Molecules of Life Chapter 2 Part 1 2




























- Slides: 89

Molecules of Life Chapter 2 Part 1

2. 1 Impacts/Issues Fear of Frying § All living things consist of the same kinds of molecules, but small differences in the ways they are put together have big effects on health § Artificial trans fats found in manufactured and fast foods raise cholesterol and increase risk of atherosclerosis, heart attack, and diabetes

Video: Fear of frying

Fear of Frying § Trans fats are made by adding hydrogen atoms to liquid vegetable oils

trans fatty acid Fig. 2 -1, p. 20

2. 2 Start With Atoms § All substances consist of atoms § Atom • Fundamental building-block particle of matter § Life’s unique characteristics start with the properties of different atoms

Subatomic Particles and Their Charge § Atoms consist of electrons moving around a nucleus of protons and neutrons § Electron (e-) • Negatively charged subatomic particle that occupies orbitals around the atomic nucleus § Charge • Electrical property of some subatomic particles • Opposite charges attract; like charges repel

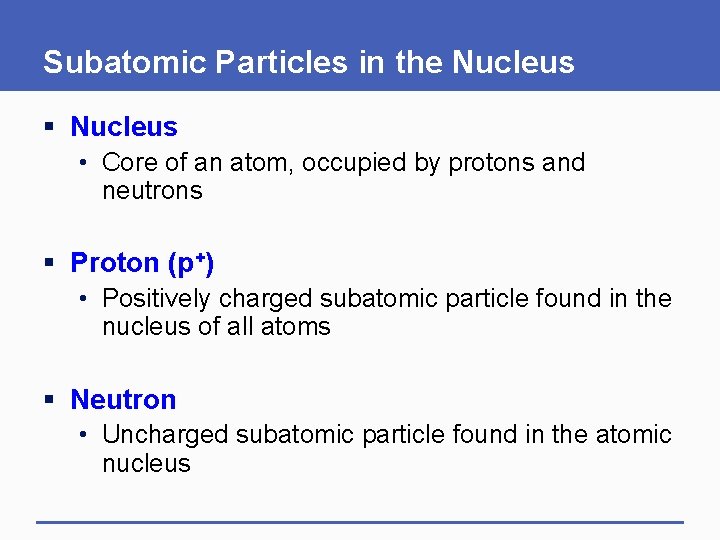
Subatomic Particles in the Nucleus § Nucleus • Core of an atom, occupied by protons and neutrons § Proton (p+) • Positively charged subatomic particle found in the nucleus of all atoms § Neutron • Uncharged subatomic particle found in the atomic nucleus

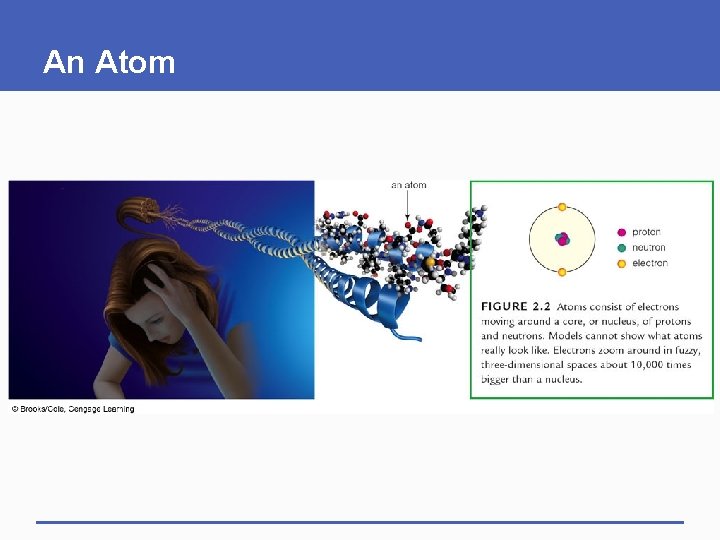
An Atom

an atom Fig. 2 -2 a, p. 21

Elements: Different Types of Atoms § Atoms differ in numbers of subatomic particles § Element • A pure substance that consists only of atoms with the same number of protons § Atomic number • Number of protons in the atomic nucleus • Determines the element

Elements in Living Things § The proportions of different elements differ between living and nonliving things § Some atoms, such as carbon, are found in greater proportions in molecules made only by living things – the molecules of life

Same Elements, Different Forms § Isotopes • Forms of an element that differ in the number of neutrons their atoms carry • Changes the mass number, but not the charge § Mass number • Total number of protons and neutrons in the nucleus of an element’s atoms

Radioactive Isotopes § Radioisotope • Isotope with an unstable nucleus, such as carbon 14 (14 C) § Radioactive decay • Process by which atoms of a radioisotope spontaneously emit energy and subatomic particles when their nucleus disintegrates

Carbon 14: A Radioisotope § Most carbon atoms have 6 protons and 6 neutrons (12 C) § Carbon 14 (14 C) is a radioisotope with six protons and eight neutrons § When 14 C decays, one neutron splits into a proton and an electron, and the atom becomes a different element – nitrogen 14 (14 N)

Radioactive Tracers § Researchers introduce radioisotope tracers into living organisms to study the way they move through a system § Tracers • Molecules with a detectable substance attached, often a radioisotope • Used in research and clinical testing

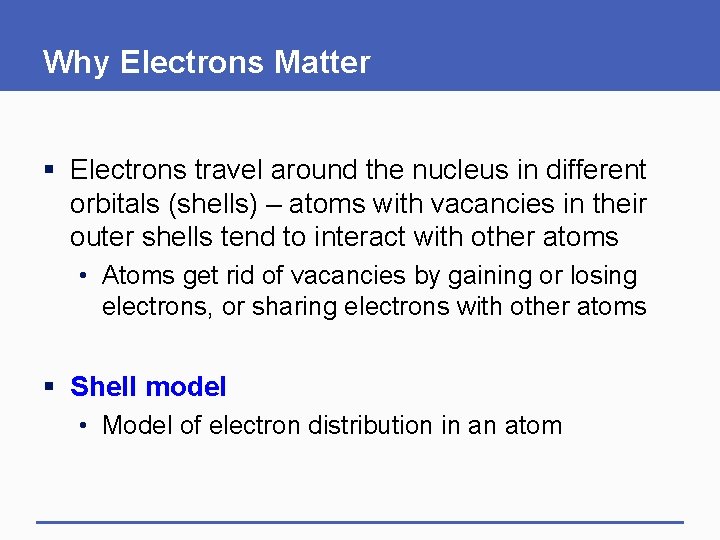
Why Electrons Matter § Electrons travel around the nucleus in different orbitals (shells) – atoms with vacancies in their outer shells tend to interact with other atoms • Atoms get rid of vacancies by gaining or losing electrons, or sharing electrons with other atoms § Shell model • Model of electron distribution in an atom

