CHEMISTRY PROJECT VSEPR THEORY Class xi CONTENT 1










- Slides: 23

CHEMISTRY PROJECT VSEPR THEORY Class xi

CONTENT 1. 2. 3. 4. 5. 6. 7. INTRODUCTION BASIC ASSUMPTIONS AXE METHOD EXAMPLES EXCEPTIONS APPENDIX BIBLIOGRAPHY

INTRODUCTION Valence shell electron pair repulsion (VSEPR) theory (1957) is a model in chemistry, which is used for predicting the shapes of individual molecules, based upon their extent of electron-pair electrostatic repulsion, determined using steric numbers. The theory is also called the Gillespie. Nyholm theory after the two main developers, and VSEPR is sometimes pronounced as "vesper" which is easier to say.

The premise of VSEPR is that a constructed Lewis structure is expanded to show all lone pairs of electrons alongside protruding and projecting bonds, for predicting the geometric shape and lone-pair behavior of a molecule through consideration of the total coordination number. VSEPR theory is based on the idea that the geometry of a molecule or polyatomic ion is determined primarily by repulsion among the pairs of electrons associated with a central atom. The pairs of electrons may be bonding or nonbonding (also called lone pairs). Only valence electrons of the central atom influence the molecular shape in a meaningful way.

BASIC ASSUMPTIONS 1. 2. 3. 4. 5. Pairs of electrons in the valence shell of a central atom repel each other. These pairs of electrons tend to occupy positions in space that minimize repulsions and maximize the distance of separation between them. The valence shell is taken as a sphere with electron pairs localizing on the spherical surface at maximum distance from one another. A multiple bond is treated as if it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair. Where two or more resonance structures can depict a molecule the VSEPR model is applicable to any such structure.

Three types of repulsion take place between the electrons of a molecule: The lone pair-lone pair repulsion The lone pair-bonding pair repulsion The bonding pair-bonding pair repulsion. A molecule must avoid these repulsions to remain stable. When repulsion cannot be avoided, the weaker repulsion (i. e. the one that causes the smallest deviation from the ideal shape) is preferred. The lone pair-lone pair (lp-lp) repulsion is considered to be stronger than the lone pairbonding pair (lp-bp) repulsion, which in turn is stronger than the bonding pair-bonding pair (bpbp) repulsion. Hence, the weaker bp-bp repulsion is preferred over the lp-lp or lp-bp

VSEPR theory is usually compared (but not part of) and contrasted with valence bond theory, which addresses molecular shape through orbitals that are energetically accessible for bonding. Valence bond theory concerns itself with the formation of sigma and pi bonds. Molecular orbital theory is another model for understanding how atoms and electrons are assembled into molecules and polyatomic ions. VSEPR theory has long been criticized for not being quantitative, and therefore limited to the generation of "crude", even though structurally accurate, molecular geometries of covalent molecules. However, molecular mechanics force fields based on VSEPR have

AXE Method The "AXE method" of electron counting is commonly used when applying the VSEPR theory. The A represents the central atom and always has an implied subscript one. The X represents how many sigma bonds are formed between the central atoms and outside atoms. Multiple covalent bonds (double, triple, etc) count as one X. The E represents the number of lone electron pairs present outside of the central atom. The sum of X and E, sometimes known as the steric number, is also associated with the total number of hybridised orbitals used by valence bond theory.


