Molecular Geometry and VSEPR Theory VSEPR Theory To







- Slides: 12

Molecular Geometry and VSEPR Theory

VSEPR Theory To understand the shapes of molecules, we use a theory called the Valence Shell Electron Pair Repulsion Theory This theory tells us how we can understand the geometry of a molecule by looking at the electron groups that surround it Electron groups can be: Single Bonds Double Bonds (1 e- group) Triple Bonds (1 e- group) Lone Pairs (still 1 e- group)

VSEPR Theory tells us that electron groups will repel each other This is because of negative charges. A negative and a negative from the electrons will repel The electron groups then move as far away from each other as possible This will determine the shape of the molecules

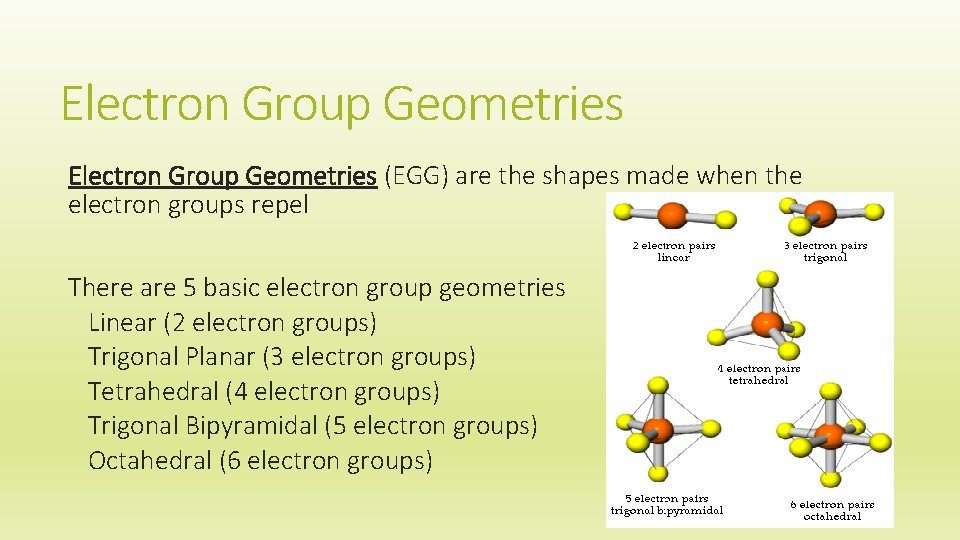
Electron Group Geometries (EGG) are the shapes made when the electron groups repel There are 5 basic electron group geometries Linear (2 electron groups) Trigonal Planar (3 electron groups) Tetrahedral (4 electron groups) Trigonal Bipyramidal (5 electron groups) Octahedral (6 electron groups)

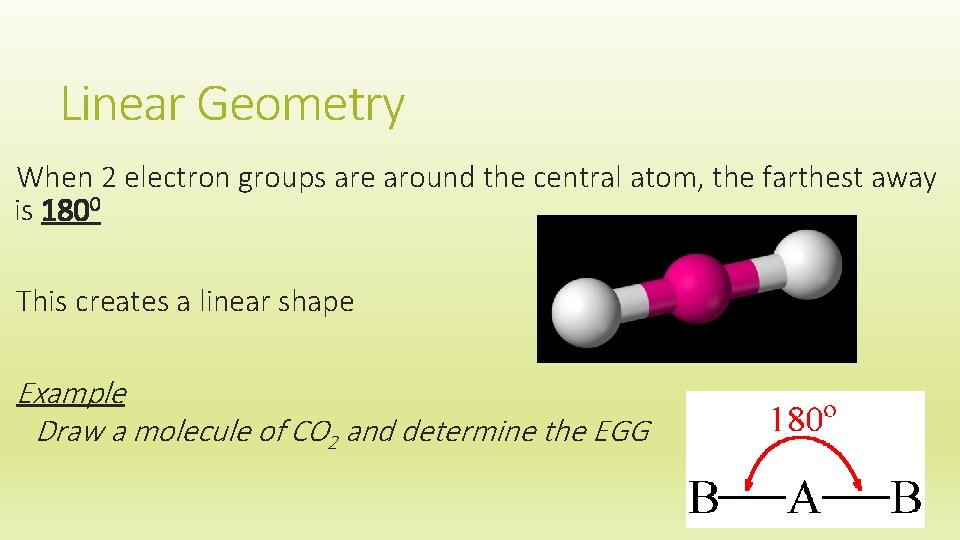
Linear Geometry When 2 electron groups are around the central atom, the farthest away is 1800 This creates a linear shape Example Draw a molecule of CO 2 and determine the EGG

Trigonal Planar With 3 electron groups around the central atom, the farthest away for all electrons to be is 1200 in the same plane Because they are in the same plane, the is called Trigonal Planar (3 in same plane) Example Draw a molecule of COCl 2 and determine the EGG

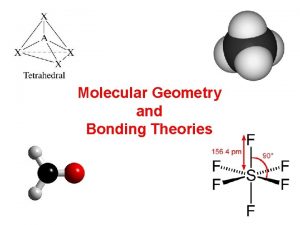
Tetrahedral With 4 Electron groups, the furthest point for electron groups is 109. 50 This may seem weird since on paper is looks like 900 but remember, molecules are 3 -dimensional and can make complex shapes This shape forms a tetrahedron and is called Tetrahedral Example Draw a molecule of NH 3 and determine the EGG