Shell Models

Fig. 2 -3 (top), p. 22

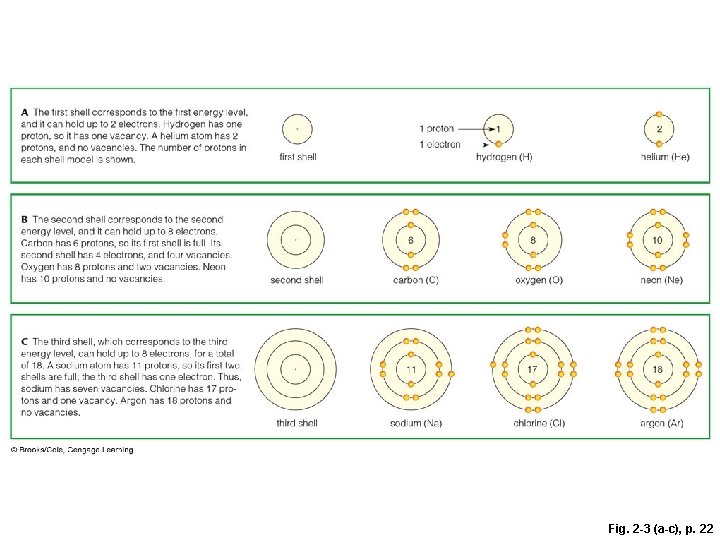
Fig. 2 -3 (a-c), p. 22

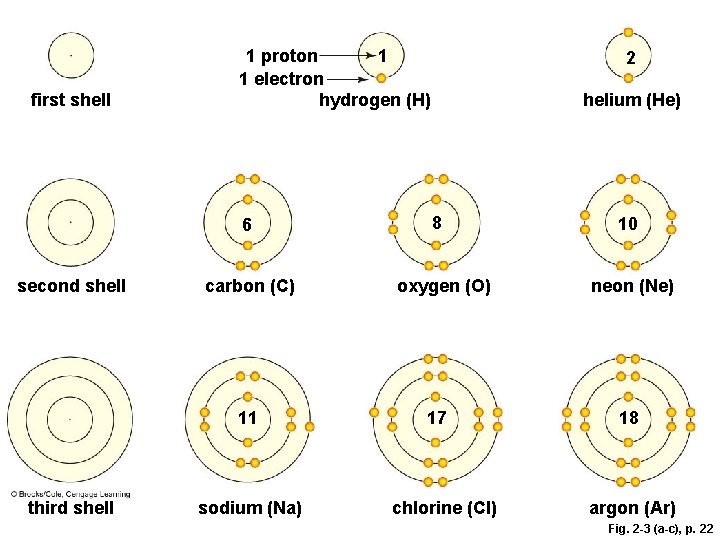
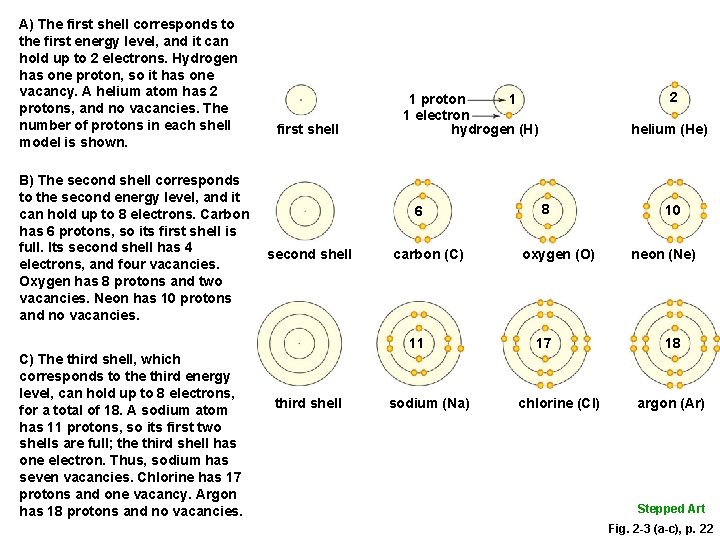
first shell 1 1 proton 1 electron hydrogen (H) 6 second shell carbon (C) 11 third shell sodium (Na) 2 helium (He) 8 oxygen (O) 17 chlorine (Cl) 10 neon (Ne) 18 argon (Ar) Fig. 2 -3 (a-c), p. 22

A) The first shell corresponds to the first energy level, and it can hold up to 2 electrons. Hydrogen has one proton, so it has one vacancy. A helium atom has 2 protons, and no vacancies. The number of protons in each shell model is shown. B) The second shell corresponds to the second energy level, and it can hold up to 8 electrons. Carbon has 6 protons, so its first shell is full. Its second shell has 4 electrons, and four vacancies. Oxygen has 8 protons and two vacancies. Neon has 10 protons and no vacancies. first shell 6 second shell carbon (C) 11 C) The third shell, which corresponds to the third energy level, can hold up to 8 electrons, for a total of 18. A sodium atom has 11 protons, so its first two shells are full; the third shell has one electron. Thus, sodium has seven vacancies. Chlorine has 17 protons and one vacancy. Argon has 18 protons and no vacancies. third shell 2 1 1 proton 1 electron hydrogen (H) sodium (Na) helium (He) 8 oxygen (O) 17 chlorine (Cl) 10 neon (Ne) 18 argon (Ar) Stepped Art Fig. 2 -3 (a-c), p. 22

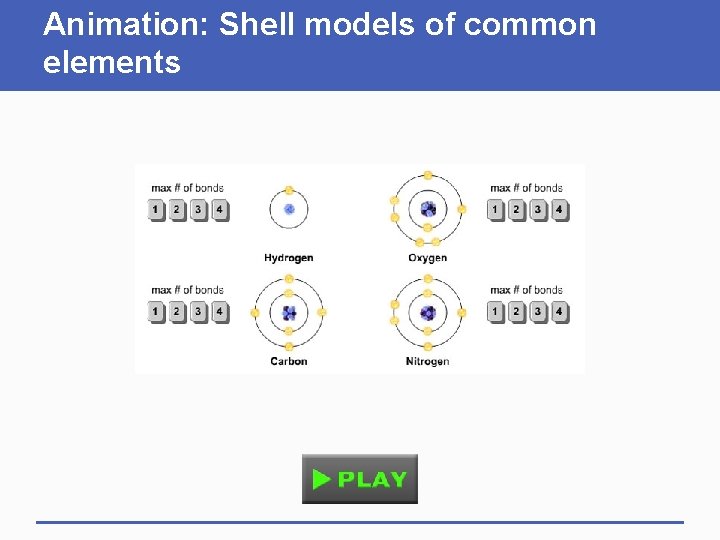
Animation: Shell models of common elements