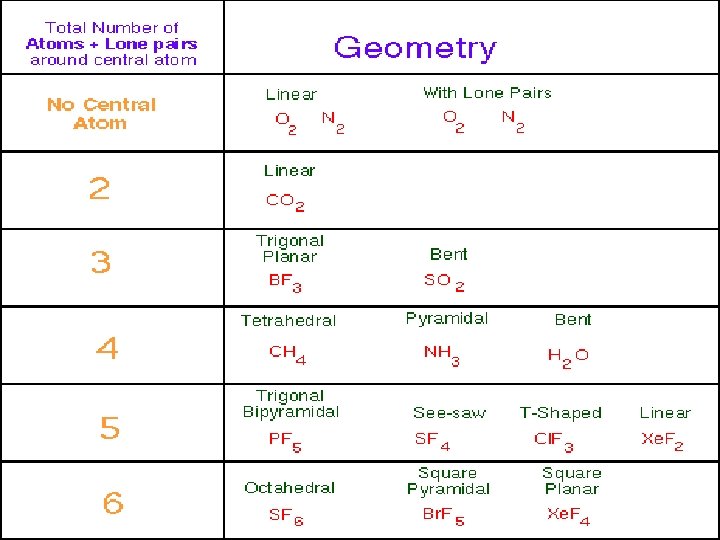
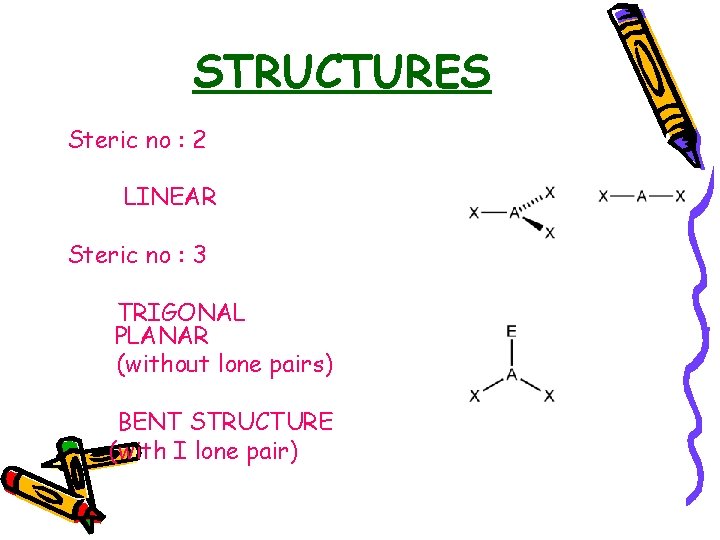
STRUCTURES Steric no : 2 LINEAR Steric no : 3 TRIGONAL PLANAR (without lone pairs) BENT STRUCTURE (with I lone pair)

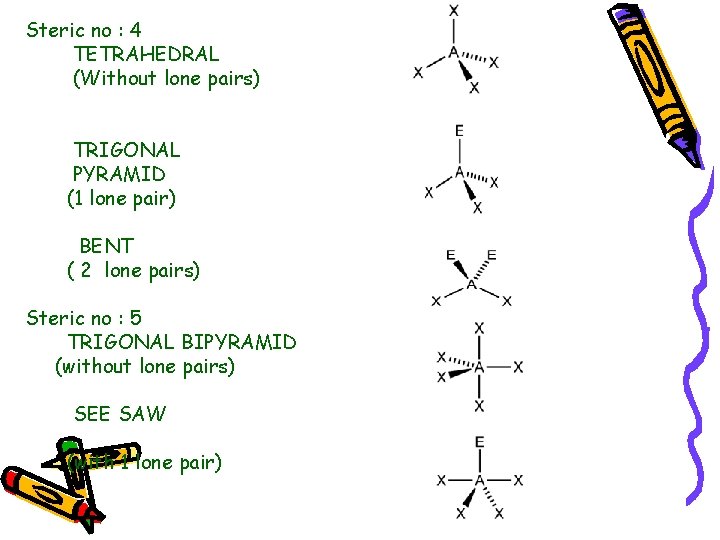
Steric no : 4 TETRAHEDRAL (Without lone pairs) TRIGONAL PYRAMID (1 lone pair) BENT ( 2 lone pairs) Steric no : 5 TRIGONAL BIPYRAMID (without lone pairs) SEE SAW (with 1 lone pair)

T SHAPED (2 lone pairs) LINEAR (3 lone pairs) Steric no : 6 OCTAHEDRAL (without lone pairs) SQUARE PYRAMID (with 1 lone pair) SQUARE PLANAR (with 2 lone pairs)

Steric no : 7 PENTAGONAL BIPYRAMID (without lone pairs) PENTAGONAL PYRAMID (with 1 lone pair)

EXAMPLES The methane molecule (CH 4) is tetrahedral because there are four pairs of electrons. The four hydrogen atoms are positioned at the vertices of a tetrahedron, and the bond angle is cos-1(-1/3) ≈ 109° 28'. This is referred to as an AX 4 type of molecule. A represents the central atom and X represents all of the outer atoms.