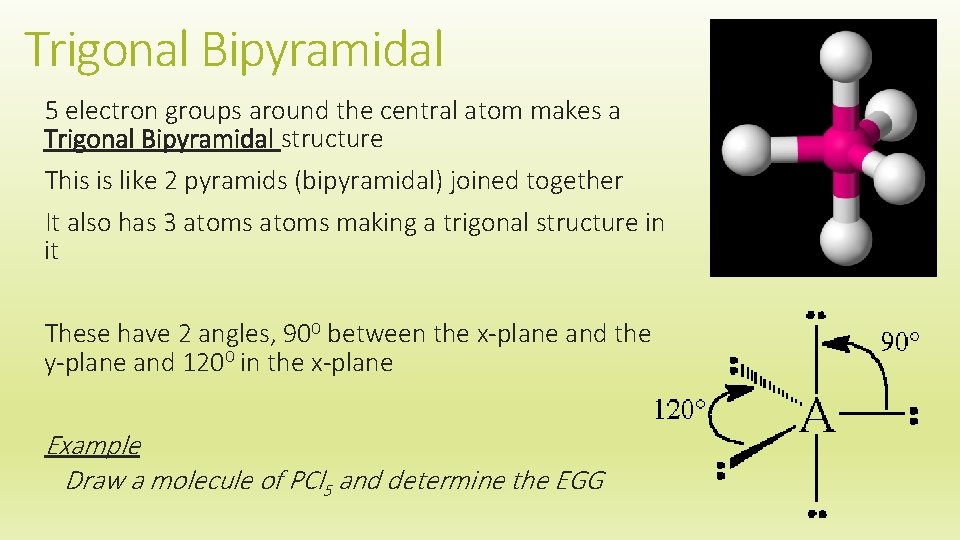
Trigonal Bipyramidal 5 electron groups around the central atom makes a Trigonal Bipyramidal structure This is like 2 pyramids (bipyramidal) joined together It also has 3 atoms making a trigonal structure in it These have 2 angles, 900 between the x-plane and the y-plane and 1200 in the x-plane Example Draw a molecule of PCl 5 and determine the EGG

Octahedral With 6 electron groups an octahedral structure is formed All angles are 900 Example Draw a molecule of SF 6 and determine the EGG

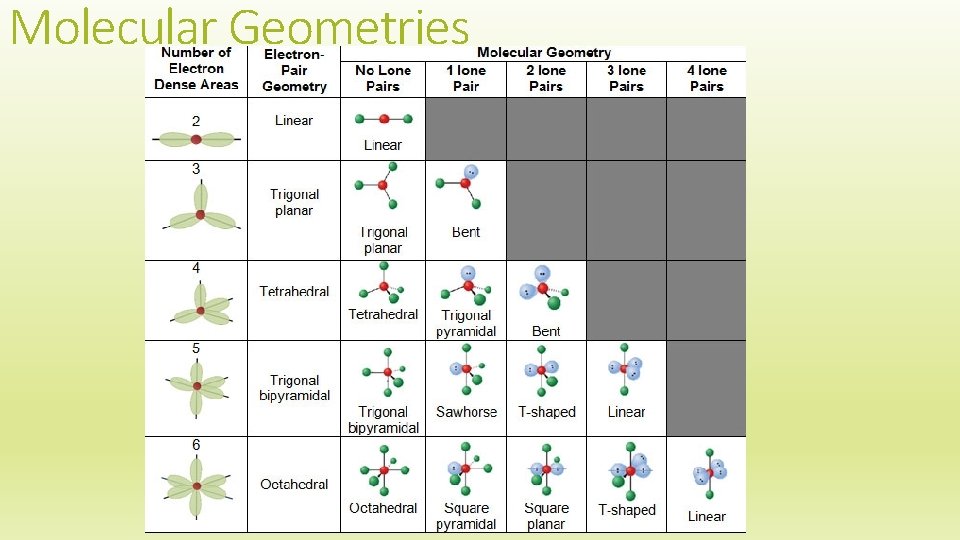
Molecular Geometry The EGG tells us the shape of ALL ELECTRON GROUPS The molecular geometry is the geometry of the atoms This is determined by the number of electron groups AND the number of bonds around a central atom

Molecular Geometries

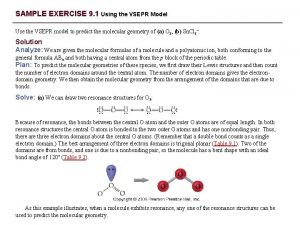
Molecular Geometry Example Draw a molecule of H 2 O Determine the EGG Use the chart provided to find the Molecular Geometry
 Pf3 molecular geometry
Pf3 molecular geometry Electron domain geometry vs molecular geometry
Electron domain geometry vs molecular geometry This molecule is
This molecule is How to memorize molecular geometry
How to memorize molecular geometry Sn lewis structure
Sn lewis structure Vsepr
Vsepr Shapes of compounds
Shapes of compounds Molecular geometry and bonding theories
Molecular geometry and bonding theories Lewis dot structure and molecular geometry
Lewis dot structure and molecular geometry Molecular shapes
Molecular shapes Molecular geometry and bonding theories
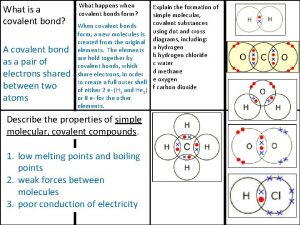
Molecular geometry and bonding theories What is a covalent bond simple definition
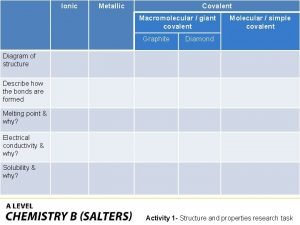
What is a covalent bond simple definition Ionic covalent metallic
Ionic covalent metallic