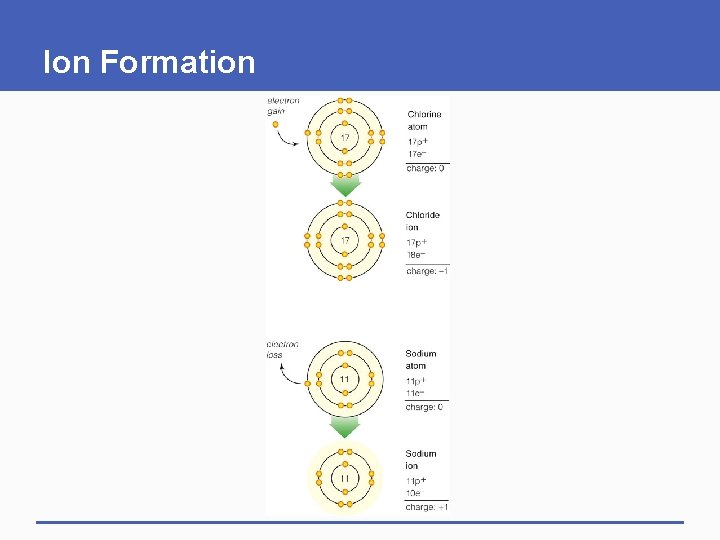
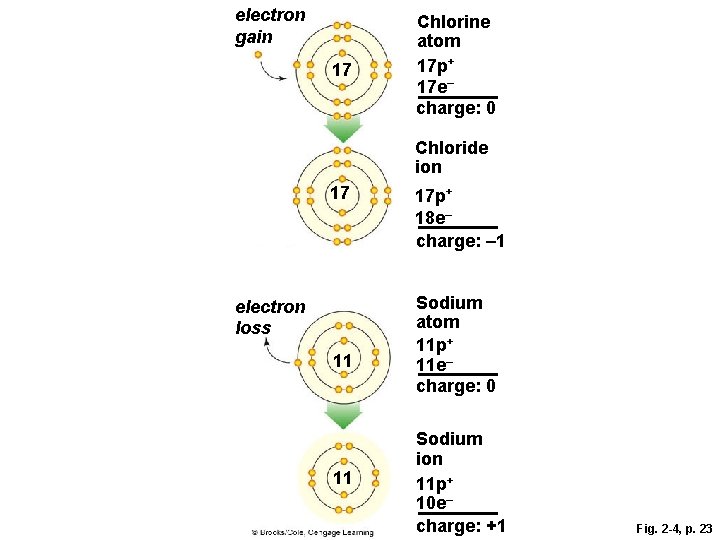
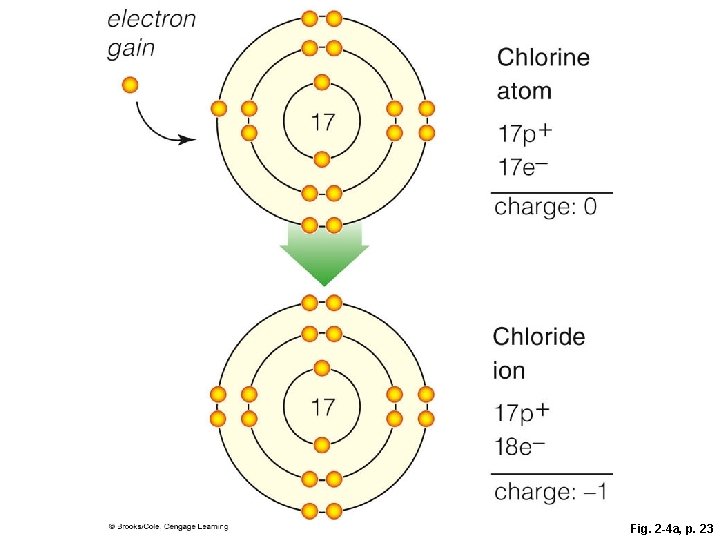
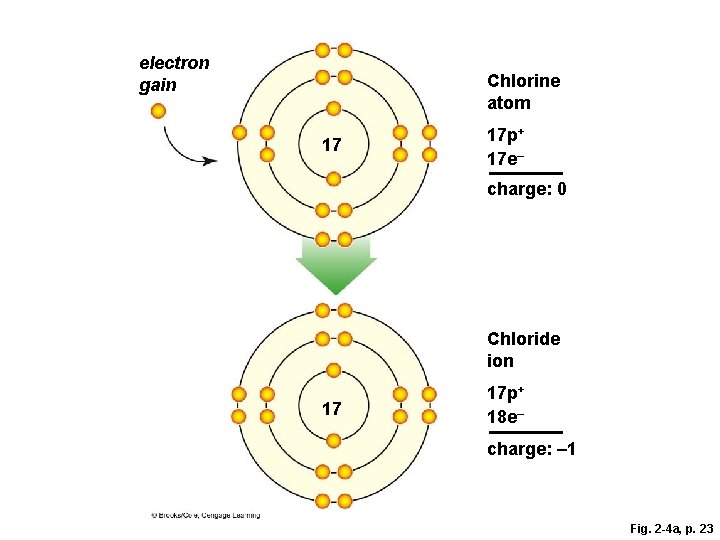
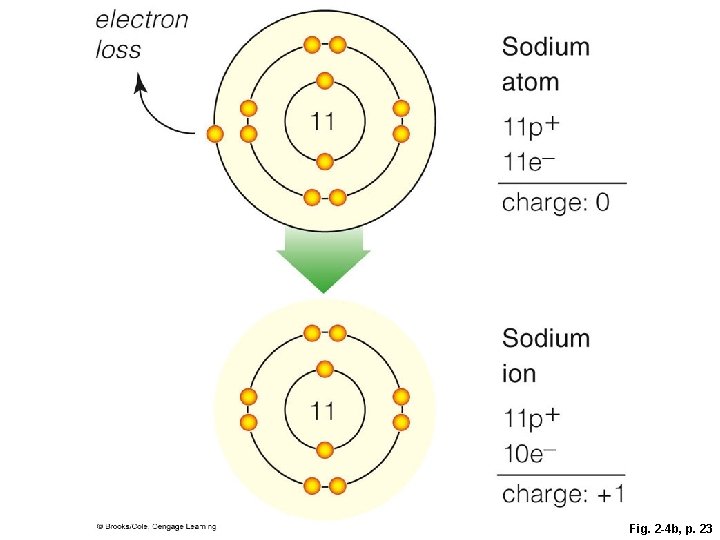
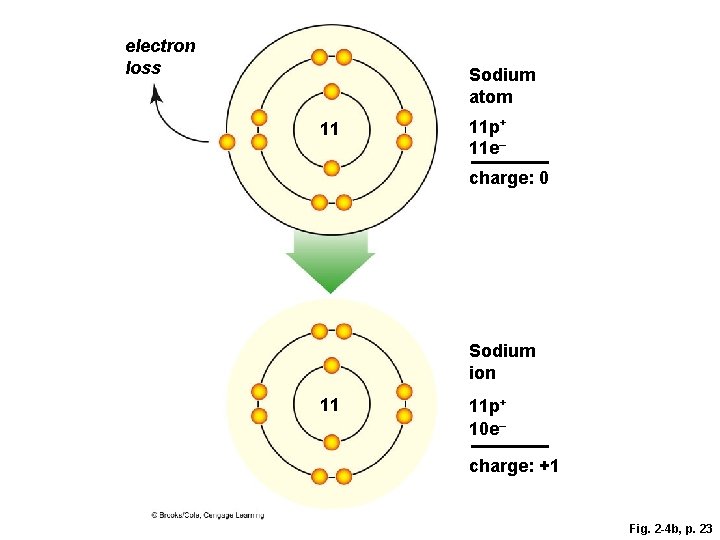
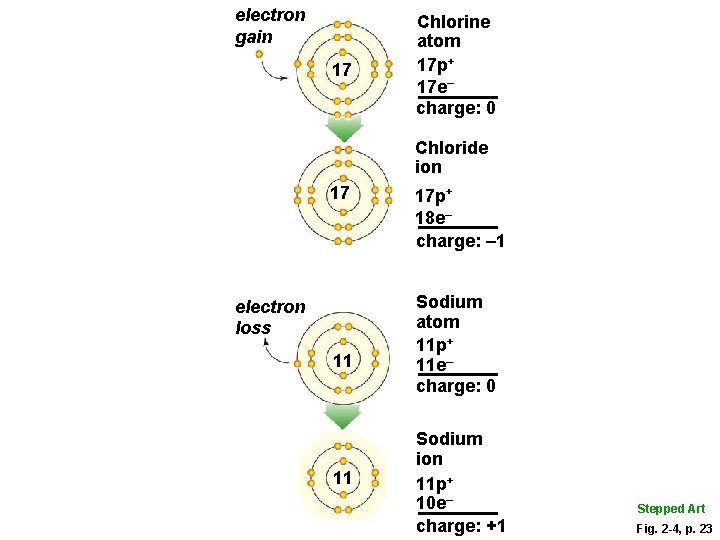
Ions § The negative charge of an electron balances the positive charge of a proton in the nucleus § Changing the number of electrons may fill its outer shell, but changes the charge of the atom § Ion • Atom that carries a charge because it has an unequal number of protons and electrons

