What Holds Molecules Together Intermolecular Forces of Attraction









- Slides: 40

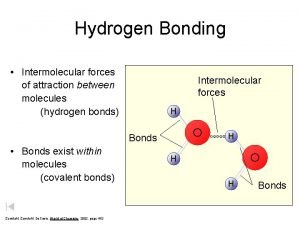
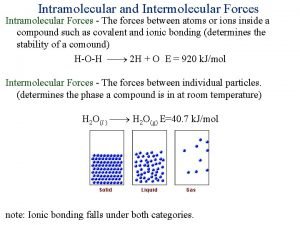
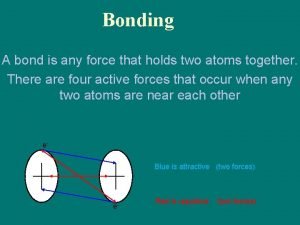
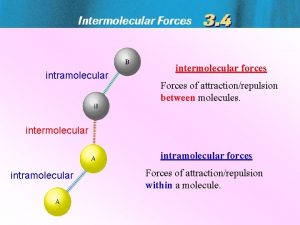
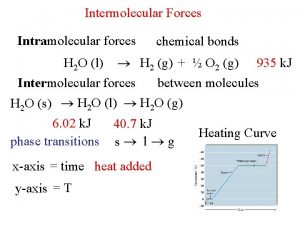
What Holds Molecules Together? Intermolecular Forces of Attraction Called “Van der Waals Forces” They arise from weak “electrostatic attractions” between molecules. l Different from “Intramolecular Forces” or “covalent bonds” that exist between atoms!

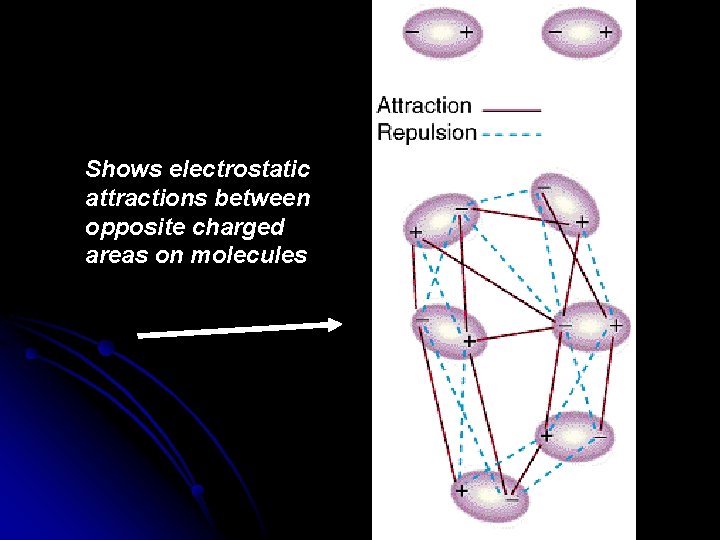
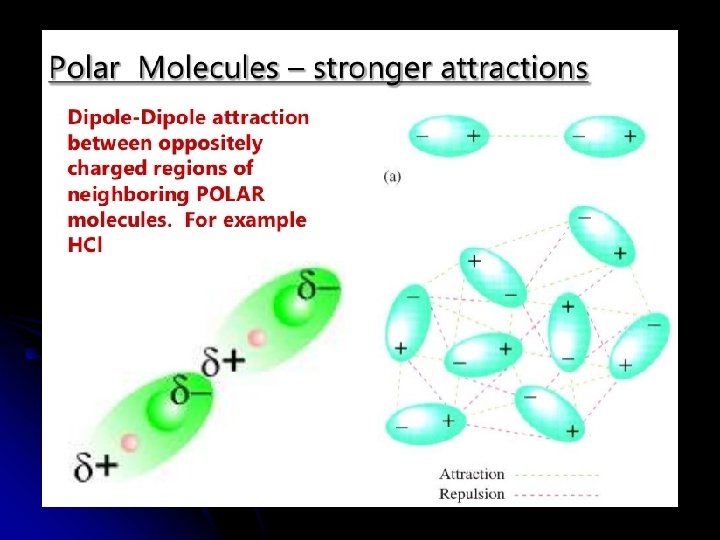
Shows electrostatic attractions between opposite charged areas on molecules

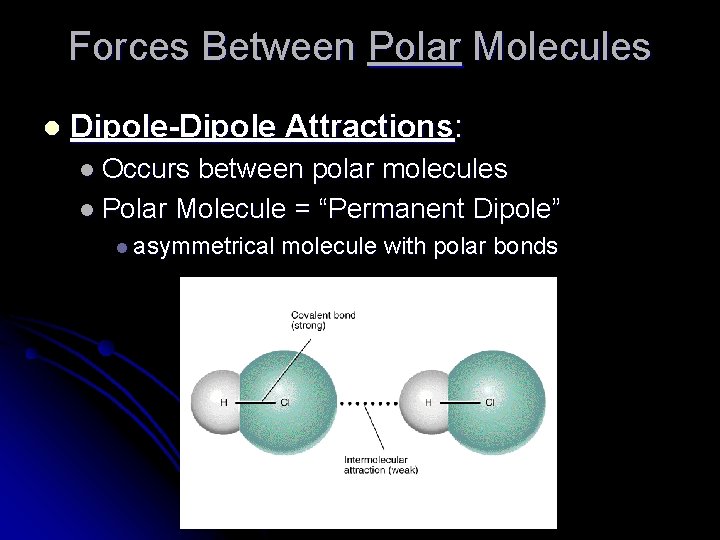
Forces Between Polar Molecules l Dipole-Dipole Attractions: l Occurs between polar molecules l Polar Molecule = “Permanent Dipole” l asymmetrical molecule with polar bonds



