Molecules of Life Introduction to Organic Molecules 2














- Slides: 38

Molecules of Life

Introduction to Organic Molecules 2


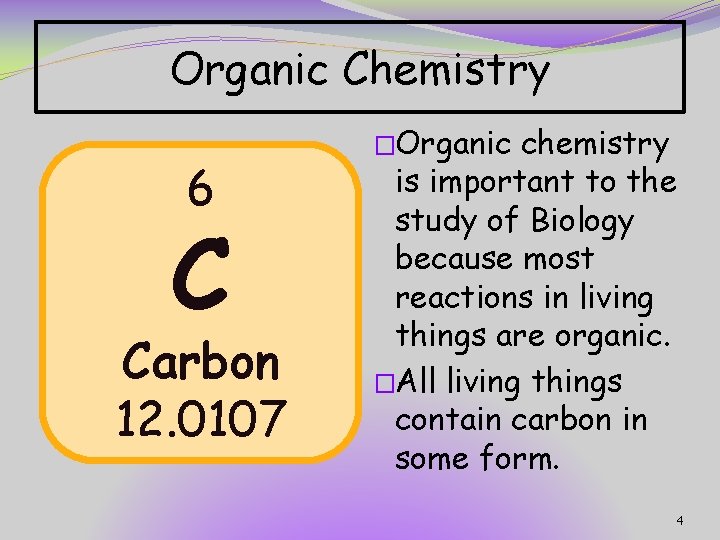
Organic Chemistry 6 C Carbon 12. 0107 �Organic chemistry is important to the study of Biology because most reactions in living things are organic. �All living things contain carbon in some form. 4

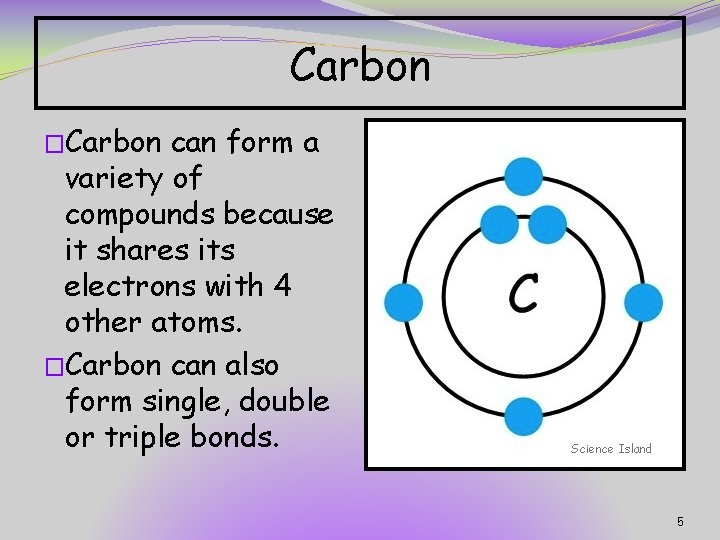
Carbon �Carbon can form a variety of compounds because it shares its electrons with 4 other atoms. �Carbon can also form single, double or triple bonds. Science Island 5

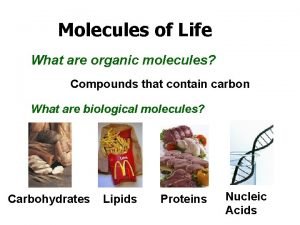
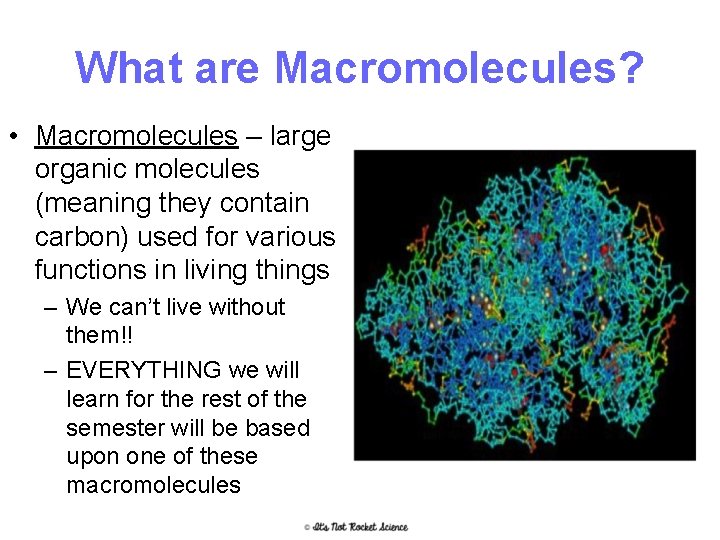
What are Macromolecules? • Macromolecules – large organic molecules (meaning they contain carbon) used for various functions in living things – We can’t live without them!! – EVERYTHING we will learn for the rest of the semester will be based upon one of these macromolecules 6

