3 2 VSEPR Theory VSEPR Theory Valence Shell



- Slides: 14


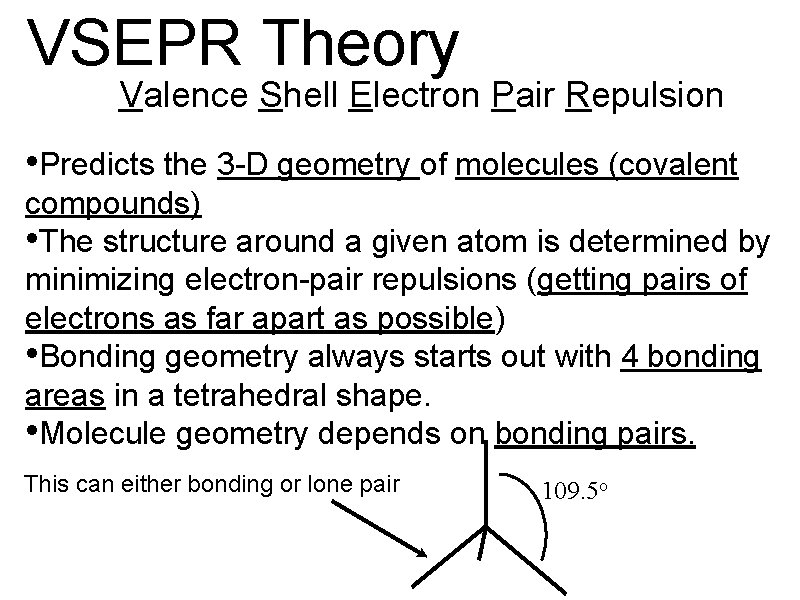
3. 2 VSEPR Theory

VSEPR Theory Valence Shell Electron Pair Repulsion • Predicts the 3 -D geometry of molecules (covalent compounds) • The structure around a given atom is determined by minimizing electron-pair repulsions (getting pairs of electrons as far apart as possible) • Bonding geometry always starts out with 4 bonding areas in a tetrahedral shape. • Molecule geometry depends on bonding pairs. This can either bonding or lone pair 109. 5 o

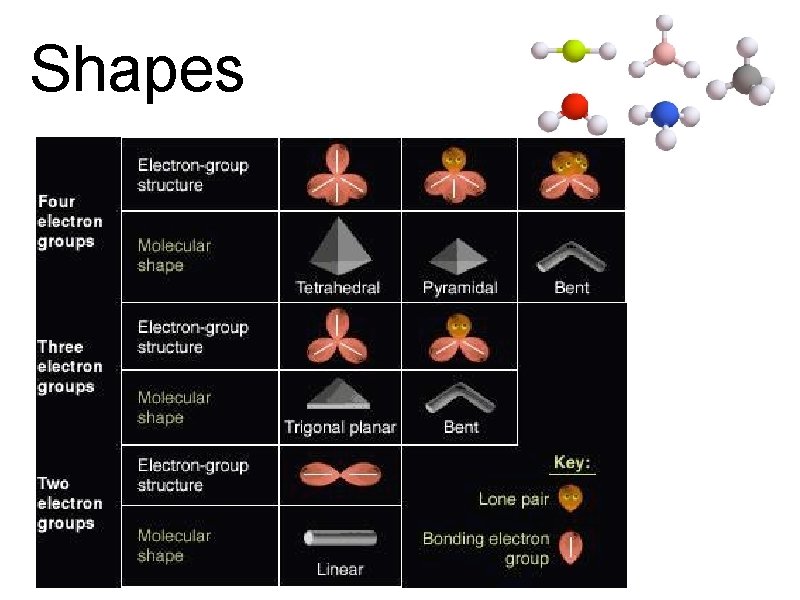
Shapes

Shapes 1. Tetrahedral – 4 bonding e- groups 2. Pyramidal – 3 bonding e- groups, 1 lone pair 3. Bent – 2 bonding e- groups, 2 lone pairs 4. Trigonal Planar – 3 bonding e- groups 5. Linear – 1 or 2 bonding e- groups

