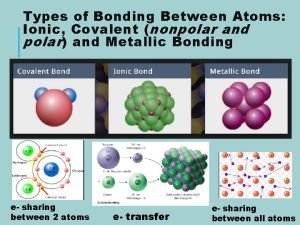
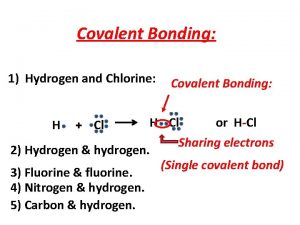
Shapes of Covalent Molecules VSEPR Theory VSEPR stands



- Slides: 12

Shapes of Covalent Molecules

VSEPR Theory • VSEPR stands for Valence shell electron pair repulsion theory • It describes how the shape of a molecule is determined by the number of pairs of electrons in the outer shell of the central atom in a bond • The electrons will always arrange themselves to be as far apart as possible as the negative charges repel eachother

The Shapes of covalent Molecules

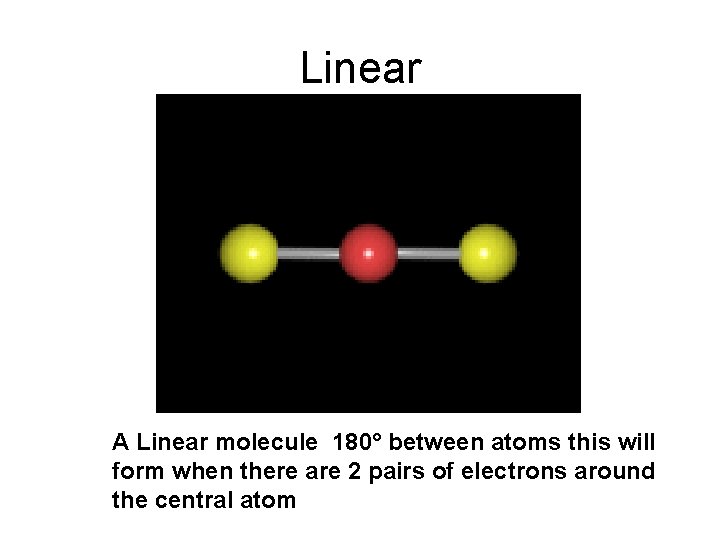
Linear A Linear molecule 180° between atoms this will form when there are 2 pairs of electrons around the central atom

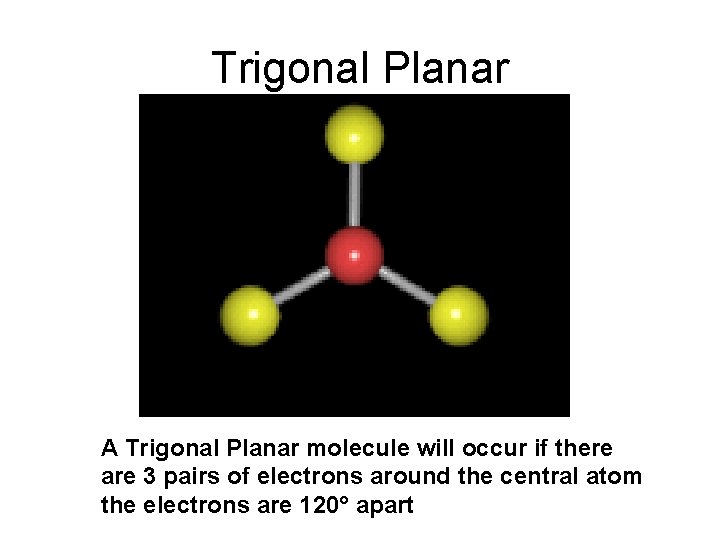
Trigonal Planar A Trigonal Planar molecule will occur if there are 3 pairs of electrons around the central atom the electrons are 120° apart

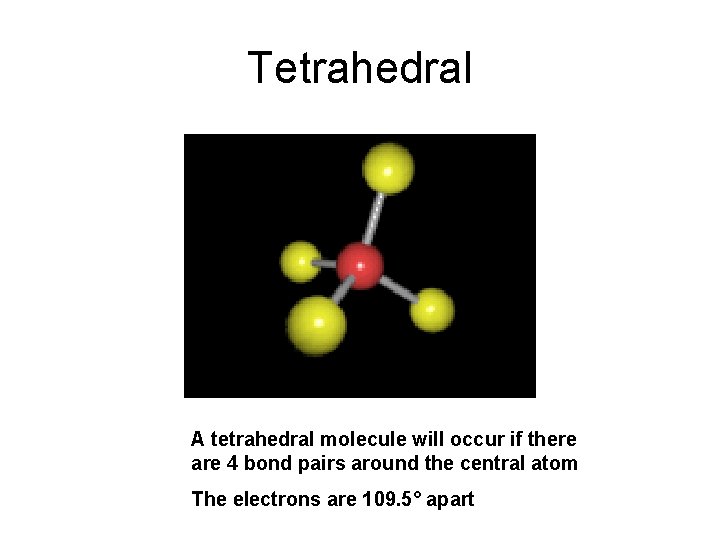
Tetrahedral A tetrahedral molecule will occur if there are 4 bond pairs around the central atom The electrons are 109. 5° apart