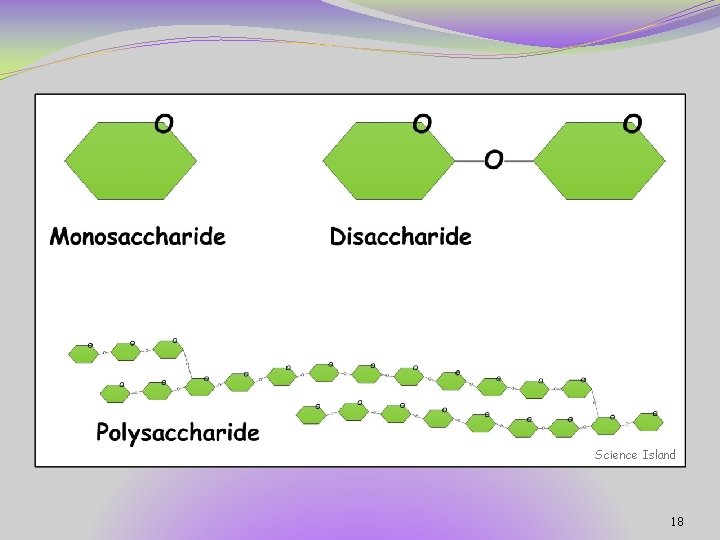
Monomers and Polymers �Monomers are small molecular units that link together to form long chains called polymers. �Example: �styrene is a monomer �polystyrene is a polymer 7

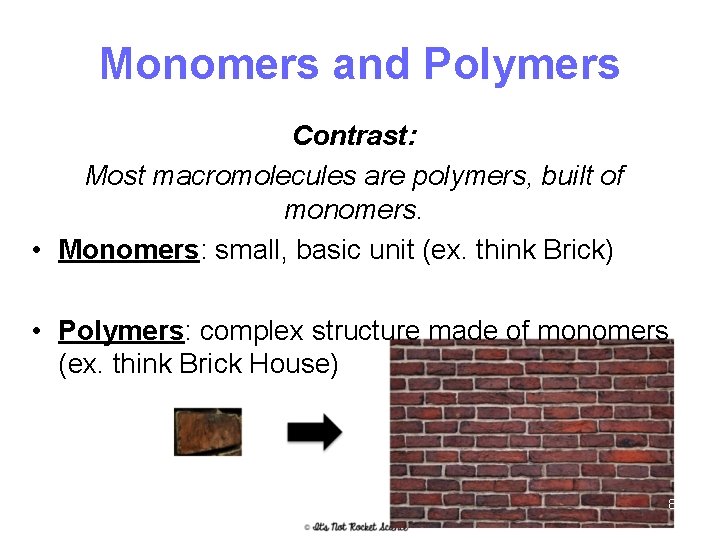
Monomers and Polymers Contrast: Most macromolecules are polymers, built of monomers. • Monomers: small, basic unit (ex. think Brick) • Polymers: complex structure made of monomers (ex. think Brick House) 8