Ion Formation

electron gain 17 Chlorine atom 17 p+ 17 e– charge: 0 Chloride ion 17 electron loss 11 11 17 p+ 18 e– charge: – 1 Sodium atom 11 p+ 11 e– charge: 0 Sodium ion 11 p+ 10 e– charge: +1 Fig. 2 -4, p. 23

Fig. 2 -4 a, p. 23

electron gain Chlorine atom 17 17 p+ 17 e– charge: 0 Chloride ion 17 17 p+ 18 e– charge: – 1 Fig. 2 -4 a, p. 23

Fig. 2 -4 b, p. 23

electron loss Sodium atom 11 11 p+ 11 e– charge: 0 Sodium ion 11 11 p+ 10 e– charge: +1 Fig. 2 -4 b, p. 23

electron gain 17 Chlorine atom 17 p+ 17 e– charge: 0 Chloride ion 17 electron loss 11 11 17 p+ 18 e– charge: – 1 Sodium atom 11 p+ 11 e– charge: 0 Sodium ion 11 p+ 10 e– charge: +1 Stepped Art Fig. 2 -4, p. 23

Animation: How atoms bond

Animation: PET scan

Animation: The shell model of electron distribution

Animation: Subatomic particles

Animation: Atomic number, mass number

Animation: Electron arrangements in atoms

Animation: Isotopes of hydrogen

Video: ABC News: Nuclear Energy

Animation: Electron distribution

2. 3 From Atoms to Molecules § Atoms can also fill their vacancies by sharing electrons with other atoms § A chemical bond forms when the electrons of two atoms interact § Chemical bond • An attractive force that arises between two atoms when their electrons interact

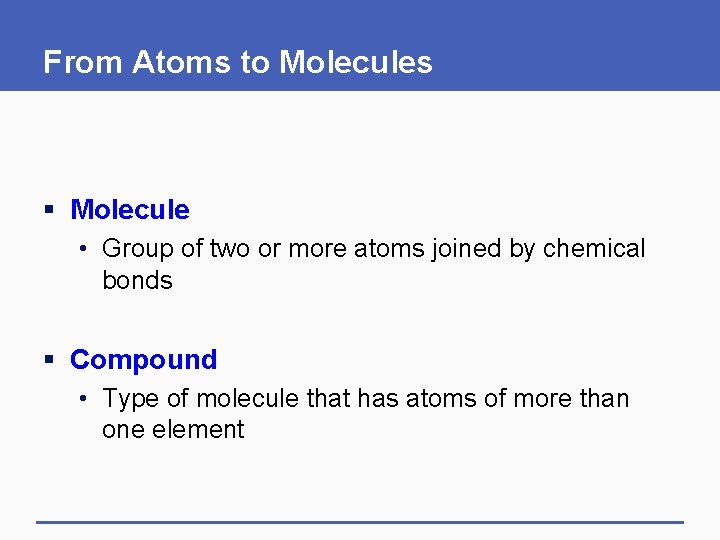
From Atoms to Molecules § Molecule • Group of two or more atoms joined by chemical bonds § Compound • Type of molecule that has atoms of more than one element

