Molecular Shapes What is VSEPR VSEPR Valence Shell




- Slides: 20

Molecular Shapes

What is VSEPR?

VSEPR • Valence Shell Electron Pair Repulsion • The main idea is that electrons don’t like to hang around near each other because they repel each other. As a result, the atoms in a molecule tend to separate as far as they can because their bonds repel each other.

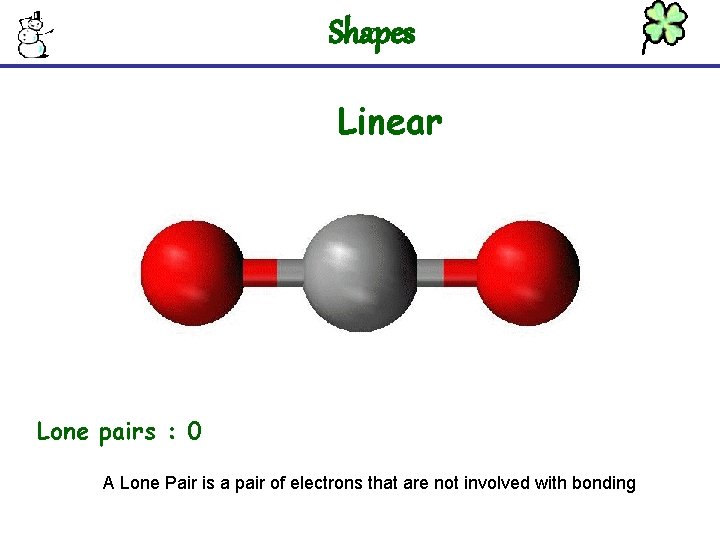
Shapes Linear Lone pairs : 0 A Lone Pair is a pair of electrons that are not involved with bonding

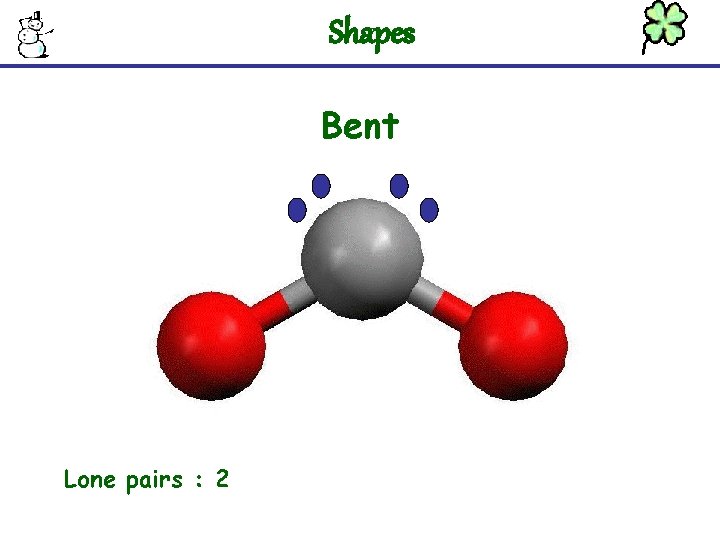
Shapes Bent Lone pairs : 2

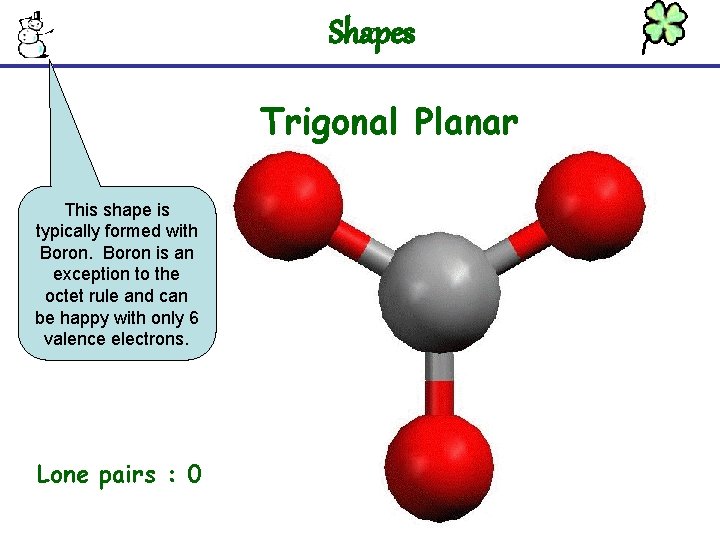
Shapes Trigonal Planar This shape is typically formed with Boron is an exception to the octet rule and can be happy with only 6 valence electrons. Lone pairs : 0