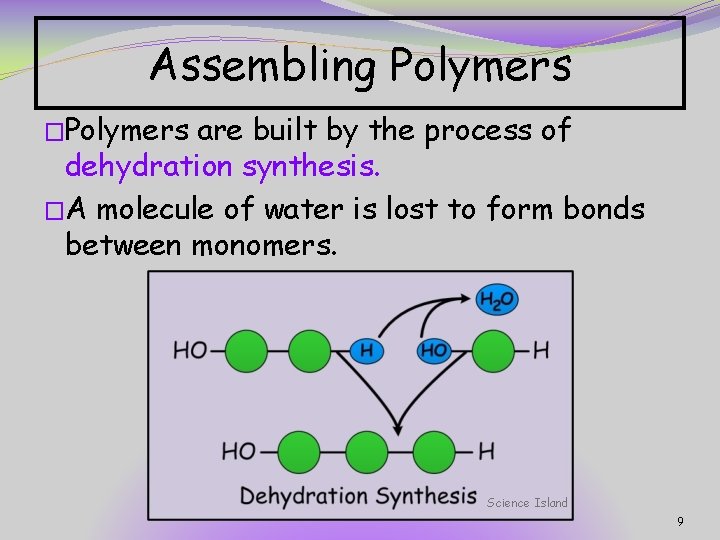
Assembling Polymers �Polymers are built by the process of dehydration synthesis. �A molecule of water is lost to form bonds between monomers. Science Island 9

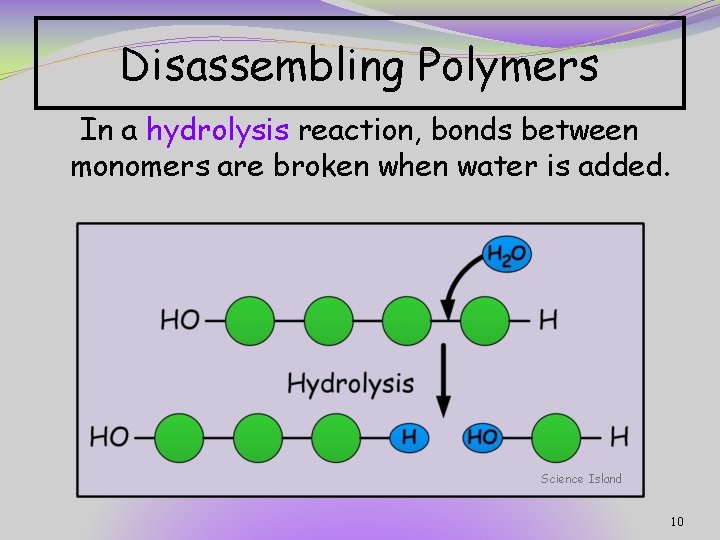
Disassembling Polymers In a hydrolysis reaction, bonds between monomers are broken when water is added. Science Island 10

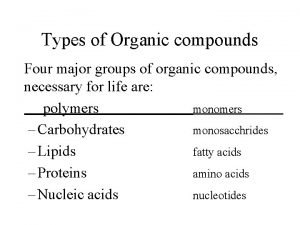
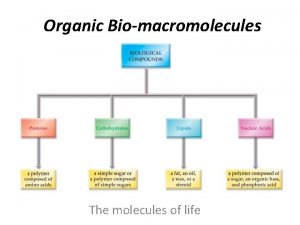
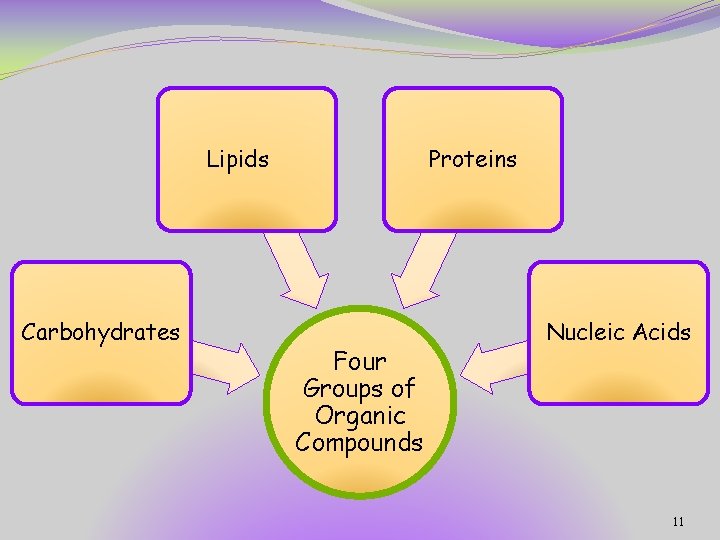
Lipids Carbohydrates Proteins Four Groups of Organic Compounds Nucleic Acids 11