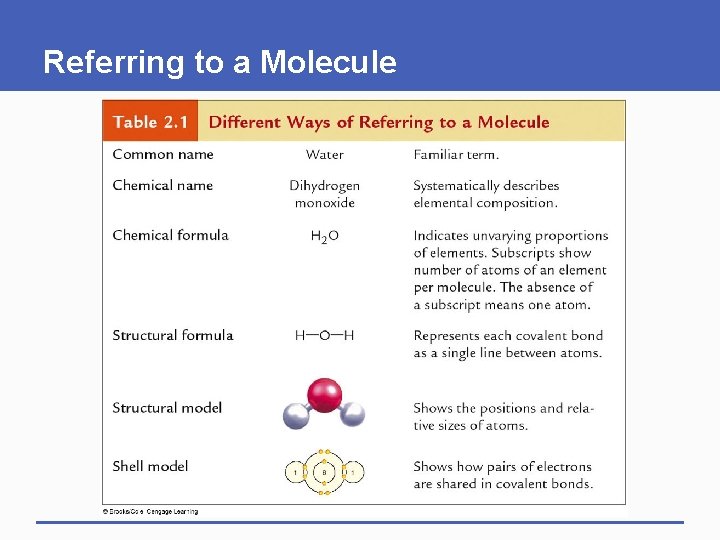
Referring to a Molecule

Same Materials, Different Results

Animation: Building blocks of life

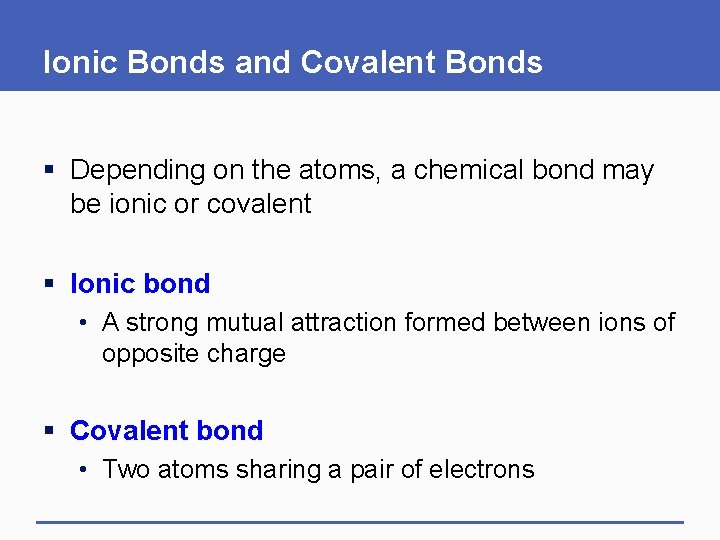
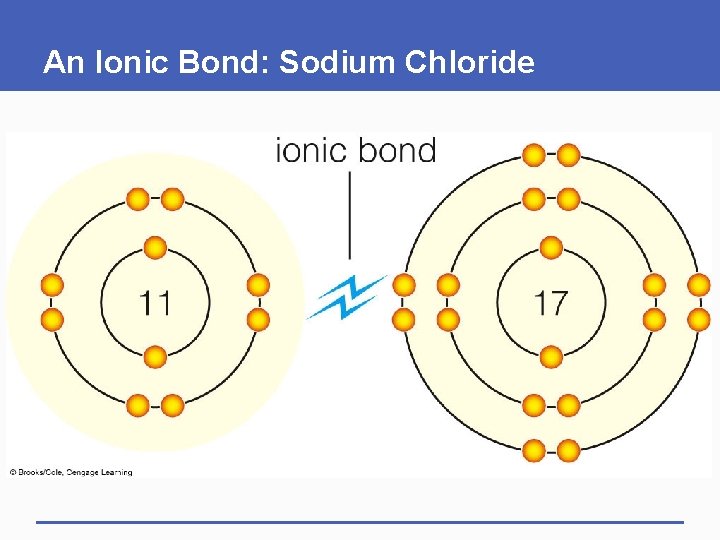
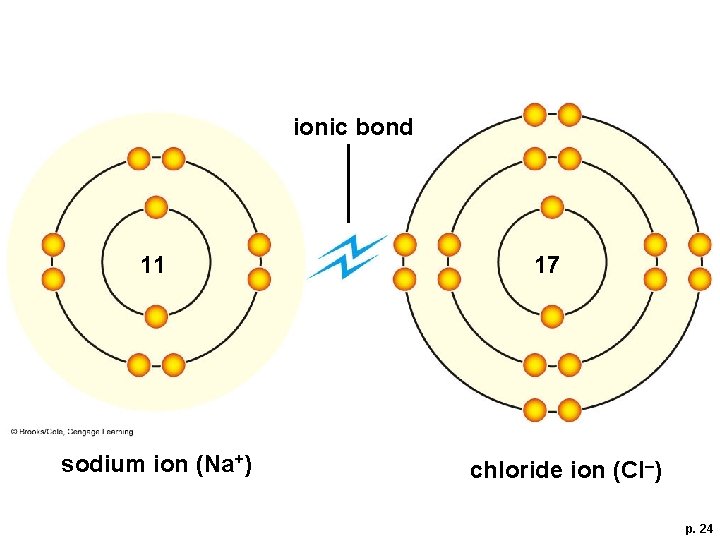
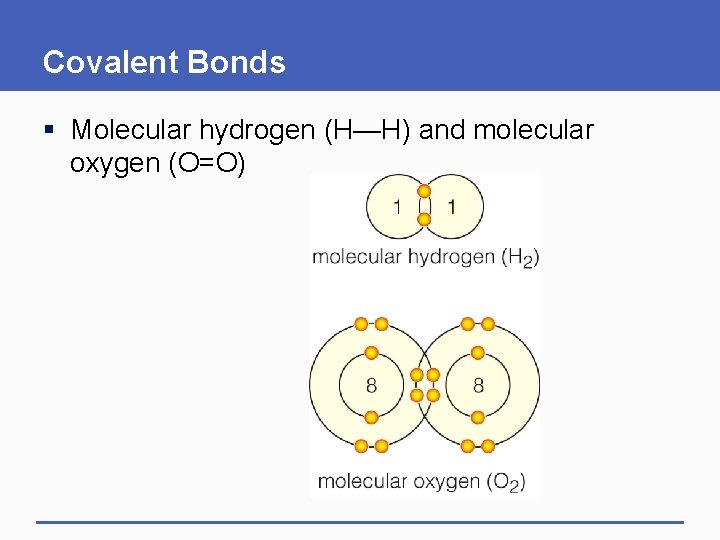
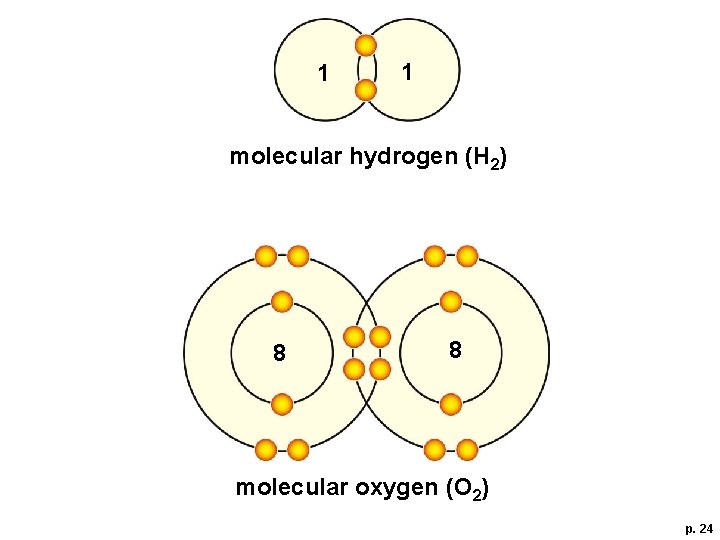
Ionic Bonds and Covalent Bonds § Depending on the atoms, a chemical bond may be ionic or covalent § Ionic bond • A strong mutual attraction formed between ions of opposite charge § Covalent bond • Two atoms sharing a pair of electrons

An Ionic Bond: Sodium Chloride

ionic bond 11 sodium ion (Na+) 17 chloride ion (Cl–) p. 24

Covalent Bonds § Molecular hydrogen (H—H) and molecular oxygen (O=O)

1 1 molecular hydrogen (H 2) 8 8 molecular oxygen (O 2) p. 24

Polarity § A covalent bond is nonpolar if electrons are shared equally, and polar if the sharing is unequal § Polarity • Any separation of charge into distinct positive and negative regions

Polar and Nonpolar Covalent Bonds § Nonpolar • Having an even distribution of charge • When atoms in a covalent bond share electrons equally, the bond is nonpolar § Polar • Having an uneven distribution of charge • When the atoms share electrons unequally, the bond is polar