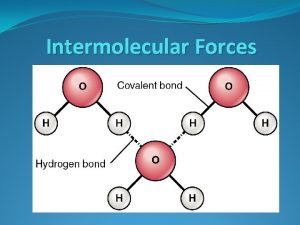
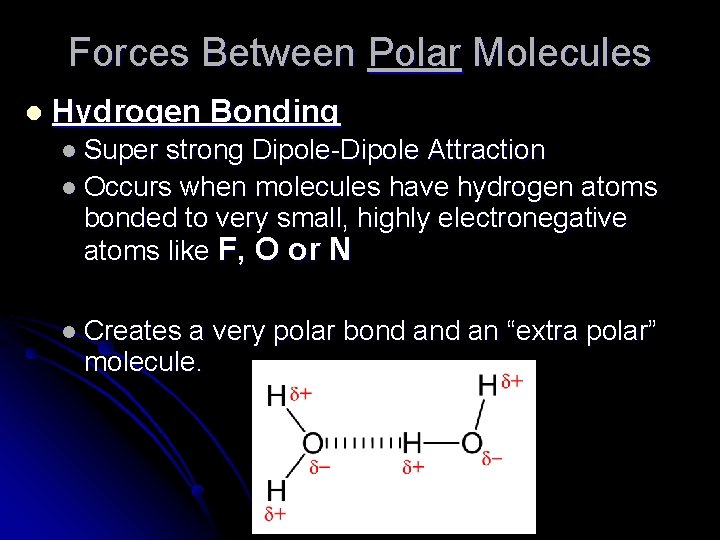
Forces Between Polar Molecules l Hydrogen Bonding l Super strong Dipole-Dipole Attraction l Occurs when molecules have hydrogen atoms bonded to very small, highly electronegative atoms like F, O or N l Creates a very polar bond an “extra polar” molecule.

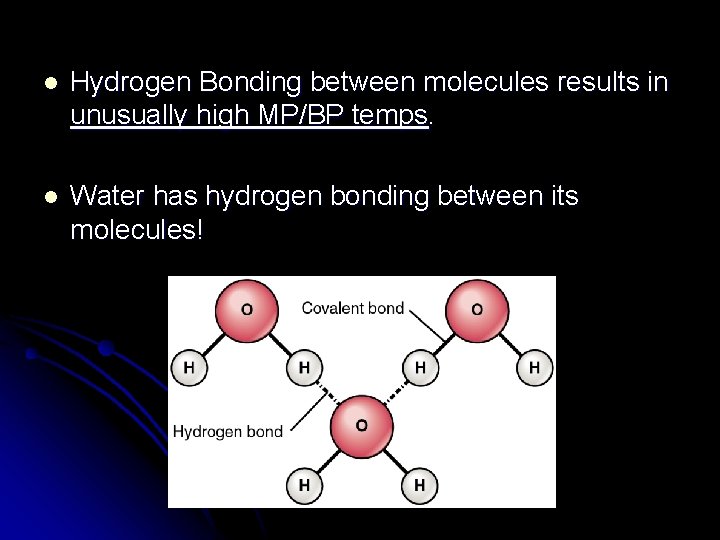
l Hydrogen Bonding between molecules results in unusually high MP/BP temps. l Water has hydrogen bonding between its molecules!

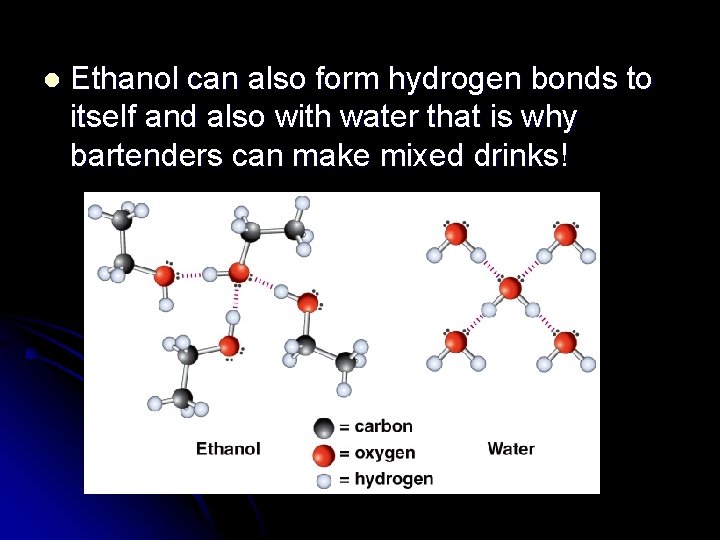
l Ethanol can also form hydrogen bonds to itself and also with water that is why bartenders can make mixed drinks!

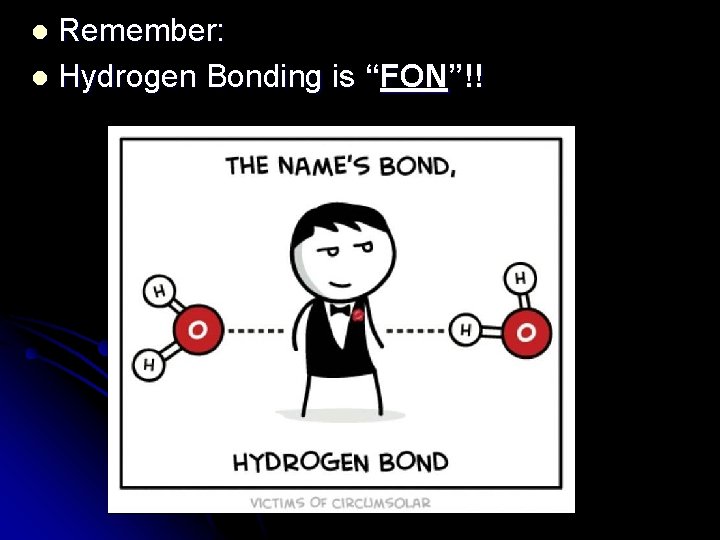
Remember: l Hydrogen Bonding is “FON”!! l