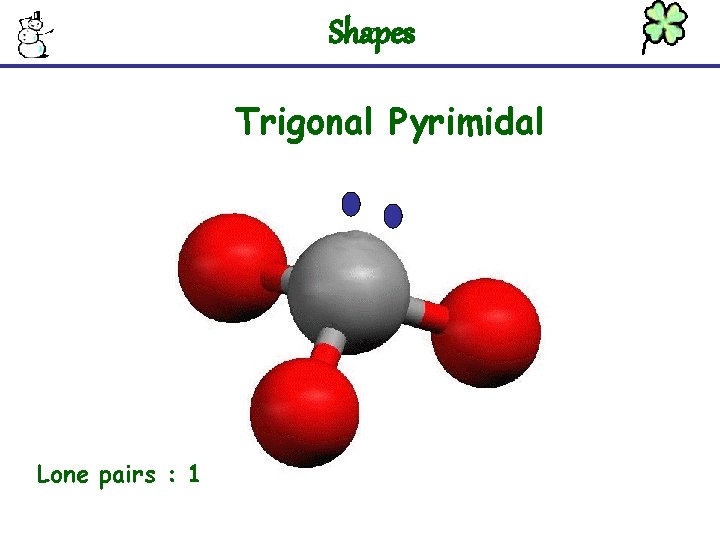
Shapes Trigonal Pyrimidal Lone pairs : 1

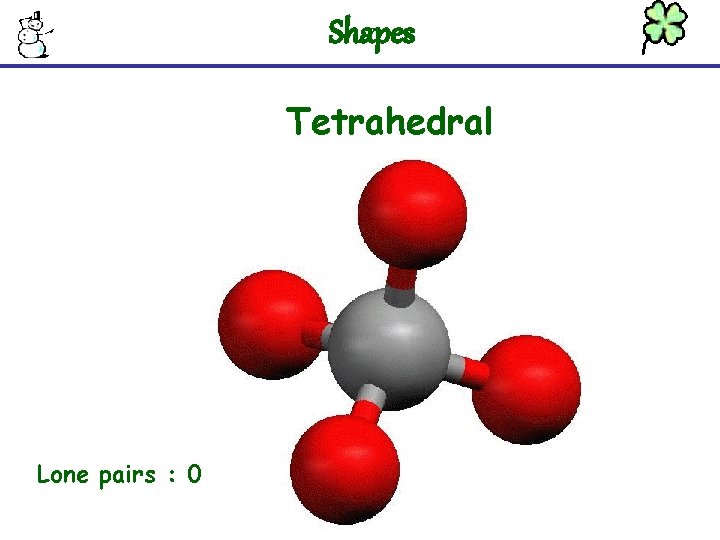
Shapes Tetrahedral Lone pairs : 0

Recap • • • Linear = 0 lone pairs Bent = 2 lone pairs Trigonal Planar = 0 lone pairs Trigonal Pyramidal = 1 lone pair Tetrahedral = 0 lone pairs

• Polar covalent molecules: A type of bond that forms when electrons are not shared equally (nonsymmetrical) • Non polar molecules: A type of bond that forms when electrons are shared equally (symmetrical)

Polarity • Go back to page 6 and for each shape, include the following examples of when the shape is polar and non polar.

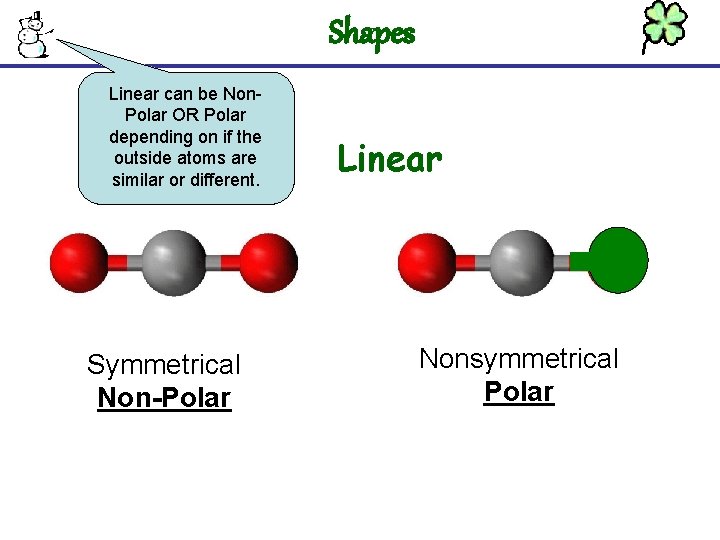
Shapes Linear can be Non. Polar OR Polar depending on if the outside atoms are similar or different. Symmetrical Non-Polar Linear Nonsymmetrical Polar