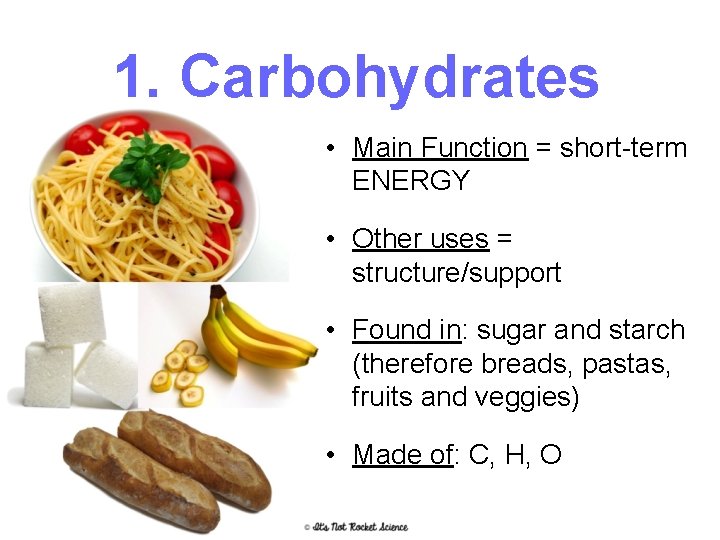
1. Carbohydrates • Main Function = short-term ENERGY • Other uses = structure/support • Found in: sugar and starch (therefore breads, pastas, fruits and veggies) • Made of: C, H, O 12

Energy Storage of Carbs • 4 calories/milligram • Because it is short term energy, your body can access it very easily so it is the FIRST thing you will break down to get energy when you need it! • • Contain carbon, hydrogen, & oxygen in a ratio of 1: 2: 1 Are usually hydrophilic (water loving) dissolve in water 13

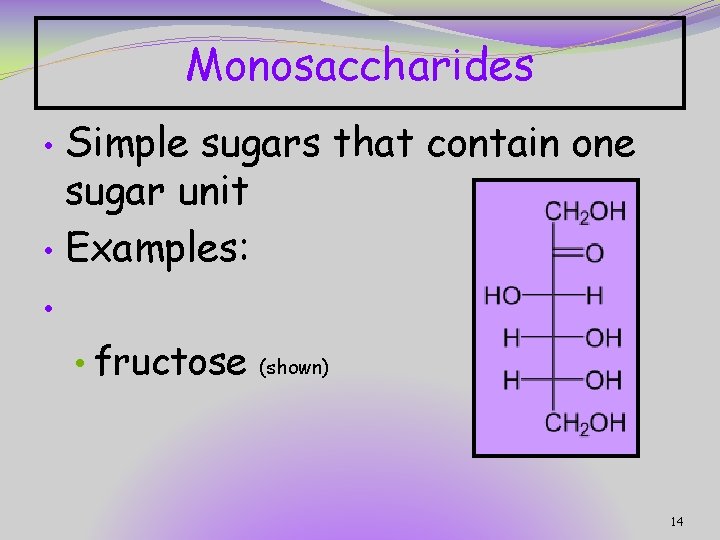
Monosaccharides Simple sugars that contain one sugar unit • Examples: • • • fructose (shown) 14

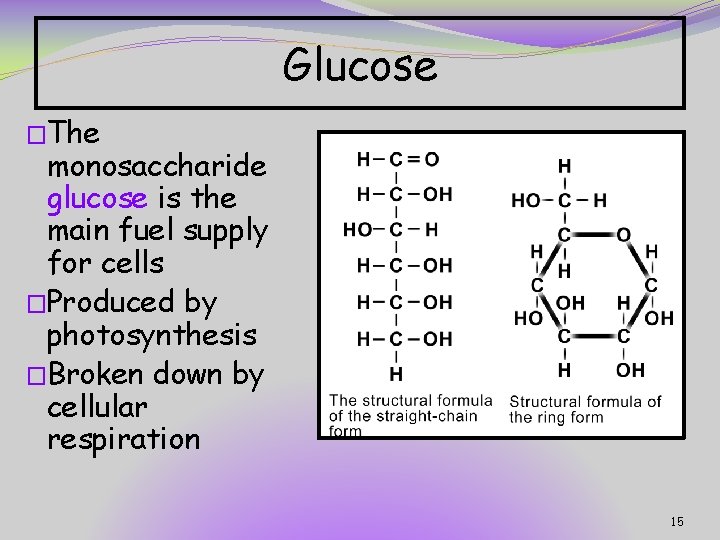
Glucose �The monosaccharide glucose is the main fuel supply for cells �Produced by photosynthesis �Broken down by cellular respiration 15