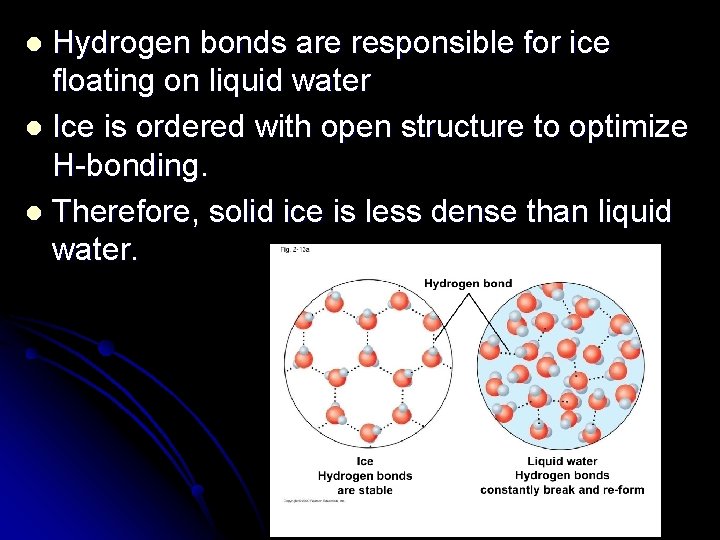
Hydrogen bonds are responsible for ice floating on liquid water l Ice is ordered with open structure to optimize H-bonding. l Therefore, solid ice is less dense than liquid water. l

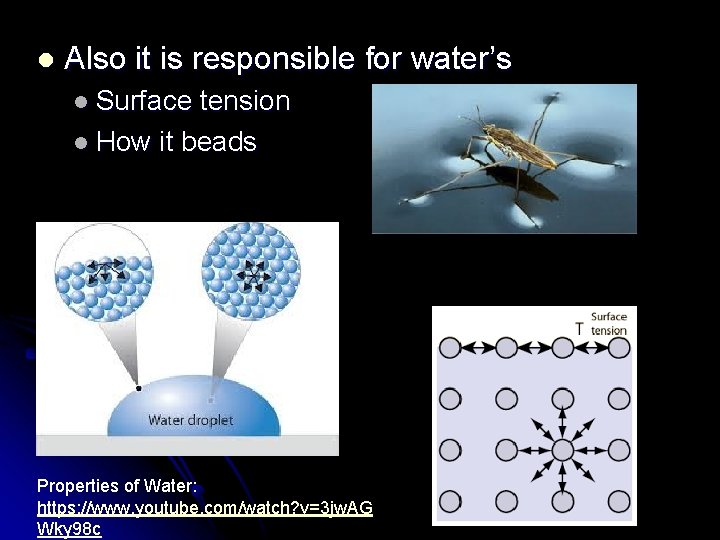
l Also it is responsible for water’s l Surface tension l How it beads Properties of Water: https: //www. youtube. com/watch? v=3 jw. AG Wky 98 c

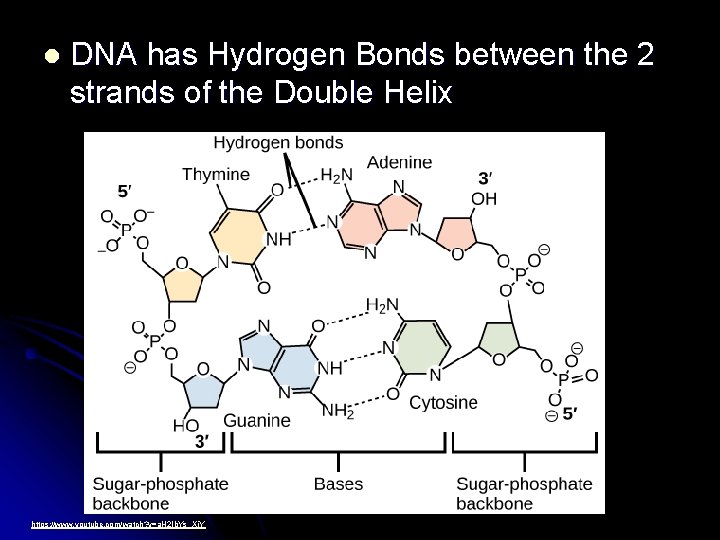
l DNA has Hydrogen Bonds between the 2 strands of the Double Helix https: //www. youtube. com/watch? v=a. H 2 Ib. Ys_Xj. Y