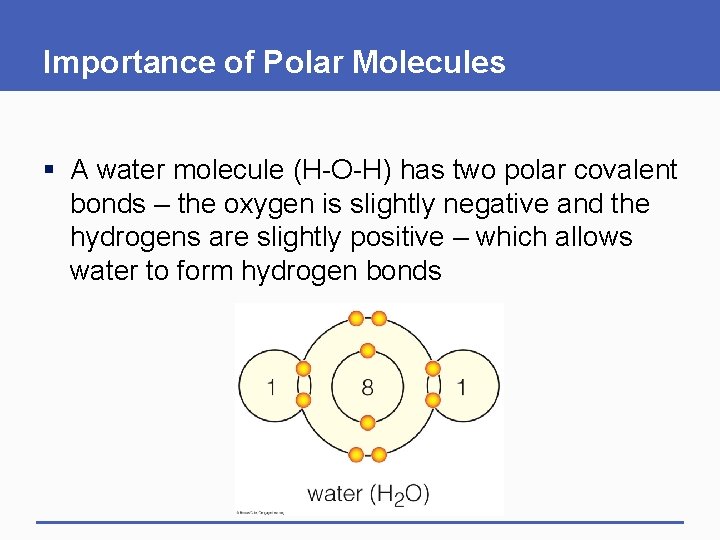
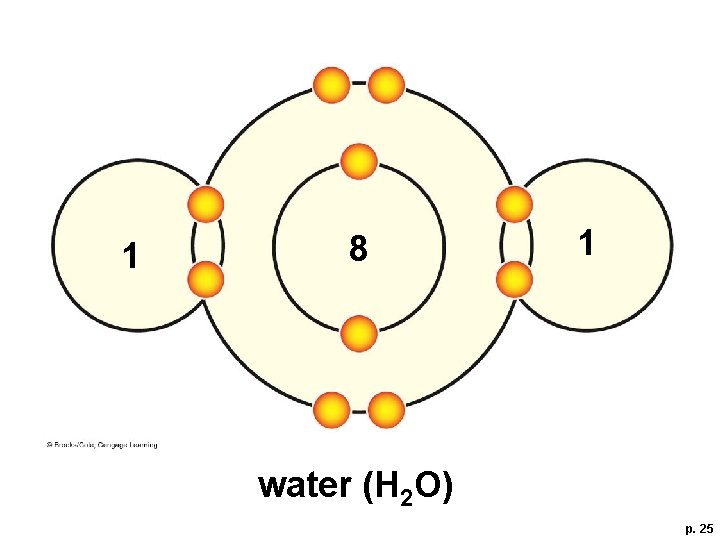
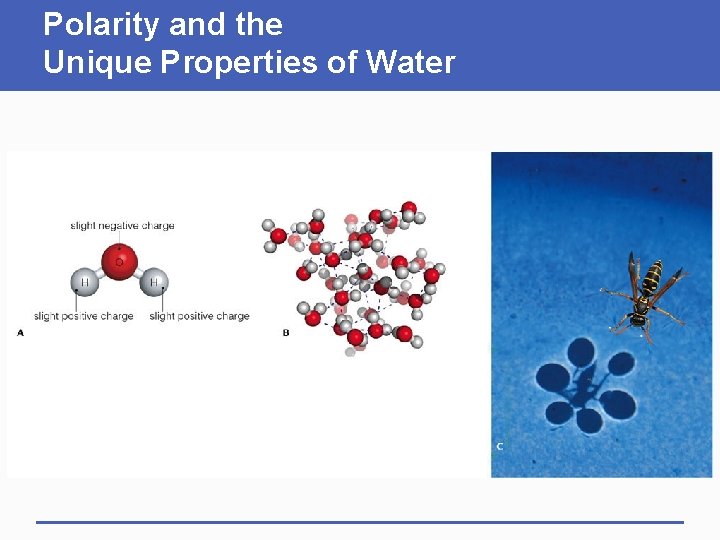
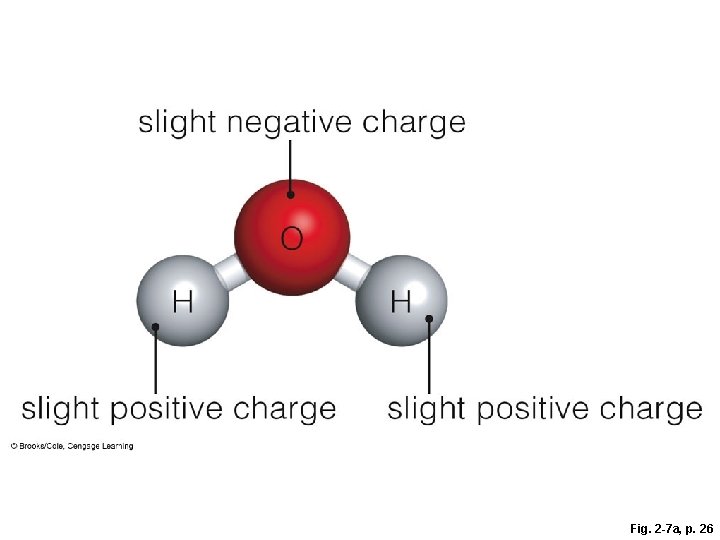
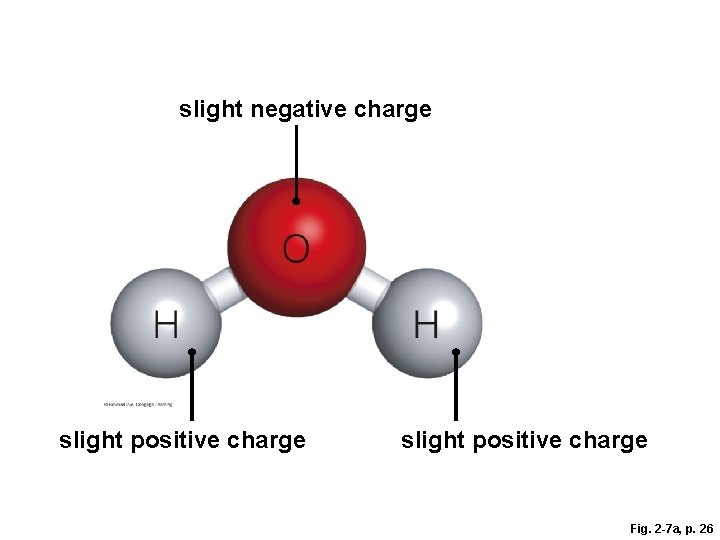
Importance of Polar Molecules § A water molecule (H-O-H) has two polar covalent bonds – the oxygen is slightly negative and the hydrogens are slightly positive – which allows water to form hydrogen bonds

p. 25

1 8 1 water (H 2 O) p. 25

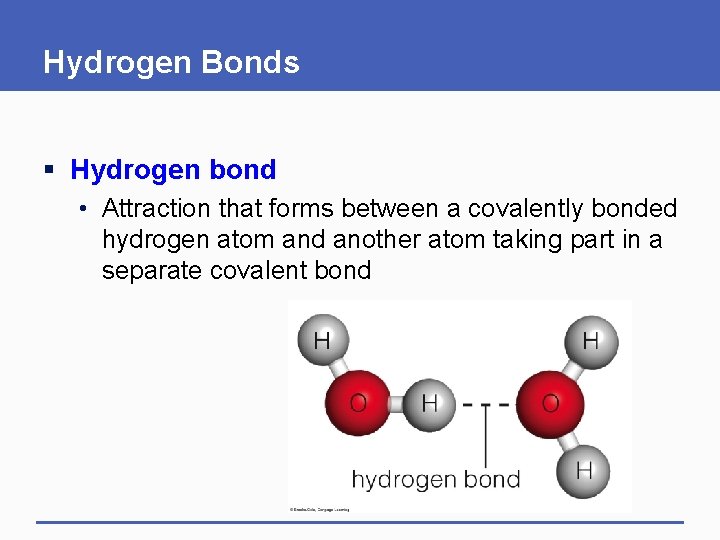
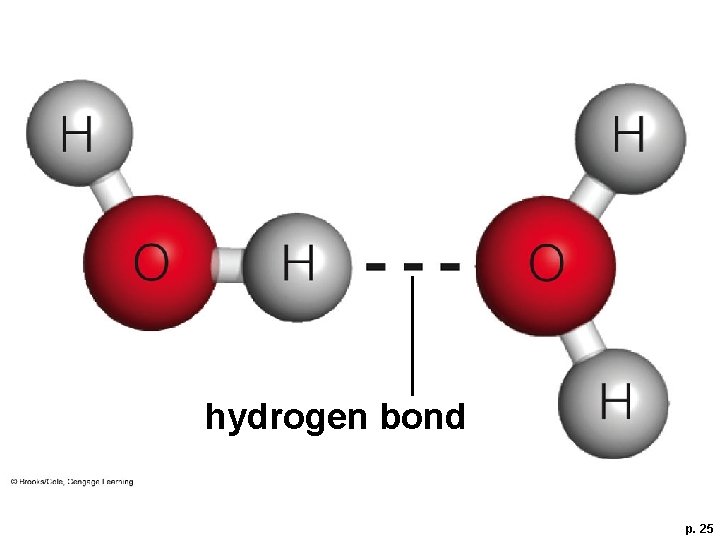
Hydrogen Bonds § Hydrogen bond • Attraction that forms between a covalently bonded hydrogen atom and another atom taking part in a separate covalent bond

