Ions Valence Shell Valence Shell The most outer
















- Slides: 28

Ions

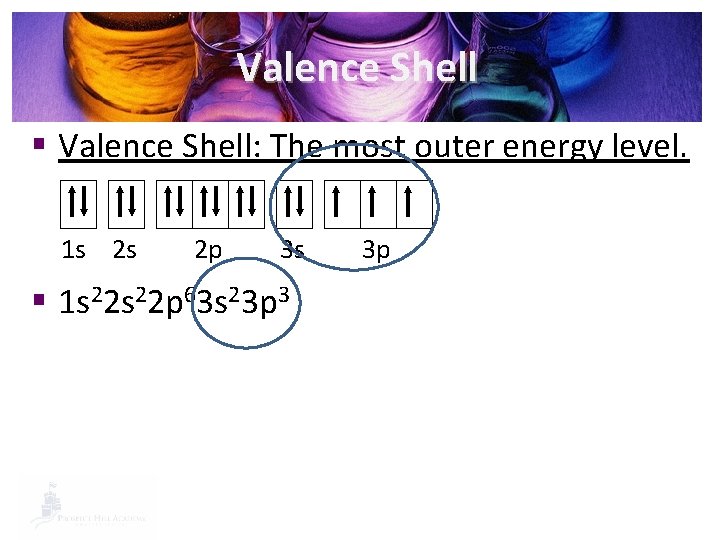
Valence Shell § Valence Shell: The most outer energy level. 1 s 2 s 2 p 3 s § 1 s 22 p 63 s 23 p 3 3 p