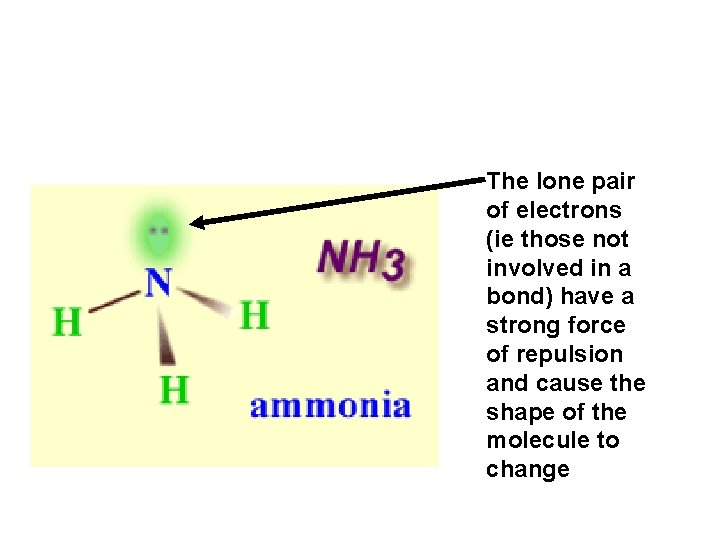
Exceptions • In all the examples we have seen there are no lone pairs of electrons in the outer shell of the central atom • If there are lone pairs they will have a stronger force of repulsion and will change the shape of a molecule • An example is seen in ammonia NH 3

The lone pair of electrons (ie those not involved in a bond) have a strong force of repulsion and cause the shape of the molecule to change

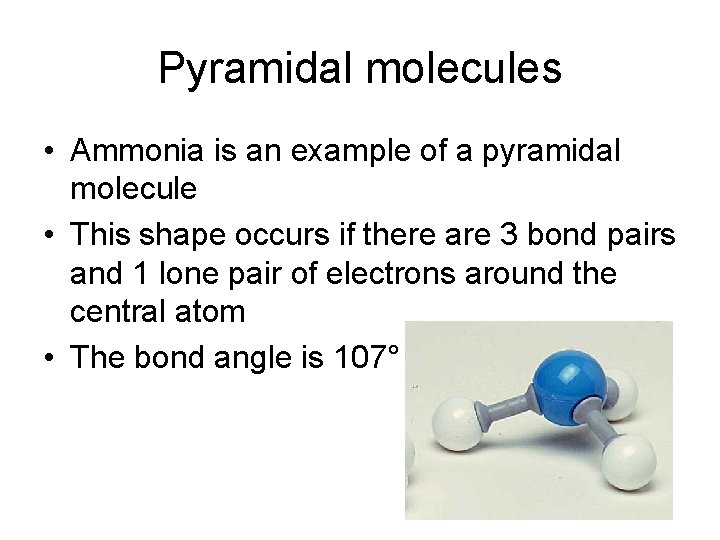
Pyramidal molecules • Ammonia is an example of a pyramidal molecule • This shape occurs if there are 3 bond pairs and 1 lone pair of electrons around the central atom • The bond angle is 107°