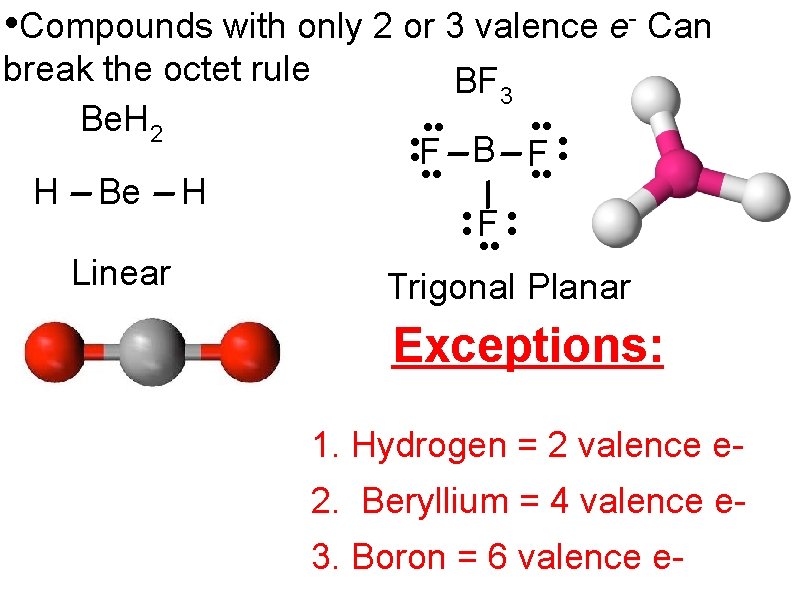
• Compounds with only 2 or 3 valence e- Can break the octet rule Be. H 2 H Be Linear H BF 3 • • F B F • • F • • Trigonal Planar Exceptions: 1. Hydrogen = 2 valence e 2. Beryllium = 4 valence e 3. Boron = 6 valence e-

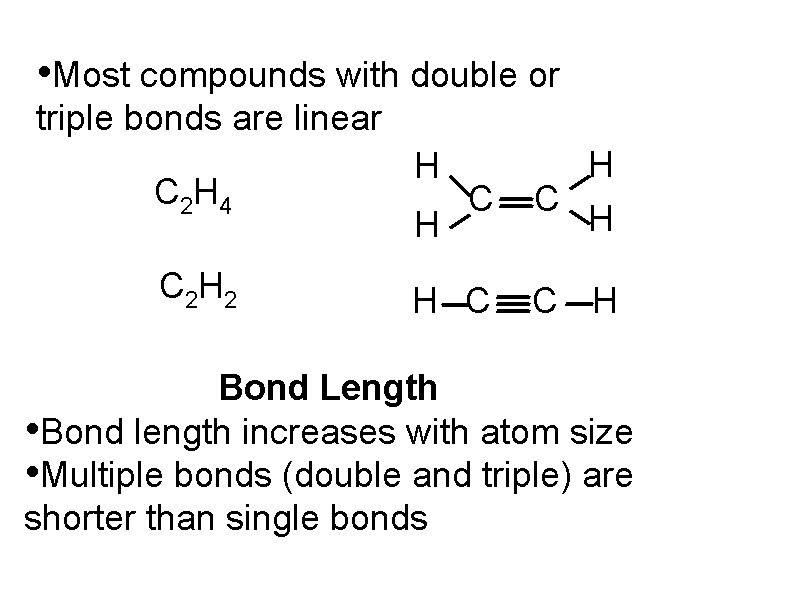
• Most compounds with double or triple bonds are linear C 2 H 4 C 2 H 2 C H H C C H H H Bond Length • Bond length increases with atom size • Multiple bonds (double and triple) are shorter than single bonds