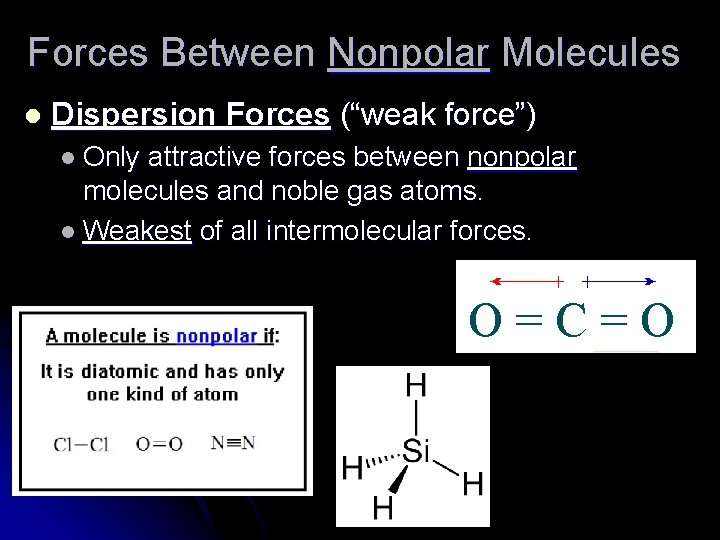
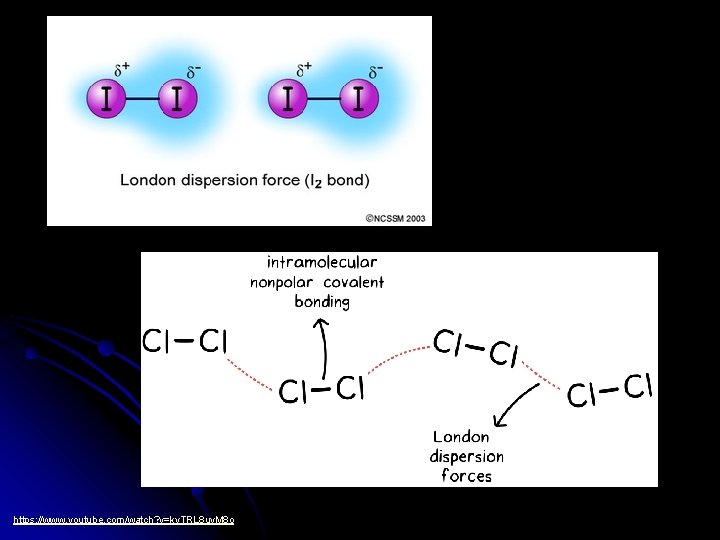
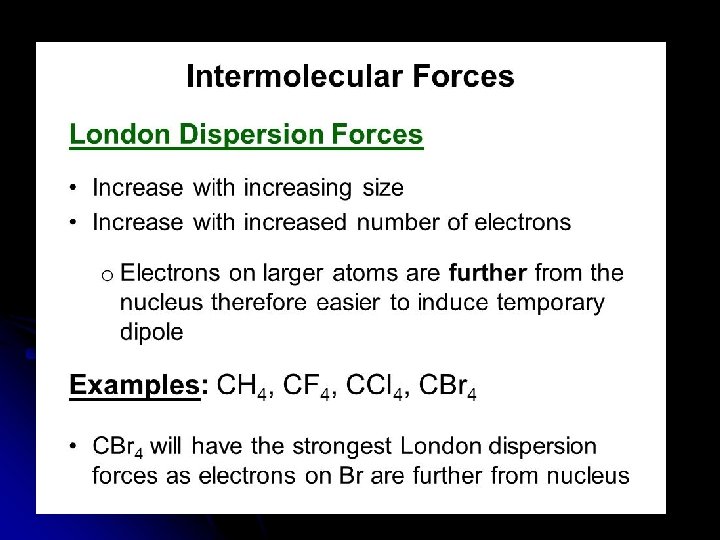
Forces Between Nonpolar Molecules l Dispersion Forces (“weak force”) l Only attractive forces between nonpolar molecules and noble gas atoms. l Weakest of all intermolecular forces.

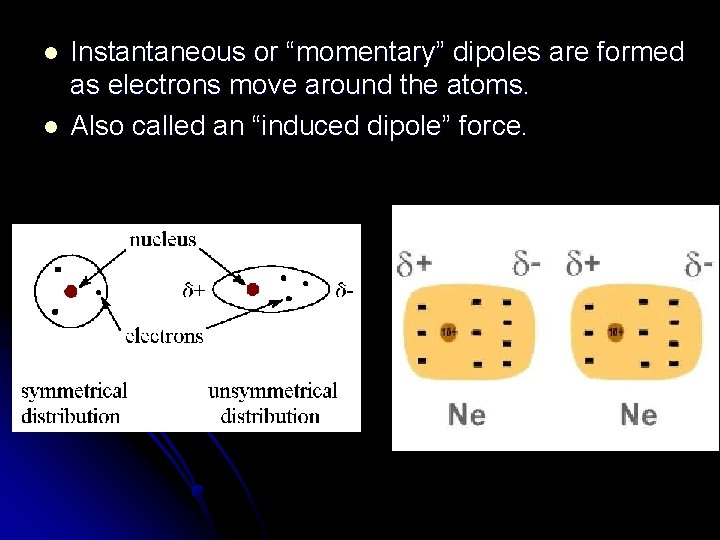
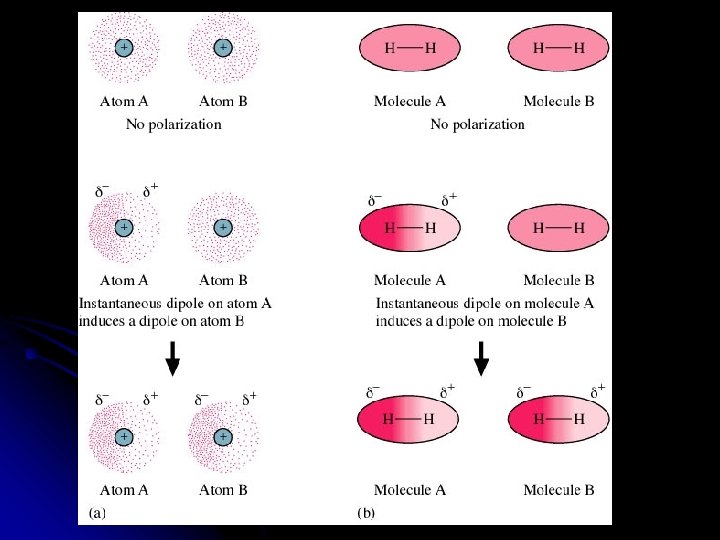
l l Instantaneous or “momentary” dipoles are formed as electrons move around the atoms. Also called an “induced dipole” force.