Disaccharides • • • Means “double sugar” di = two Two simple sugars combine together in a dehydration reaction Examples: sucrose, lactose, maltose 16

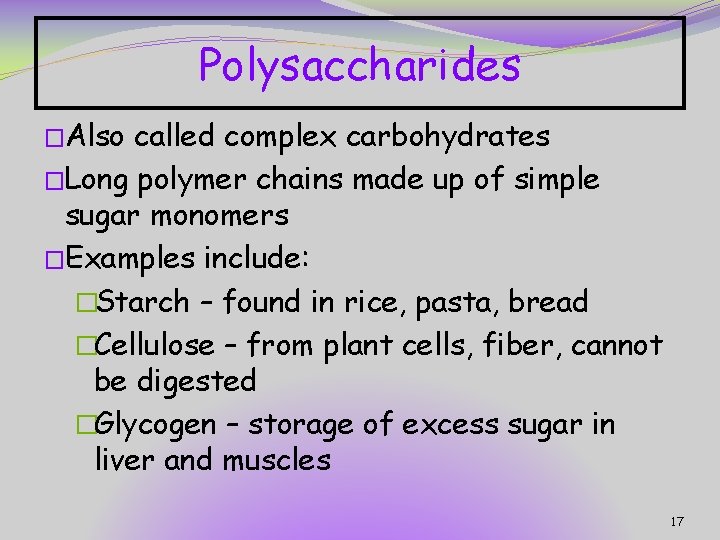
Polysaccharides �Also called complex carbohydrates �Long polymer chains made up of simple sugar monomers �Examples include: �Starch – found in rice, pasta, bread �Cellulose – from plant cells, fiber, cannot be digested �Glycogen – storage of excess sugar in liver and muscles 17

Science Island 18

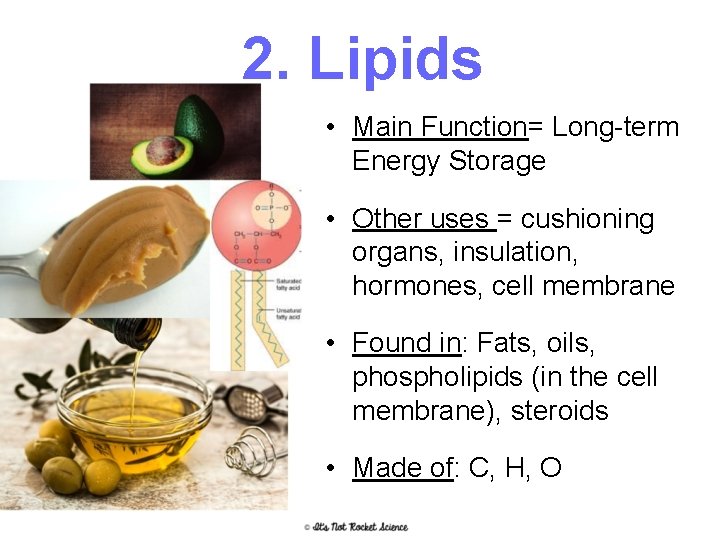
2. Lipids • Main Function= Long-term Energy Storage • Other uses = cushioning organs, insulation, hormones, cell membrane • Found in: Fats, oils, phospholipids (in the cell membrane), steroids • Made of: C, H, O 19

Lipids �Water avoiding molecules that are said to be “water-fearing” or hydrophobic �They do not dissolve in water. �Examples include: fats, waxes, phospholipids & steroids 20