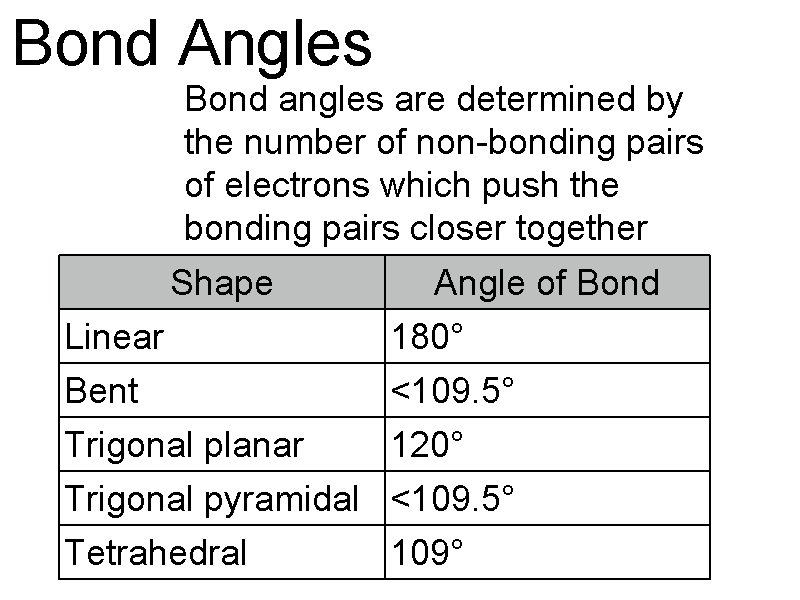
Bond Angles Bond angles are determined by the number of non-bonding pairs of electrons which push the bonding pairs closer together Shape Angle of Bond Linear 180° Bent <109. 5° Trigonal planar 120° Trigonal pyramidal <109. 5° Tetrahedral 109°

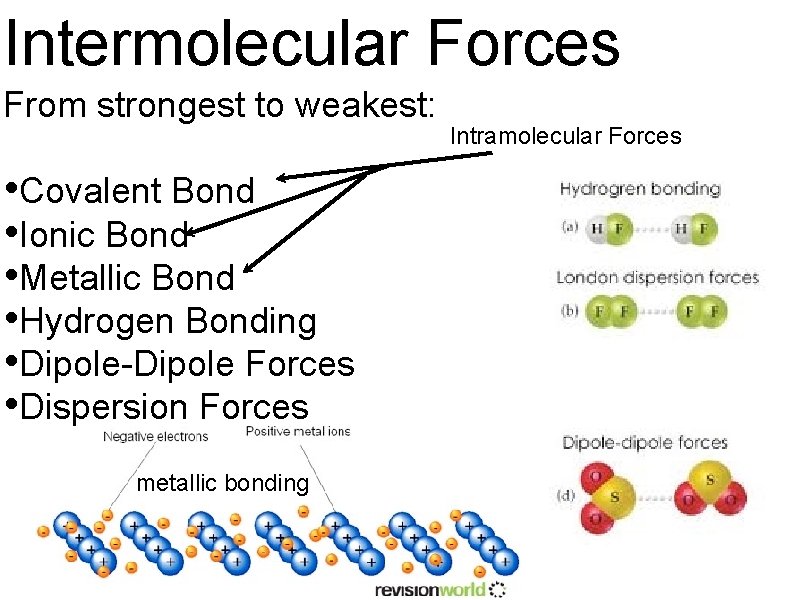
Intermolecular Forces From strongest to weakest: • Covalent Bond • Ionic Bond • Metallic Bond • Hydrogen Bonding • Dipole-Dipole Forces • Dispersion Forces metallic bonding Intramolecular Forces

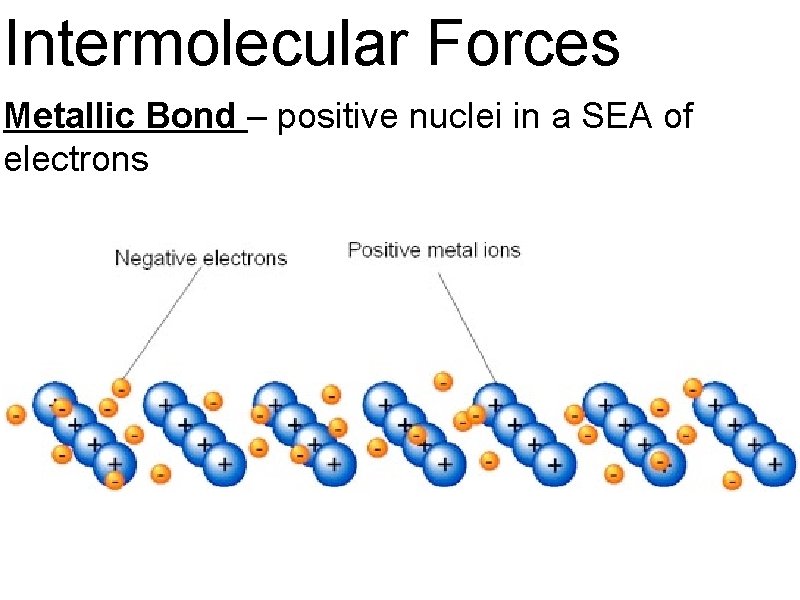
Intermolecular Forces Metallic Bond – positive nuclei in a SEA of electrons