https: //www. youtube. com/watch? v=kv. TRL 8 uy. M 8 o

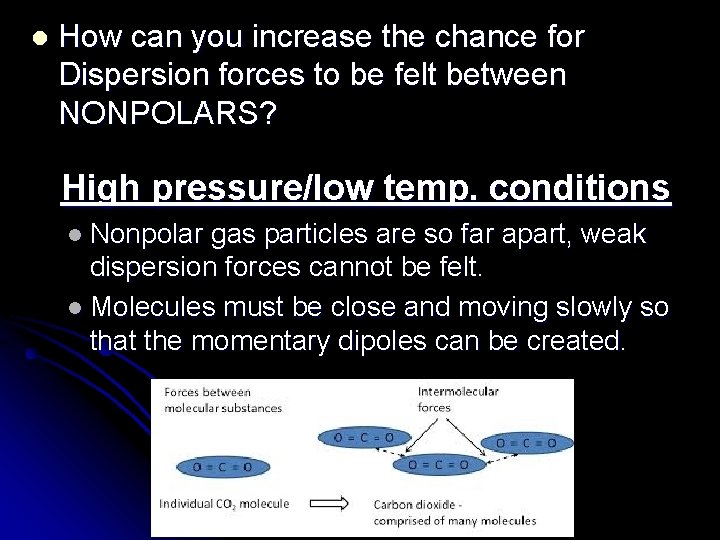
l How can you increase the chance for Dispersion forces to be felt between NONPOLARS? High pressure/low temp. conditions l Nonpolar gas particles are so far apart, weak dispersion forces cannot be felt. l Molecules must be close and moving slowly so that the momentary dipoles can be created.

l Dispersion Forces have different strengths. l The more total electrons in a molecule, the greater the force can get. l Ex: Cl 2 has 34 electrons (it’s a gas) Br 2 has 70 electrons (it’s a liquid) I 2 has 106 electrons (it’s a solid)


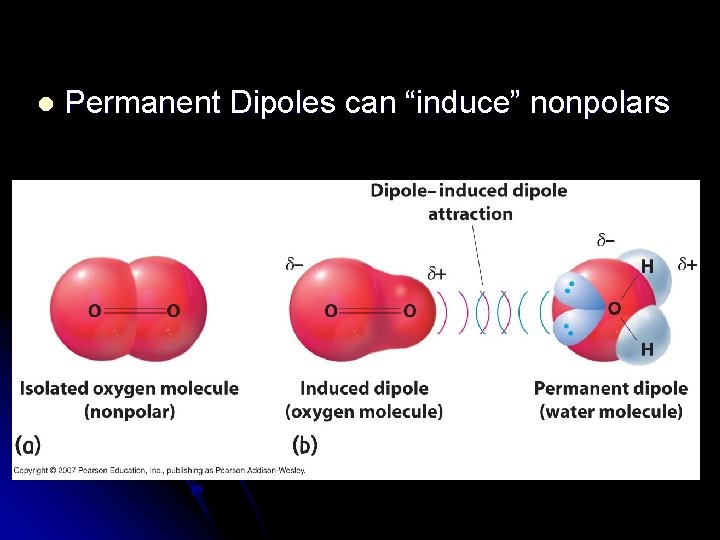
l Permanent Dipoles can “induce” nonpolars

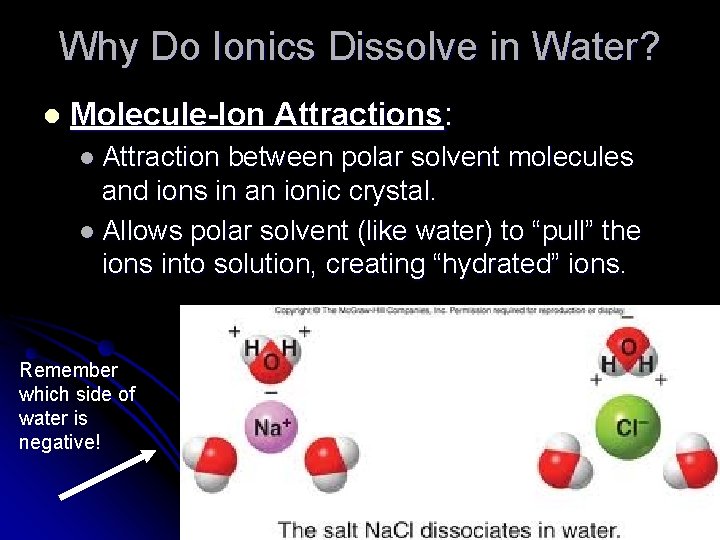
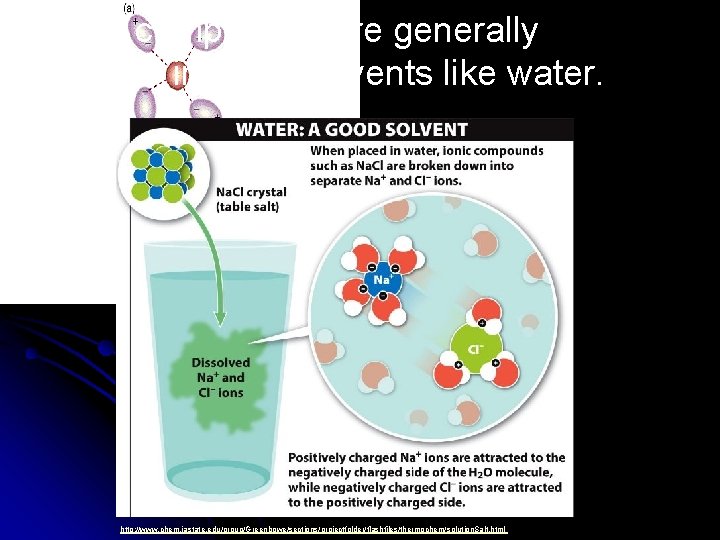
Why Do Ionics Dissolve in Water? l Molecule-Ion Attractions: l Attraction between polar solvent molecules and ions in an ionic crystal. l Allows polar solvent (like water) to “pull” the ions into solution, creating “hydrated” ions. Remember which side of water is negative!