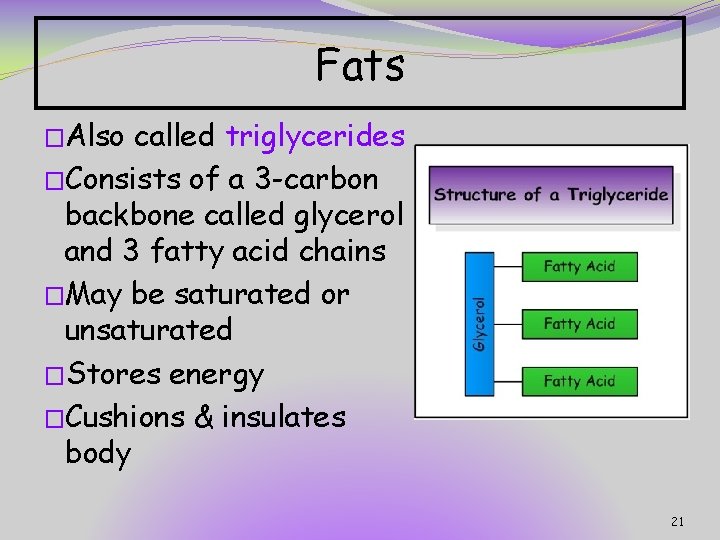
Fats �Also called triglycerides �Consists of a 3 -carbon backbone called glycerol and 3 fatty acid chains �May be saturated or unsaturated �Stores energy �Cushions & insulates body 21

Saturated vs. Unsaturated Fats �Saturated – all single bonds (C-H), from animals, solid at room temperature �Unsaturated – at least one double bond, from plants, liquid at room temperature 22

Waxes �Used for waterproofing in plants and animals �Human ear wax helps keep foreign material out of ear canal �Waxy coatings help plants maintain moisture 23

Proteins • Most diverse macromolecule • Most abundant macromolecule (make up 50% of cell’s biomass) • They literally RUN your body!! 24

Functions of Proteins 1. Enzymes control the rate of chemical reactions 2. Hormones regulate cell processes (ex. Insulin) 3. Used to form bones and muscles (ex. Collagen) 4. Transports substances in & out of cells (ex. Hemoglobin) 5. Antibodies help fight diseases 25

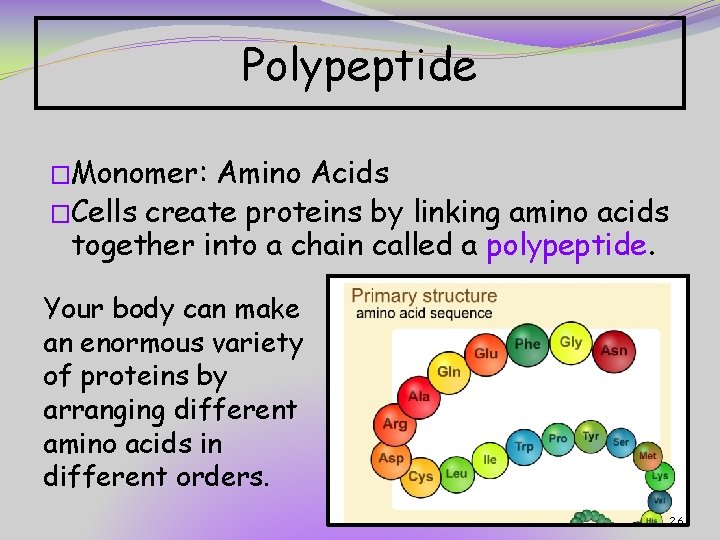
Polypeptide �Monomer: Amino Acids �Cells create proteins by linking amino acids together into a chain called a polypeptide. Your body can make an enormous variety of proteins by arranging different amino acids in different orders. 26

Functional Protein Just like a bundle of yarn needs to be carefully knitted to make a sweater, the polypeptides of a protein are twisted and folded into a specific shape so that it can function properly. 27