Dispersion Forces • Found between nonpolar covalent molecules • They occur when the electrons around a molecule become unevenly distributed, causing a slight dipole (positive end & negative end)

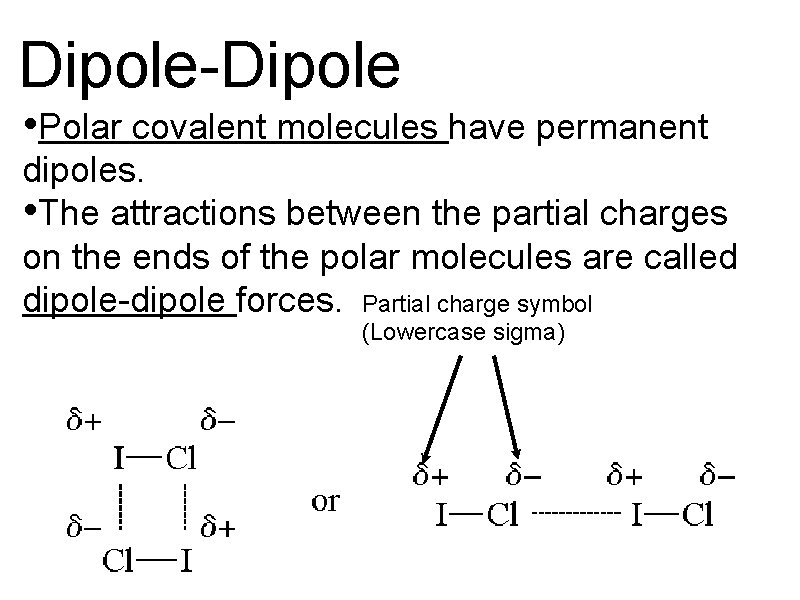
Dipole-Dipole • Polar covalent molecules have permanent dipoles. • The attractions between the partial charges on the ends of the polar molecules are called dipole-dipole forces. Partial charge symbol (Lowercase sigma)

Polarity (add these to your VSEPR shapes notes) 1. Tetrahedral – nonpolar 2. Pyramidal – polar 3. Bent – polar 4. Trigonal Planar – nonpolar 5. Linear – nonpolar

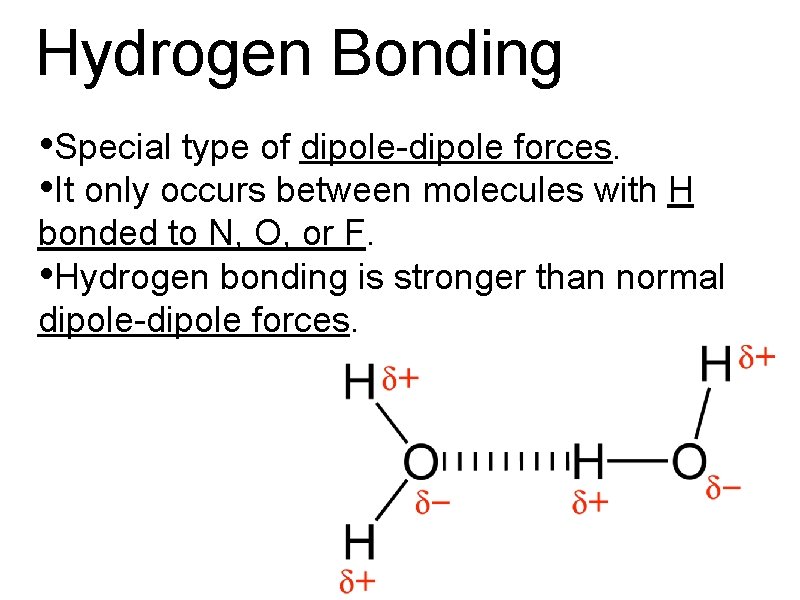
Hydrogen Bonding • Special type of dipole-dipole forces. • It only occurs between molecules with H bonded to N, O, or F. • Hydrogen bonding is stronger than normal dipole-dipole forces.