hydrogen bond p. 25

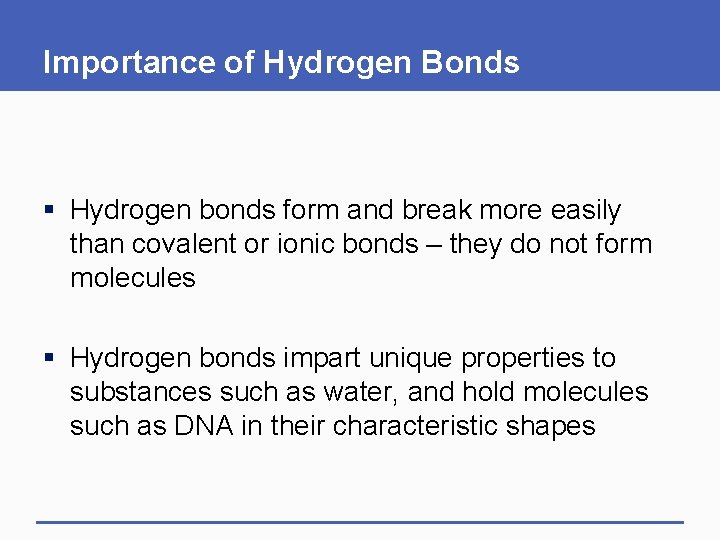
Importance of Hydrogen Bonds § Hydrogen bonds form and break more easily than covalent or ionic bonds – they do not form molecules § Hydrogen bonds impart unique properties to substances such as water, and hold molecules such as DNA in their characteristic shapes

Animation: Ionic bonding

Animation: Examples of hydrogen bonds

Video: ABC News: Fuel Cell Vehicles

Animation: Sucrose synthesis

Animation: Covalent bonds

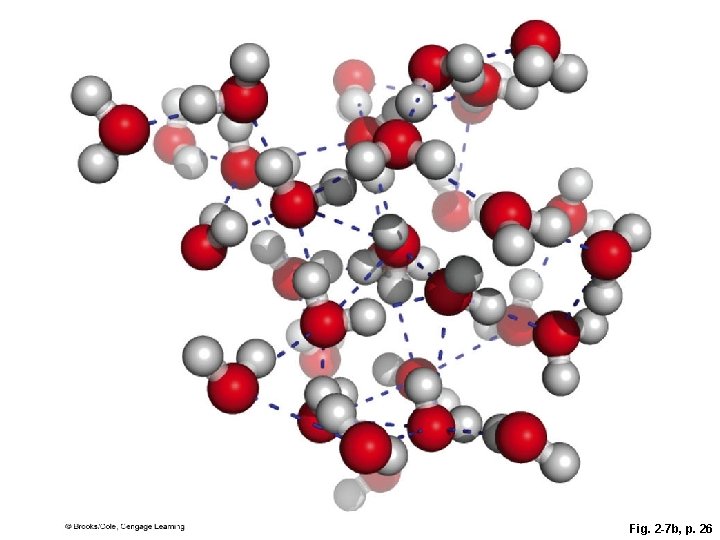
2. 4 Water § All living organisms are mostly water, and all chemical reactions of life are carried out in water § Hydrogen bonds between water molecules give water unique properties that make life possible • Capacity to dissolve many substances • Cohesion (surface tension) • Temperature stability

Polarity and the Unique Properties of Water

Fig. 2 -7 a, p. 26

slight negative charge slight positive charge Fig. 2 -7 a, p. 26

Fig. 2 -7 b, p. 26

Fig. 2 -7 c, p. 26

Animation: Structure of water

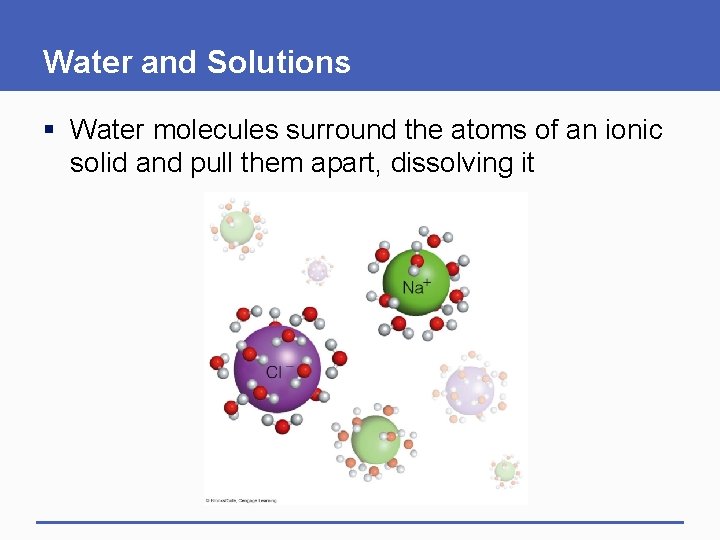
Water and Solutions § Polar water molecules hydrogen-bond to other polar (hydrophilic) substances, and repel nonpolar (hydrophobic) substances § Hydrophilic (water-loving) • A substance that dissolves easily in water § Hydrophobic (water-dreading) • A substance that resists dissolving in water

Water and Solutions § Water is an excellent solvent § Solvent • Liquid that can dissolve other substances § Solute • A dissolved substance

Water and Solutions § Salts, sugars, and many polar molecules dissolve easily in water § Salt • Compound that dissolves easily in water and releases ions other than H+ and OH • Example: sodium chloride (Na. Cl)

Water and Solutions § Water molecules surround the atoms of an ionic solid and pull them apart, dissolving it

Animation: Spheres of hydration

Temperature Stability § Temperature stability is an important part of homeostasis • Water absorbs more heat than other liquids before temperature rises • Hydrogen bonds hold ice together in a rigid pattern that makes ice float § Temperature • Measure of molecular motion

Cohesion § Cohesion helps sustain multicelled bodies and resists evaporation § Cohesion • Tendency of water molecules to stick together § Evaporation • Transition of liquid to gas • Absorbs heat energy (cooling effect)