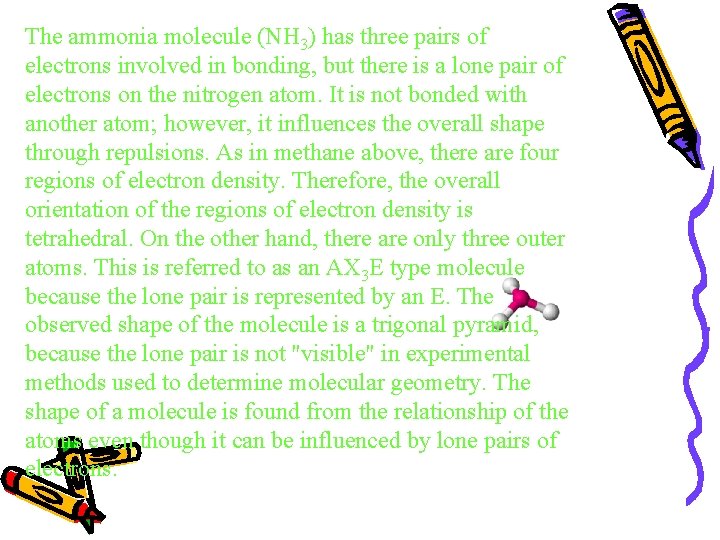
The ammonia molecule (NH 3) has three pairs of electrons involved in bonding, but there is a lone pair of electrons on the nitrogen atom. It is not bonded with another atom; however, it influences the overall shape through repulsions. As in methane above, there are four regions of electron density. Therefore, the overall orientation of the regions of electron density is tetrahedral. On the other hand, there are only three outer atoms. This is referred to as an AX 3 E type molecule because the lone pair is represented by an E. The observed shape of the molecule is a trigonal pyramid, because the lone pair is not "visible" in experimental methods used to determine molecular geometry. The shape of a molecule is found from the relationship of the atoms even though it can be influenced by lone pairs of electrons.

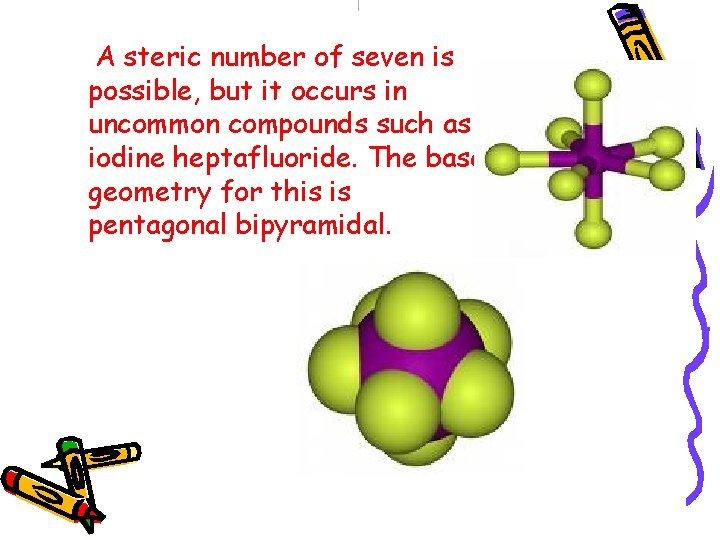
A steric number of seven is possible, but it occurs in uncommon compounds such as iodine heptafluoride. The base geometry for this is pentagonal bipyramidal.

EXCEPTIONS Transition metal compounds Many transition metal compounds do not have geometries explained by VSEPR which can be ascribed to there being no lone pairs in the valence shell and the interaction of core d electrons with the ligands. The structure of some of these compounds, including metal hydrides and alkyl complexes such as hexamethyltungsten, can be predicted correctly using the VALBOND theory, which is based on sd hybrid orbitals and the 3 -center-4 -electron bonding model. Crystal field theory is another theory that can often predict the geometry of coordination complexes.

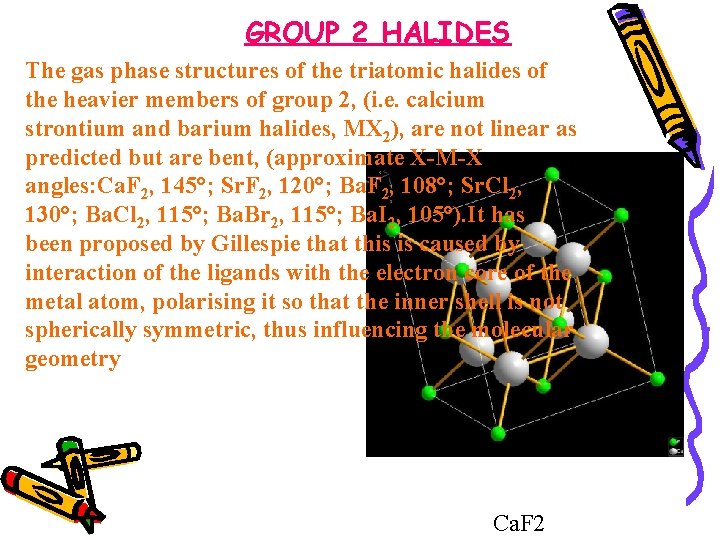
GROUP 2 HALIDES The gas phase structures of the triatomic halides of the heavier members of group 2, (i. e. calcium strontium and barium halides, MX 2), are not linear as predicted but are bent, (approximate X-M-X angles: Ca. F 2, 145°; Sr. F 2, 120°; Ba. F 2, 108°; Sr. Cl 2, 130°; Ba. Cl 2, 115°; Ba. Br 2, 115°; Ba. I 2, 105°). It has been proposed by Gillespie that this is caused by interaction of the ligands with the electron core of the metal atom, polarising it so that the inner shell is not spherically symmetric, thus influencing the molecular geometry Ca. F 2