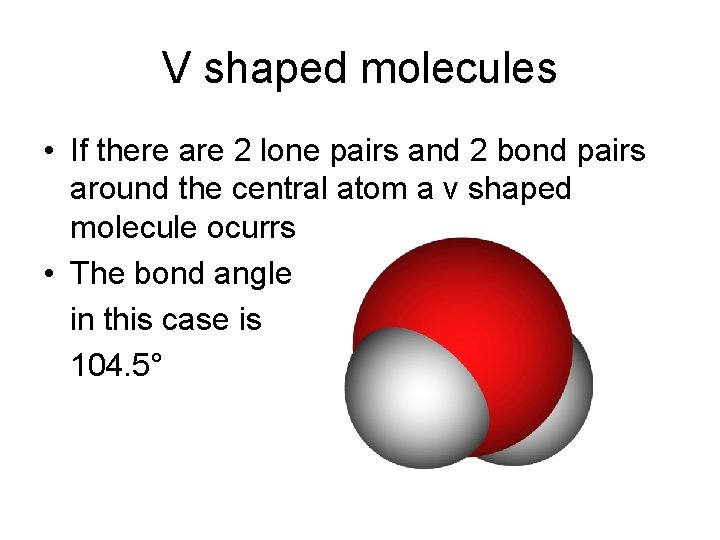
V shaped molecules • If there are 2 lone pairs and 2 bond pairs around the central atom a v shaped molecule ocurrs • The bond angle in this case is 104. 5°

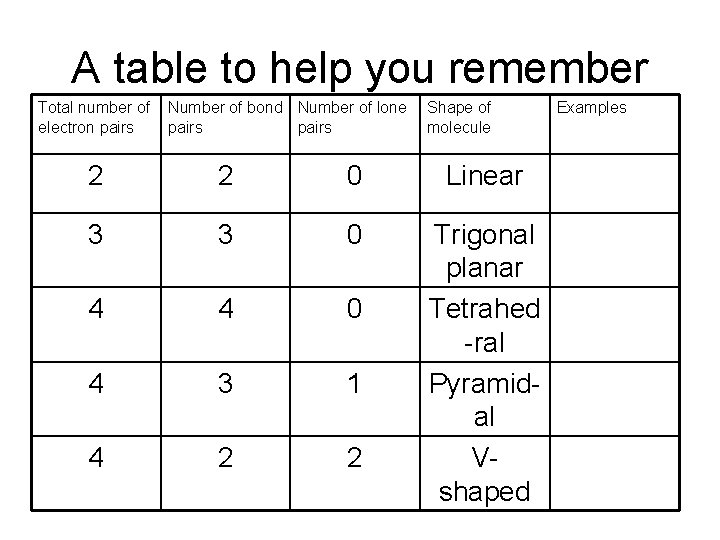
A table to help you remember Total number of electron pairs Number of bond Number of lone pairs Shape of molecule 2 2 0 Linear 3 3 0 4 4 0 4 3 1 4 2 2 Trigonal planar Tetrahed -ral Pyramidal Vshaped Examples

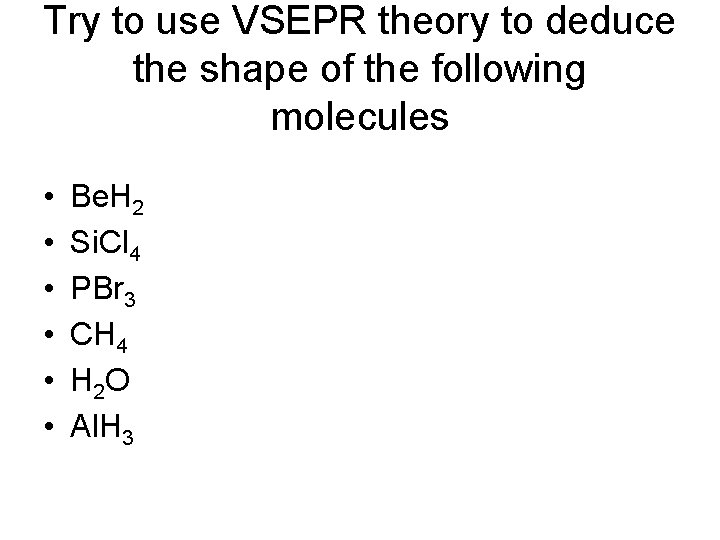
Try to use VSEPR theory to deduce the shape of the following molecules • • • Be. H 2 Si. Cl 4 PBr 3 CH 4 H 2 O Al. H 3
 Shape of ab3e molecule
Shape of ab3e molecule How to memorize vsepr theory
How to memorize vsepr theory Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Molecules with single covalent bonds
Molecules with single covalent bonds Covalent molecules
Covalent molecules Polar nonpolar ionic
Polar nonpolar ionic Coordinate covalent bond vs covalent bond
Coordinate covalent bond vs covalent bond Covalent molecular and covalent network
Covalent molecular and covalent network Water is polar or nonpolar
Water is polar or nonpolar Covalent network solid vs molecular solid
Covalent network solid vs molecular solid Network of bonded ions
Network of bonded ions Silicon carbide covalent network
Silicon carbide covalent network Electronegativity bond type chart
Electronegativity bond type chart