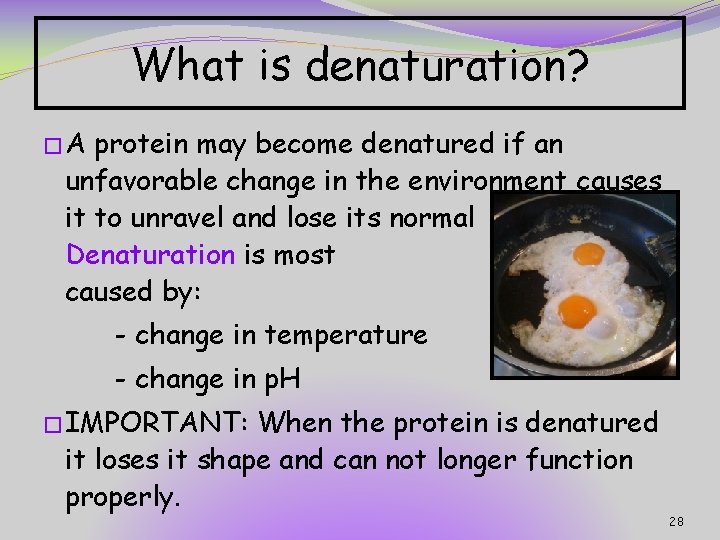
What is denaturation? �A protein may become denatured if an unfavorable change in the environment causes it to unravel and lose its normal shape. Denaturation is most often caused by: - change in temperature - change in p. H �IMPORTANT: When the protein is denatured it loses it shape and can not longer function properly. 28

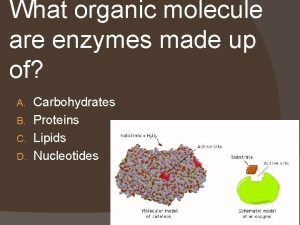
Enzymes are Specialized Proteins �Cellular reactions depend on the assistance of catalysts, compounds that speed up chemical reactions. �The main catalysts of chemical reactions in organisms are specialized proteins called enzymes. 29

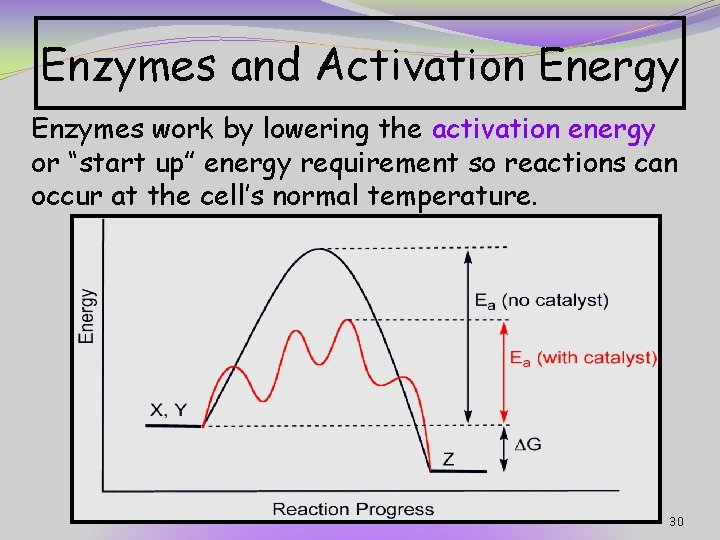
Enzymes and Activation Energy Enzymes work by lowering the activation energy or “start up” energy requirement so reactions can occur at the cell’s normal temperature. 30

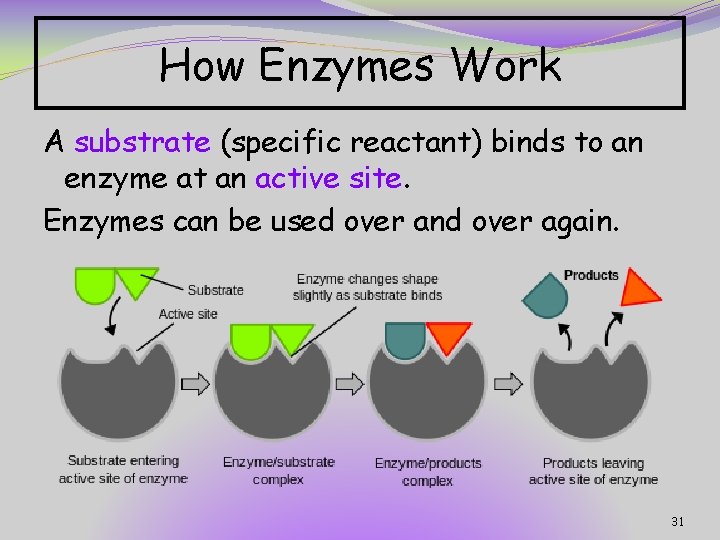
How Enzymes Work A substrate (specific reactant) binds to an enzyme at an active site. Enzymes can be used over and over again. 31

