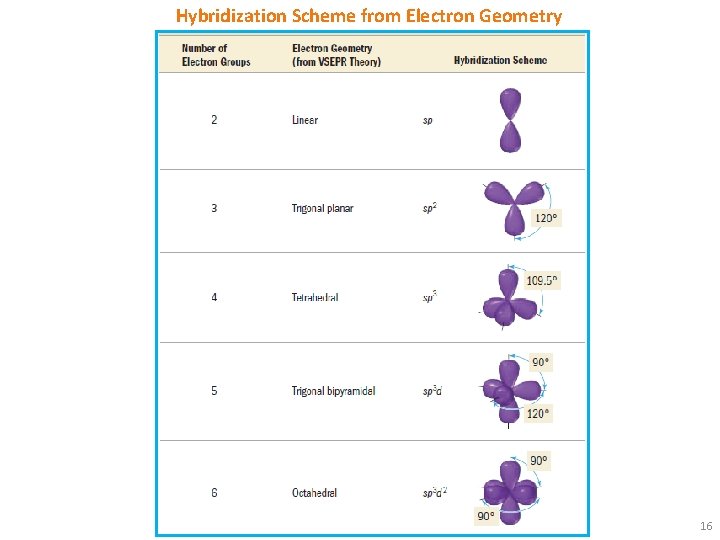
Hybridization Scheme from Electron Geometry 16
 What does the vsepr theory stand for
What does the vsepr theory stand for Vsepr theory formula
Vsepr theory formula How to memorize molecular geometry
How to memorize molecular geometry Explain vsepr theory
Explain vsepr theory Vsepr theory formula
Vsepr theory formula Vsepr theory angles
Vsepr theory angles Vsepr theory project
Vsepr theory project Vsepr theory assignment
Vsepr theory assignment Vserp pia
Vserp pia The shape of ab3e
The shape of ab3e Ammonia bond angle and shape
Ammonia bond angle and shape Importance of vsepr theory
Importance of vsepr theory Bond 8 generacji
Bond 8 generacji Steve ward calm counts
Steve ward calm counts Tdot traffic count
Tdot traffic count Cage questionnaire
Cage questionnaire It consists of numbers representing counts or measurements.
It consists of numbers representing counts or measurements.