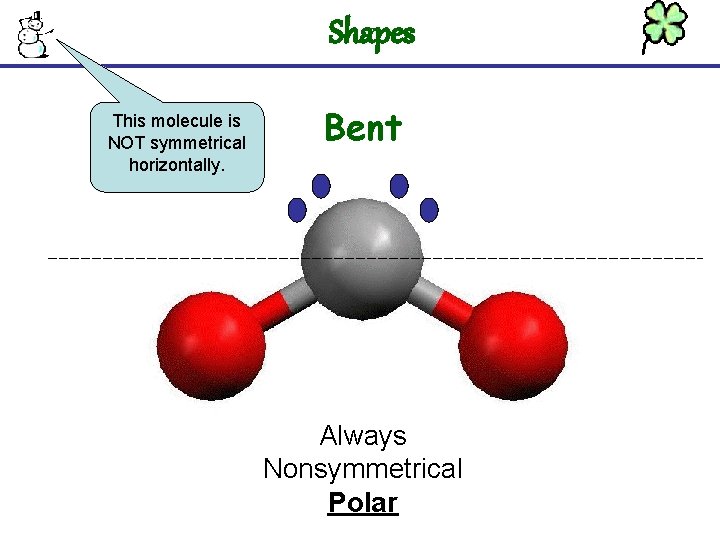
Shapes This molecule is NOT symmetrical horizontally. Bent Always Nonsymmetrical Polar

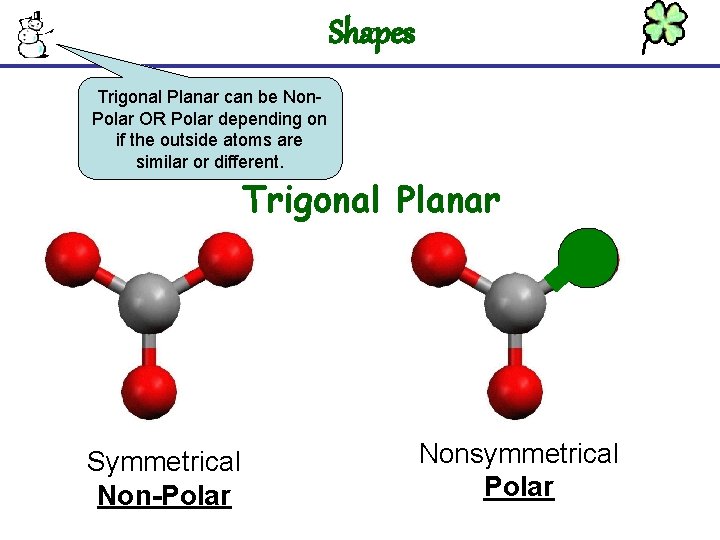
Shapes Trigonal Planar can be Non. Polar OR Polar depending on if the outside atoms are similar or different. Trigonal Planar Symmetrical Non-Polar Nonsymmetrical Polar

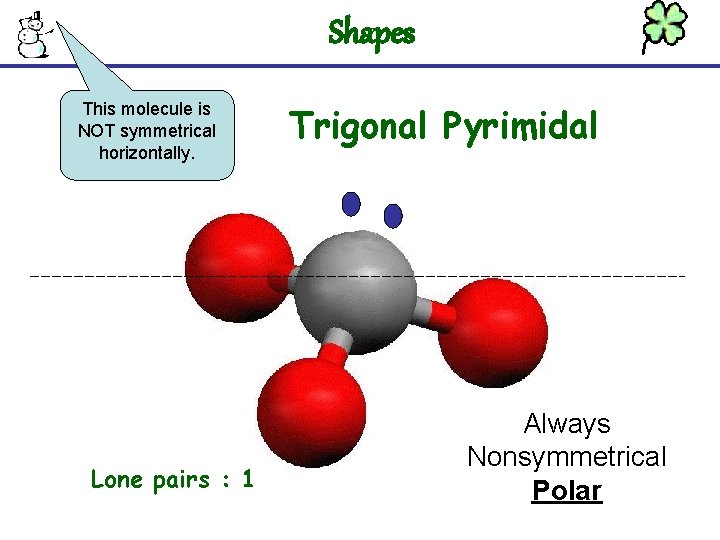
Shapes This molecule is NOT symmetrical horizontally. Lone pairs : 1 Trigonal Pyrimidal Always Nonsymmetrical Polar