Nucleic Acids - Main Function: store & transmit hereditary or genetic information - Ex. DNA, RNA - You get them from your PARENTS not your food! - Made of: C, H, O, P, and N 32

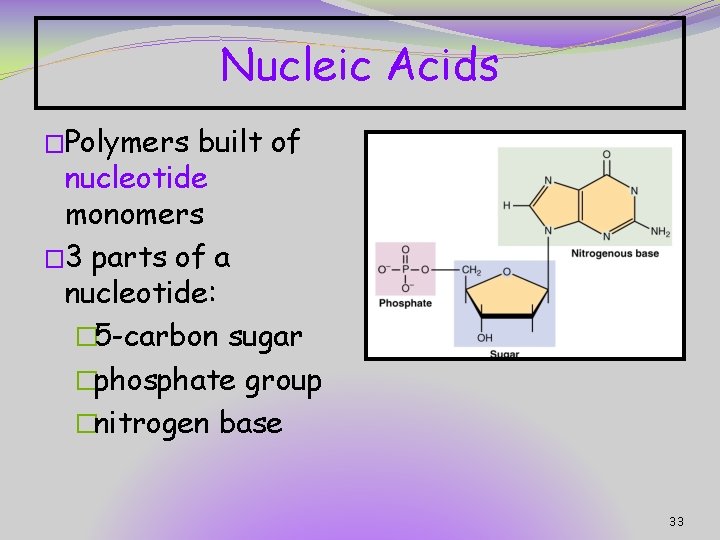
Nucleic Acids �Polymers built of nucleotide monomers � 3 parts of a nucleotide: � 5 -carbon sugar �phosphate group �nitrogen base 33

Examples of Nucleic Acids �DNA – deoxyribonucleic acid �RNA – ribonucleic acid �ATP – adenosine triphosphate Do you recognize this molecule? 34

DNA & RNA � All living things contain DNA and/or RNA. � Carry genetic information � Direct the process of protein synthesis which uses inherited information to determine the characteristics of an organism 35


Question: Name the four groups of organic compounds. 37

Answer: 1. 2. 3. 4. Carbohydrates Lipids Proteins Nucleic Acids 38
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules The four major groups of organic compounds are
The four major groups of organic compounds are Organic vs inorganic
Organic vs inorganic Four types of organic compounds
Four types of organic compounds What is organic chemistry
What is organic chemistry Organic compounds grade 10 life science
Organic compounds grade 10 life science Biological molecules what are the building blocks of life
Biological molecules what are the building blocks of life Chapter 3 molecules of life
Chapter 3 molecules of life Conclusion paragraph format
Conclusion paragraph format Enzymes are composed of what organic molecule
Enzymes are composed of what organic molecule Organic analogy parsons
Organic analogy parsons Organic solidarity
Organic solidarity Weathering and soil erosion
Weathering and soil erosion Where vitamins are absorbed
Where vitamins are absorbed Vitamin flowchart
Vitamin flowchart Numbering carbon chains
Numbering carbon chains Organic solidarity
Organic solidarity Pros and cons of organic food
Pros and cons of organic food Organic structure characteristics
Organic structure characteristics Swot analysis of organic products
Swot analysis of organic products Basic organic nomenclature packet
Basic organic nomenclature packet Soil is a mixture of weathered rock and
Soil is a mixture of weathered rock and Decomposition of organic compounds
Decomposition of organic compounds Organic social media
Organic social media Canola oil
Canola oil Smear layer in dentistry
Smear layer in dentistry Showeet
Showeet Functional groups ib chemistry
Functional groups ib chemistry Ester organic chemistry
Ester organic chemistry Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Osp entek
Osp entek Inorganic vs organic chemistry
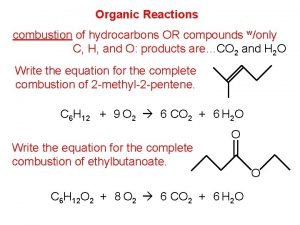
Inorganic vs organic chemistry Organic combustion reaction
Organic combustion reaction Qualitative analysis of organic functional groups
Qualitative analysis of organic functional groups Grapes
Grapes What is organic polymer
What is organic polymer Organic growth
Organic growth Swot analysis of organic products
Swot analysis of organic products Organic compound made by living things
Organic compound made by living things