Some AX 2 E 2 molecules One example is molecular lithium oxide, Li 2 O, which is linear rather than being bent, and this has been ascribed to the bonding being essentially ionic leading to strong repulsion between the lithium atoms. Another example is O(Si. H 3)2 with an Si-O-Si angle of 144. 1° which compares to the angles in Cl 2 O (110. 9°), (CH 3)2 O (111. 7°)and N(CH 3)3 (110. 9°). Gillespies rationalisation is that the localisation of the lone pairs, and therefore their ability to repel other electron pairs, is greatest when the ligand has an electronegativity similar to, or greater than, the central atom. When the central atom is more electronegative, as in O(Si. H 3)2, the lone pairs are less well localised, have a weaker repulsive effect and this combined with the stronger ligand-ligand repulsion (-Si. H 3 is a relatively large ligand compared to the examples above) gives the larger than expected Si-O-Si bond angle

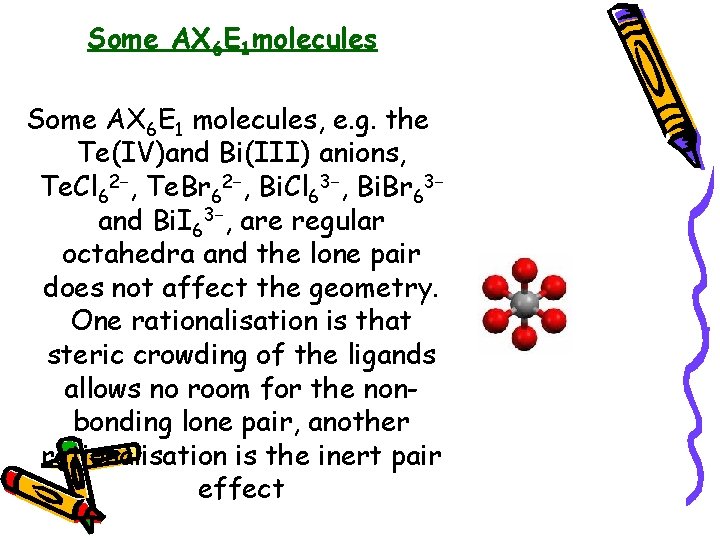
Some AX 6 E 1 molecules Some AX 6 E 1 molecules, e. g. the Te(IV)and Bi(III) anions, Te. Cl 62−, Te. Br 62−, Bi. Cl 63−, Bi. Br 63− and Bi. I 63−, are regular octahedra and the lone pair does not affect the geometry. One rationalisation is that steric crowding of the ligands allows no room for the nonbonding lone pair, another rationalisation is the inert pair effect

GLOSSARY 1. 2. Steric number : The steric number of a molecule is the number of atoms bonded to the central atom of a molecule plus the number of lone pairs on the central atom. Valence Bond Theory : Valence bond theory explains the nature of a chemical bond in a molecule in terms of atomic valencies. [ Valence bond theory summarizes the rule that the central atom in a molecule tends to form electron pair bonds in accordance with geometric constraints as defined by the octet rule, approximately.

3. Molecular Orbital Theory : Molecular orbital theory (MO theory) is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule. 4. Force field : A force field (also called a force field) refers to the functional form and parameter sets used to describe the potential energy of a system of particles (typically but not necessarily atoms).

5. 6. 7. Sigma Bond : Sigma Bond is the strongest type of covalent bond, that is symmetrical with respect to rotation about the bond axis. VALBOND Theory : In molecular mechanics, VALBOND is a method for computing the angle bending energy that is based on valence bond theory. The VALBOND functions are suitable for describing the energy of bond angle distortion not only around the equilibrium angles, but also at very large distortions. Crystal Field Theory : Crystal field theory (CFT) is a model that describes the electronic structure of transition metal compounds, all of which can be considered coordination complexes. CFT successfully accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes, but it does not attempt to describe bonding.