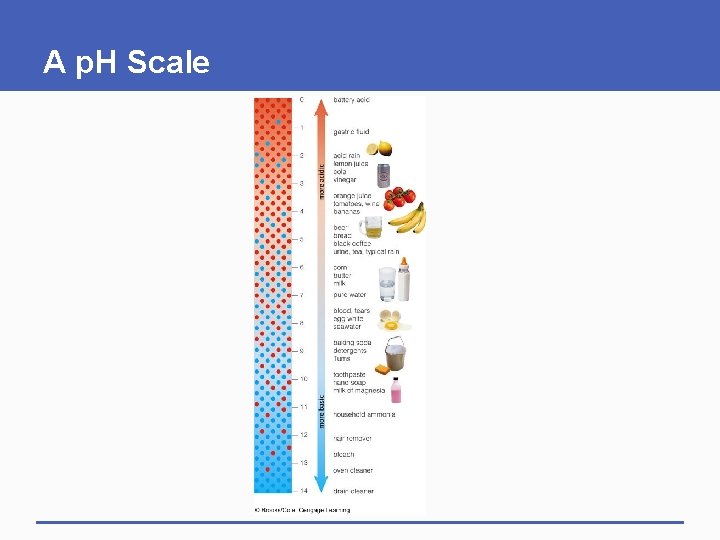
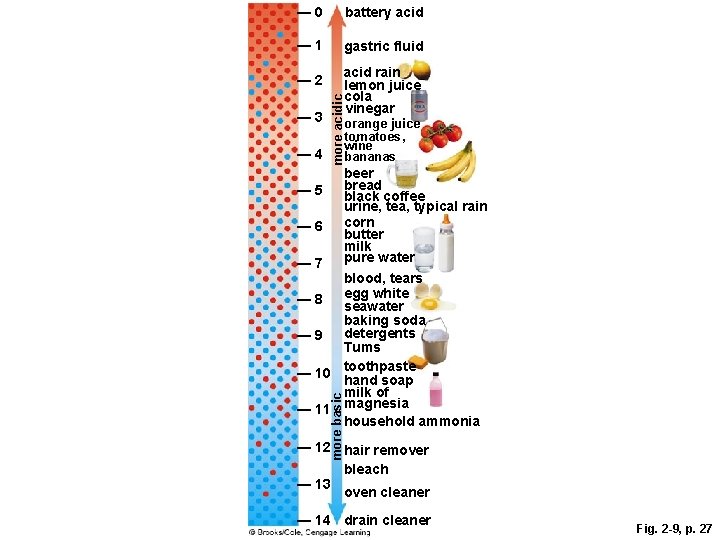
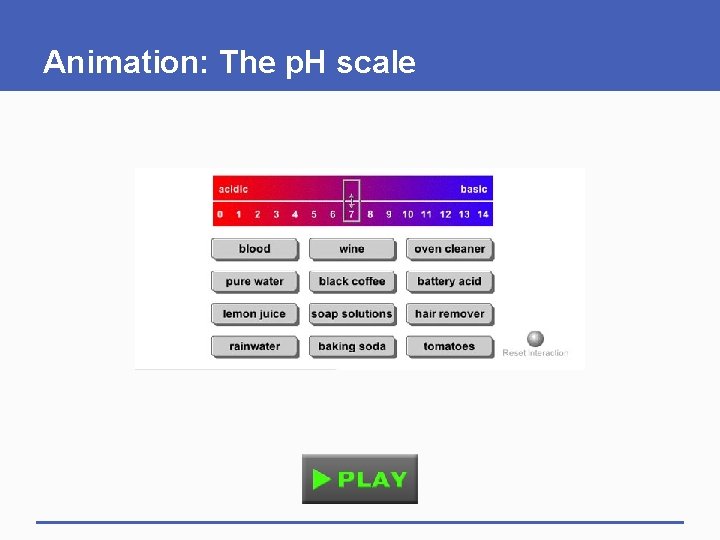
2. 5 Acids and Bases § Water molecules separate into hydrogen ions (H+) and hydroxide ions (OH-) § p. H • A measure of the number of hydrogen ions (H+) in a solution • The more hydrogen ions, the lower the p. H § Pure water has neutral p. H (p. H=7) • Number of H+ ions = OH- ions

Acids and Bases § Acid • Substance that releases hydrogen ions in water • p. H less than 7 § Base • Substance that releases hydroxide ions (accepts hydrogen ions) in water • p. H greater than 7

A p. H Scale

— 0 battery acid — 1 gastric fluid — 3 — 4 acid rain lemon juice cola vinegar more acidic — 2 orange juice tomatoes, wine bananas more basic beer bread — 5 black coffee urine, tea, typical rain corn — 6 butter milk pure water — 7 blood, tears egg white — 8 seawater baking soda detergents — 9 Tums toothpaste — 10 hand soap milk of — 11 magnesia household ammonia — 12 hair remover bleach — 13 oven cleaner — 14 drain cleaner Fig. 2 -9, p. 27

Animation: The p. H scale

Acid Rain § Sulfur dioxide and other airborne pollutants dissolve in water vapor to form acid rain

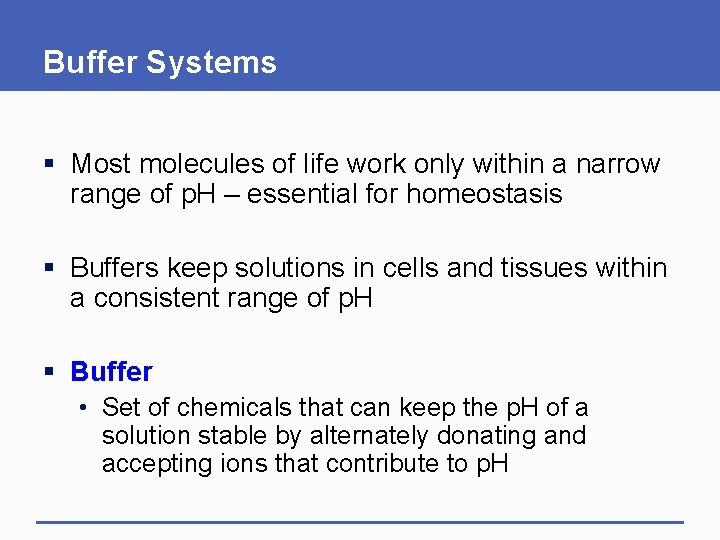
Buffer Systems § Most molecules of life work only within a narrow range of p. H – essential for homeostasis § Buffers keep solutions in cells and tissues within a consistent range of p. H § Buffer • Set of chemicals that can keep the p. H of a solution stable by alternately donating and accepting ions that contribute to p. H

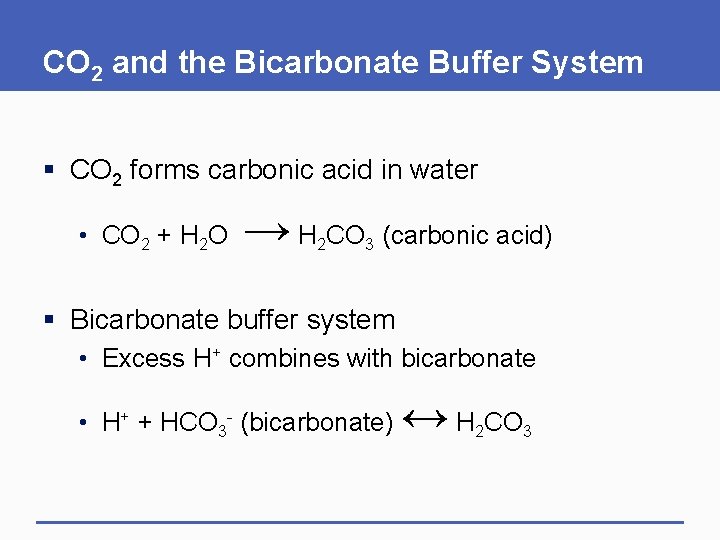
CO 2 and the Bicarbonate Buffer System § CO 2 forms carbonic acid in water • CO 2 + H 2 O → H CO 2 3 (carbonic acid) § Bicarbonate buffer system • Excess H+ combines with bicarbonate • H+ + HCO 3 - (bicarbonate) ↔ H CO 2 3

Video: ABC News: Bottle Backlash

Video: ABC News: Water Use

Video: ABC News: Water Wars

3 D Animation: Dissolution