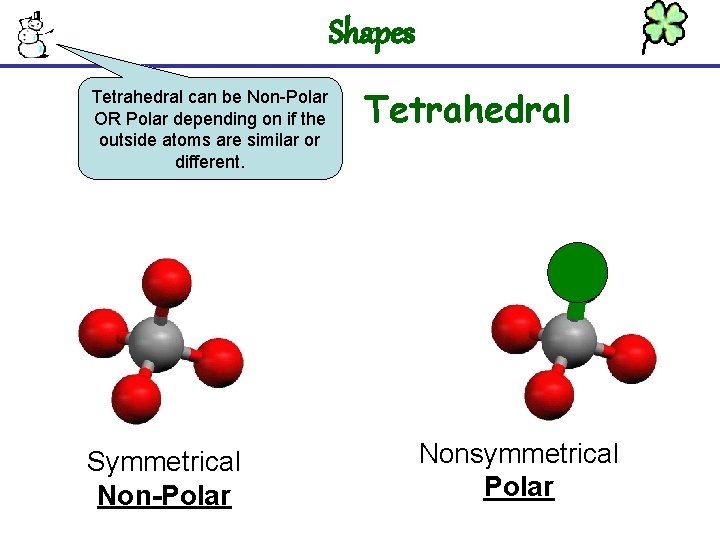
Shapes Tetrahedral can be Non-Polar OR Polar depending on if the outside atoms are similar or different. Symmetrical Non-Polar Tetrahedral Nonsymmetrical Polar

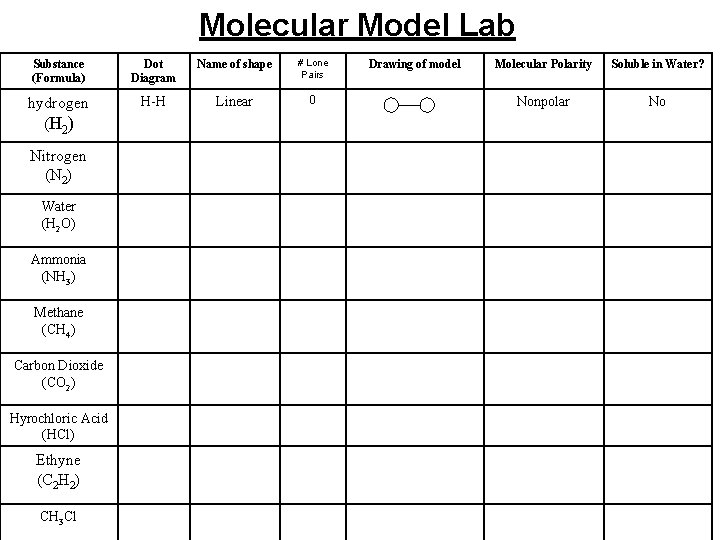
Molecular Model Lab Substance (Formula) Dot Diagram Name of shape # Lone Pairs hydrogen (H 2 ) H-H Linear 0 Nitrogen (N 2) Water (H 2 O) Ammonia (NH 3) Methane (CH 4) Carbon Dioxide (CO 2) Hyrochloric Acid (HCl) Ethyne (C 2 H 2) CH 3 Cl Drawing of model Molecular Polarity Soluble in Water? Nonpolar No

Molecular Model Lab • On the next page in the Unit 6 Packet. • You will draw electron dot diagrams, and then build the molecules using the plastic models. • All of the holes need to be filled. • Use the longer “bendy” ones to make double bonds. • Each kit should have the exact number of atoms in order to make all of the molecules without having to take any apart. • Next Slide has the Color Key

Color Codes • • • White = hydrogen Red = oxygen Black = carbon Blue = nitrogen Green = chlorine

When you finish… In the space below the chart, draw these and determine the shape and polarity of these molecules without building them. 1. BF 3 2. CF 4 3. N 2 4. H 2 S 5. CS 2 6. C 2 H 2