Ionic compounds are generally soluble in polar solvents like water. http: //www. chem. iastate. edu/group/Greenbowe/sections/projectfolder/flashfiles/thermochem/solution. Salt. html

What Effects do IMF Have?

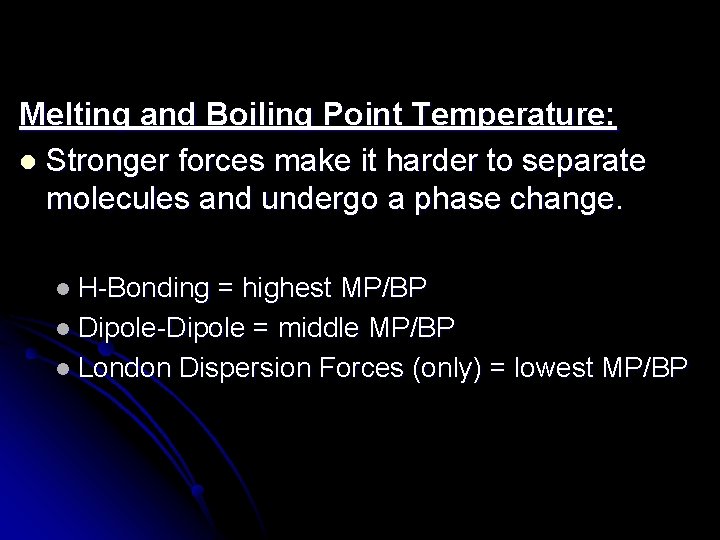
Melting and Boiling Point Temperature: l Stronger forces make it harder to separate molecules and undergo a phase change. l H-Bonding = highest MP/BP l Dipole-Dipole = middle MP/BP l London Dispersion Forces (only) = lowest MP/BP

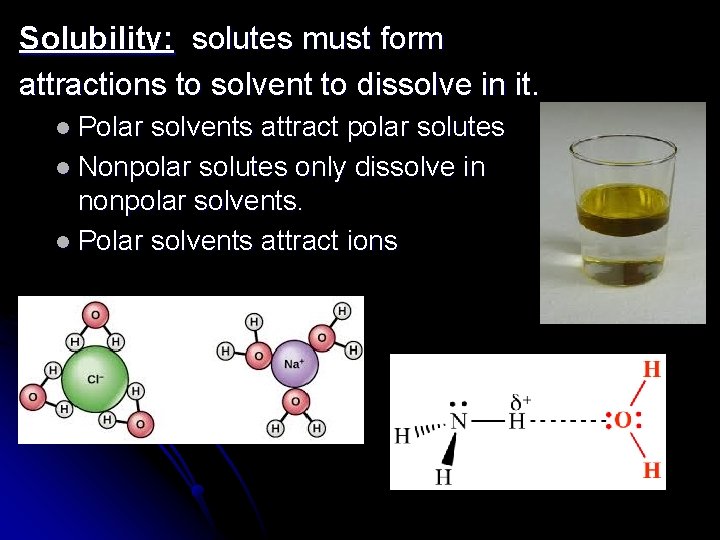
Solubility: solutes must form attractions to solvent to dissolve in it. l Polar solvents attract polar solutes l Nonpolar solutes only dissolve in nonpolar solvents. l Polar solvents attract ions

l A brown and a white bear jump into the water at the same time, which one dissolves faster?

Viscosity: measure of resistance of a fluid to flow. l Stronger IMF = more viscosity l Since there are cohesive forces between the molecules of liquid, like intermolecular forces, these forces create an "internal friction" which reduces the rate of flow of that fluid, so when a substance has greater IMF, these frictional forces are stronger. Therefore, it has more resistance toward moving. l http: //www. youtube. com/watch? v=f 6 sp. Bk. Ve. Q 4 w

Vapor Pressure: l Pressure exerted by collisions of vapor particles above a liquid l The weaker the attractions between molecules in a liquid, the easier to become a gas. Therefore: l Nonpolars with weaker attractions have higher VP than polars

Vapor Pressure l Measured in a closed system at a specific temperature. http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/vaporv 3. swf

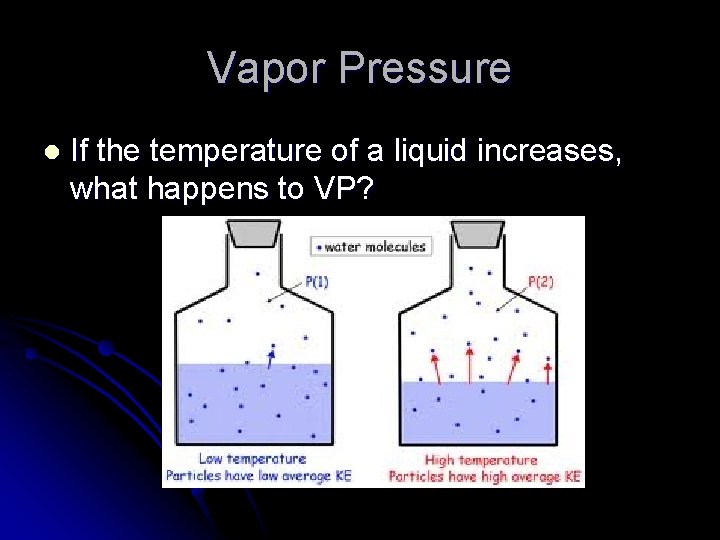
Vapor Pressure l If the temperature of a liquid increases, what happens to VP?