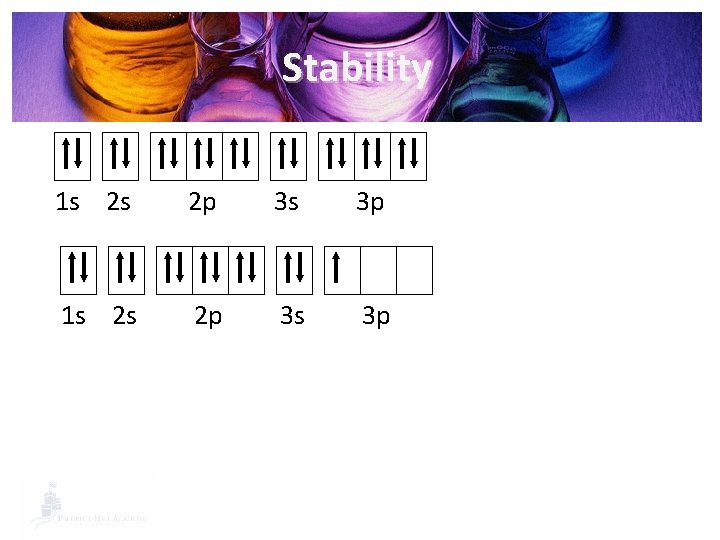
Stability 1 s 2 s 2 p 3 s 3 p

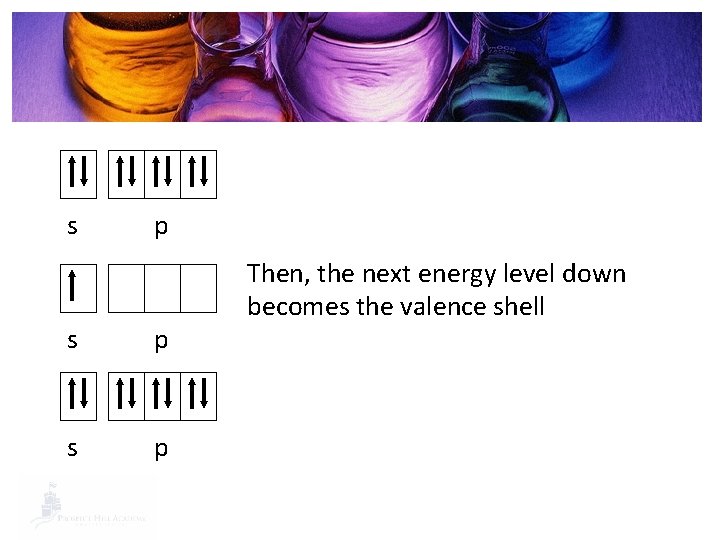
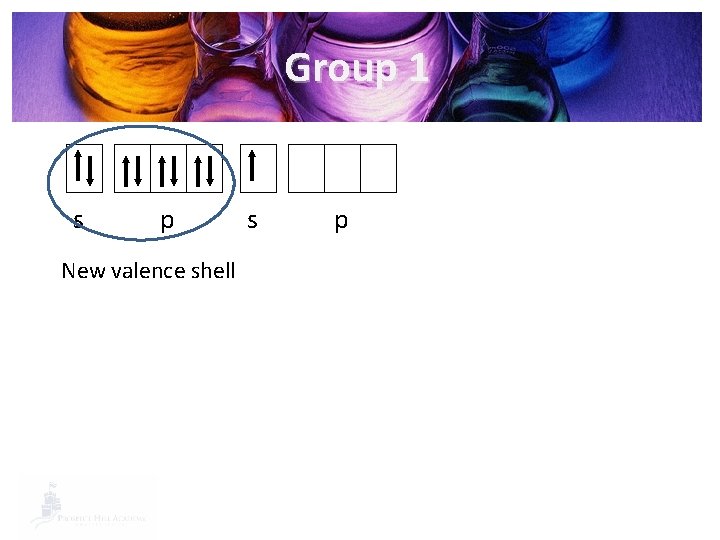
s p s p Then, the next energy level down becomes the valence shell

Valence Electrons: Label on your PTE 1 8 2 3 4 5 6 7

Group 1 s p New valence shell s p

Ions § Ion: An atom that has lost or gained one or more electrons § Cation: An atom that has lost an electron § Positive Charge § Anion: An atom that has gained an electron § Negative Charge




§ So, how do you figure out what ion an atom makes? § Look at the electron configuration.

Octet Rule § Octet Rule: All atoms lose and gain electrons to form a full valence shell § H, He = 2 § All other elements = 8 § “Oct” = 8

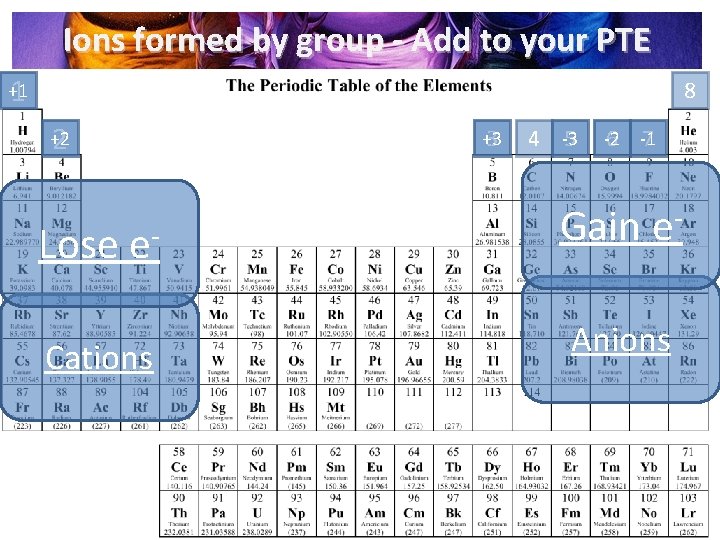
Ions formed by group - Add to your PTE 1 8 +1 2 +2 Lose e. Cations +3 3 4 -3 5 -2 6 -1 7 Gain e. Anions

Set up a table: Element # Valence Loses or Cation or Charge EGains E- Anion? of Ion 14 lines… … … Symbol of Ion Name of Ion

Number of Valence Electrons 1. 2. 3. 4. 5. 6. 7. Li Be B F O N Cl 8. 9. 10. 11. 12. 13. 14. P K Ca Al Se Br Kr

Lost or Gained Electrons? 1. 2. 3. 4. 5. 6. 7. Li Be B F O N Cl 8. 9. 10. 11. 12. 13. 14. P K Ca Al Se Br Kr

What ion is formed? 1. 2. 3. 4. 5. 6. 7. Li Be B F O N Cl 8. 9. 10. 11. 12. 13. 14. P K Ca Al Se Br Kr

Anion or Cation? § Cation is positive because it has LOST electrons. § It is a positive thing to have a cat, and it would be sad if you lost your cat. § Anion is negative because it has GAINED electrons § Mnemonic?