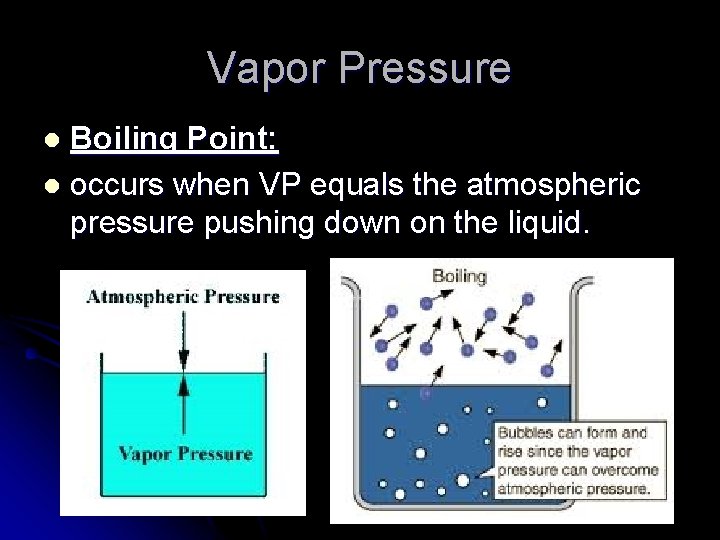
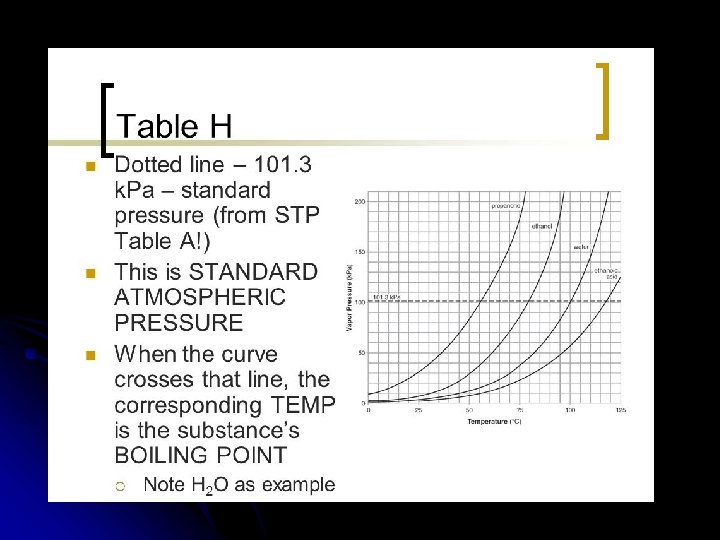
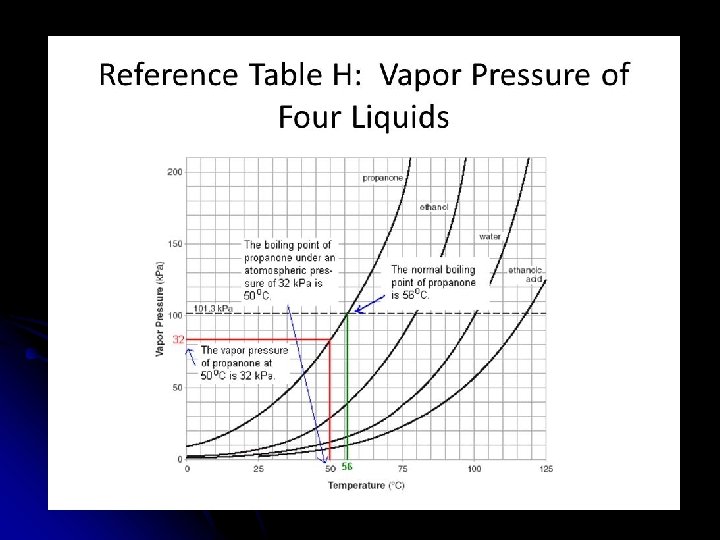
Vapor Pressure Boiling Point: l occurs when VP equals the atmospheric pressure pushing down on the liquid. l

l Normal Boiling Point: l The temp. a liquid boils at standard pressure l 1 atm l 101. 3 KPa l 760 mm. Hg (torr) For water it is 100°C.

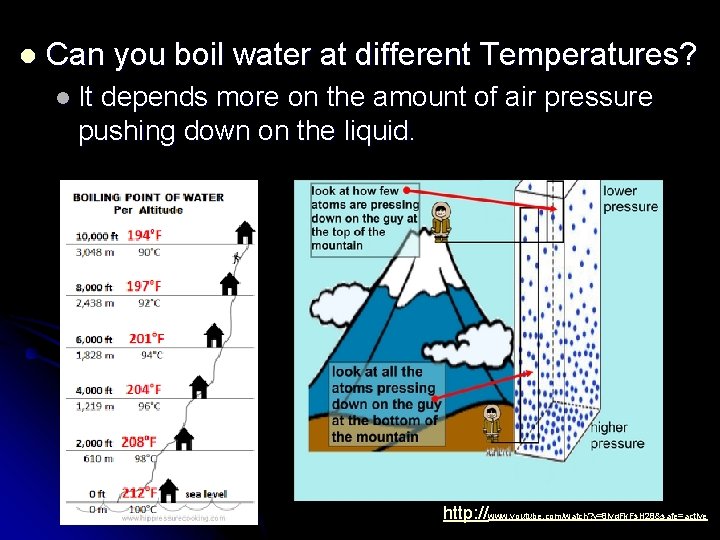
l Can you boil water at different Temperatures? l It depends more on the amount of air pressure pushing down on the liquid. http: //www. youtube. com/watch? v=8 lyq. Fk. Fs. H 28&safe=active

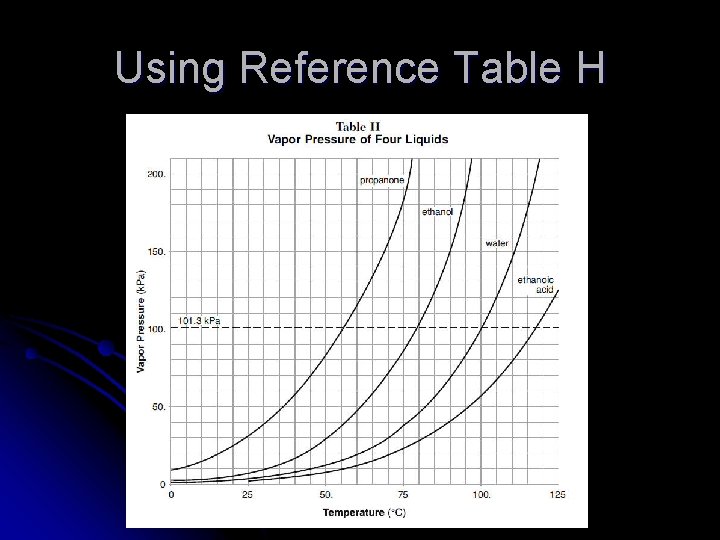
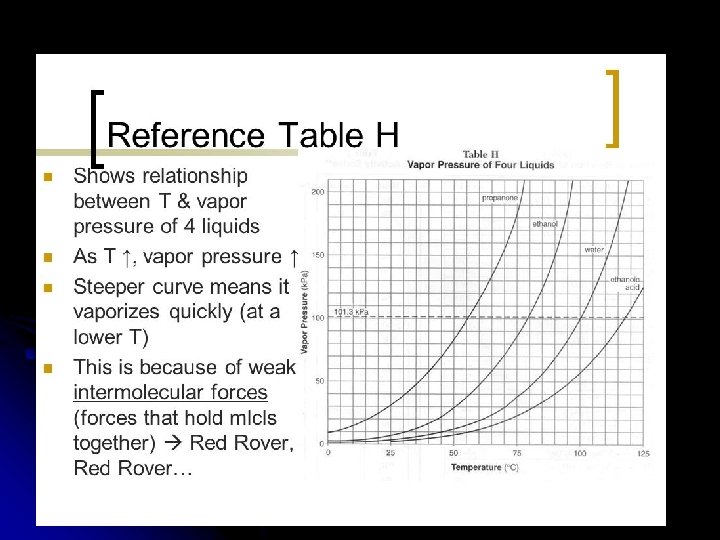
Using Reference Table H





Significant Figures Click for Powerpoint: l http: //www. slideshare. net/crespiryan/signifi cant-figures-550789 l Sig Figs Practice: l http: //www. sciencegeek. net/Chemistry/tate rs/Unit 0 Sigfigs. htm l

Accuracy vs. Precision l https: //www. youtube. com/watch? v=8 Cl 5 C ei. T 7 h. U
 Intermolecular forces of attraction
Intermolecular forces of attraction H-bonding intermolecular forces
H-bonding intermolecular forces What holds molecules together?
What holds molecules together? What holds molecules together
What holds molecules together Intermolecular forces from strongest to weakest
Intermolecular forces from strongest to weakest Intermolecular forces ranked
Intermolecular forces ranked Intramolecular forces vs intermolecular forces
Intramolecular forces vs intermolecular forces For plucking or handling small objects
For plucking or handling small objects Intramolecular forces
Intramolecular forces Intermolecular attraction
Intermolecular attraction Intermolecular forces and boiling point
Intermolecular forces and boiling point Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules The force that holds two atoms together
The force that holds two atoms together Conclusion transitions
Conclusion transitions What holds an atom together
What holds an atom together Ionic forces of attraction
Ionic forces of attraction Mind map of types of forces
Mind map of types of forces Sucrose intermolecular forces
Sucrose intermolecular forces Intermolecular forces in matter
Intermolecular forces in matter Hydrogen bromide intermolecular forces
Hydrogen bromide intermolecular forces London dispersion force
London dispersion force London dispersion force
London dispersion force How intermolecular forces affect solvation
How intermolecular forces affect solvation Carbonyl sulfide intermolecular forces
Carbonyl sulfide intermolecular forces Comic strip about intermolecular forces
Comic strip about intermolecular forces Type of intermolecular forces
Type of intermolecular forces Coh2 intermolecular forces
Coh2 intermolecular forces Intermolecular forces
Intermolecular forces Intermolecular forces symbol
Intermolecular forces symbol Intermolecular forces
Intermolecular forces Intermolecular forces
Intermolecular forces Poem about intermolecular forces
Poem about intermolecular forces Hco2h intermolecular forces
Hco2h intermolecular forces Hybrid bonding
Hybrid bonding Intermolecular forces
Intermolecular forces Solid
Solid Induced dipole
Induced dipole Surface tension intermolecular forces
Surface tension intermolecular forces Strongest intramolecular force
Strongest intramolecular force Unit 3 intermolecular forces and properties
Unit 3 intermolecular forces and properties Viscosity and intermolecular forces
Viscosity and intermolecular forces