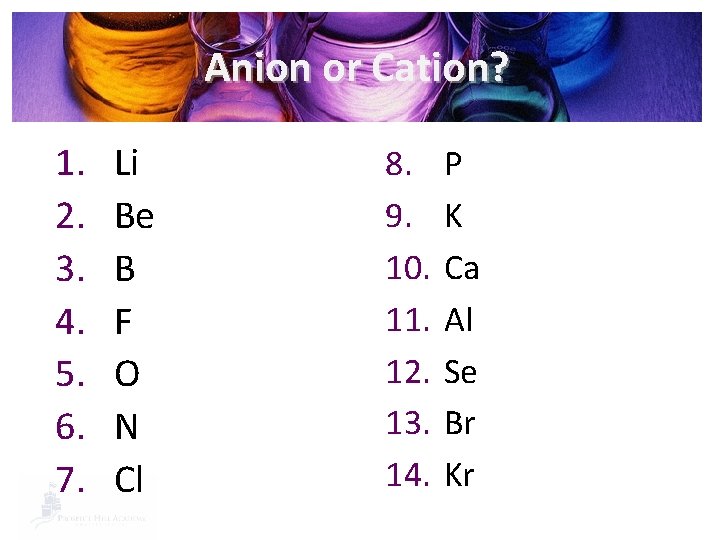
Anion or Cation? 1. 2. 3. 4. 5. 6. 7. Li Be B F O N Cl 8. 9. 10. 11. 12. 13. 14. P K Ca Al Se Br Kr

Symbols for Ions § Element Symbol. Charge § Li+ § Cl§ Al 3+ § Number, then + or – § No need to write a “ 1” § 1 is often invisible in chemistry

Write the Symbol 1. 2. 3. 4. 5. 6. 7. Li Be B F O N Cl 8. 9. 10. 11. 12. 13. 14. P K Ca Al Se Br Kr

Names for Ions § Cations: Same name as the element § Li+: lithium ion § Sr 2+: strontium ion

Naming Ions § Anions: Ending changes to “-ide” § N 3 -: nitride ion § O 2 -: oxide ion § F-: fluoride ion § S 2 -: sulfide ion § Cl-: chloride ion § Br-: bromide ion § I-: iodide ion

Name the ions formed by these elements 1. 2. 3. 4. 5. 6. 7. Li Be B F O N Cl 8. 9. 10. 11. 12. 13. 14. P K Ca Al Se Br Kr

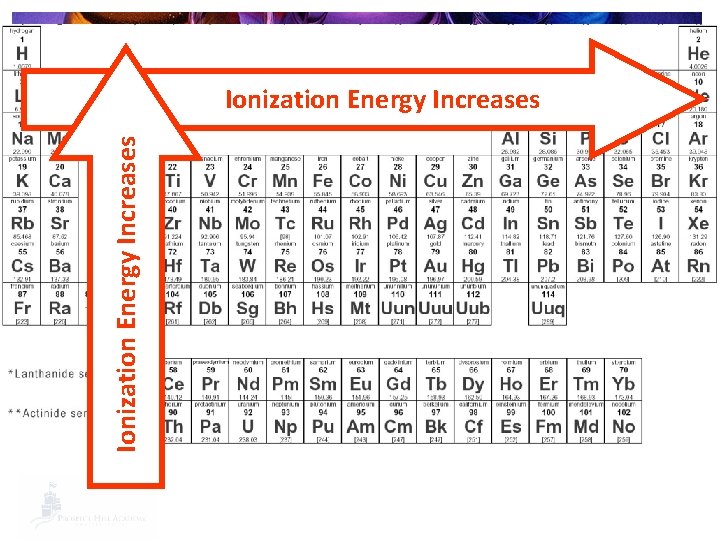
How easy is it to make an ion? § Ionization Energy: The amount of energy required to remove an electron to form a positive (+1) ion. § Increases going UP a group and across a period to the RIGHT.

Ionization Energy Increases

Trends in Ionization Energy § Why? § The smaller the atom, the closer the valence shell is to the nucleus. § The closer the valence shell is held to the nucleus, the harder it is to remove an electron. § Harder to remove = more ionization energy

Atomic Radius Increases