Chapter 10 Gases Soo Jeon 10 1 Characteristics





































- Slides: 53

Chapter. 10 Gases Soo Jeon

10. 1 Characteristics of Gases n are composed entirely of nonmetal elements. n expand spontaneously to fill its container( volume of gas = volume of its container). n n are highly compressible. form homogeneous mixtures with each other regardless of the identities. molecules are far apart. different gases behave similarly.

10. 2 Pressure n n n Pressure conveys the idea of a force, a push that tends to move something else in a given direction. Pressure = Force / Given area Force(Newton) = Mass * Acceleration) Pressure = 1*10^5 N/1 m^2 = 1*10^5 N/M^ = 1*10^5 Pascal = 100 kpa = 1 bar Blaise Pascal(1623 -1662) : French Scientist, discovered pascal(N/m^2)

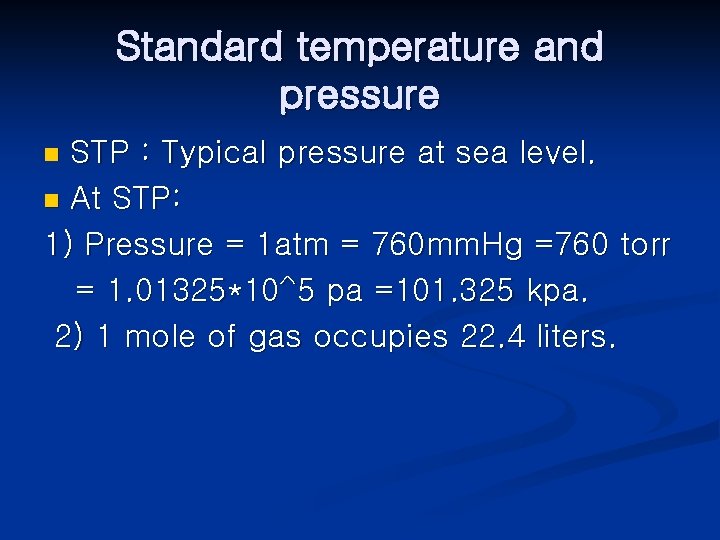
Standard temperature and pressure STP : Typical pressure at sea level. n At STP: 1) Pressure = 1 atm = 760 mm. Hg =760 torr = 1. 01325*10^5 pa =101. 325 kpa. 2) 1 mole of gas occupies 22. 4 liters. n

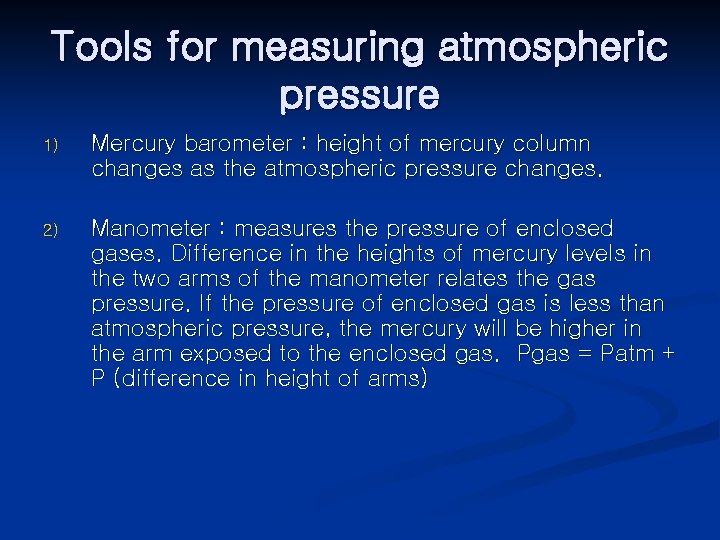
Tools for measuring atmospheric pressure 1) Mercury barometer : height of mercury column changes as the atmospheric pressure changes. 2) Manometer : measures the pressure of enclosed gases. Difference in the heights of mercury levels in the two arms of the manometer relates the gas pressure. If the pressure of enclosed gas is less than atmospheric pressure, the mercury will be higher in the arm exposed to the enclosed gas. Pgas = Patm + P (difference in height of arms)

Problems 1 Convert a) 0. 357 atm to torr. b) 6. 6*10^-2 torr to atm. c) 147. 2 k. Pa to mm. Hg. 1.

Solution 1 a) b) c) (0. 357 atm)(760 torr/1 atm) = 271 torr (6. 6*10^-2 torr)(1 atm/760 torr) = 8. 7*10^-5 atm (147. 2 k. Pa)(760 mm. Hg/101. 325 k. Pa) = 1104 torr

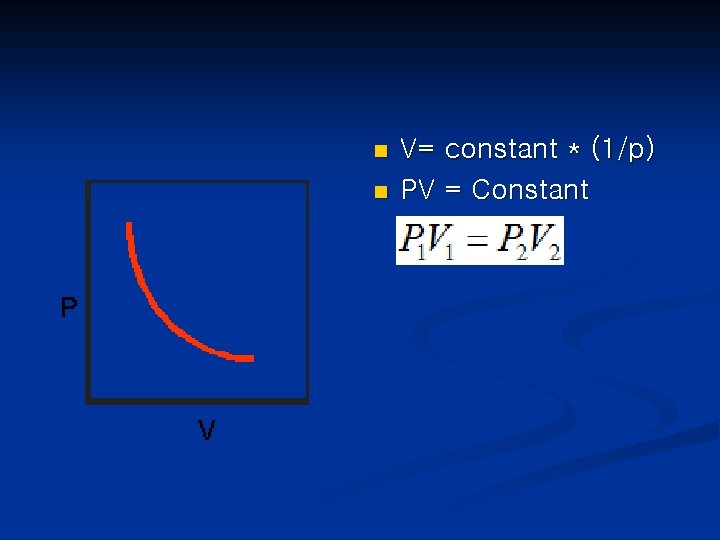
10. 3 The Gas Laws • • Boyle’s Law( the pressure-volume Relationship) : when a volume of gas is compressed, the pressure of gas increase. The volume of a fixed quantity of gas maintained at constant temperature is inversely proportional to the pressure. British Chemist Robert Boyle(1627 -1691) : first investigated the relationship between pressure of gas and volume.

n n V= constant * (1/p) PV = Constant

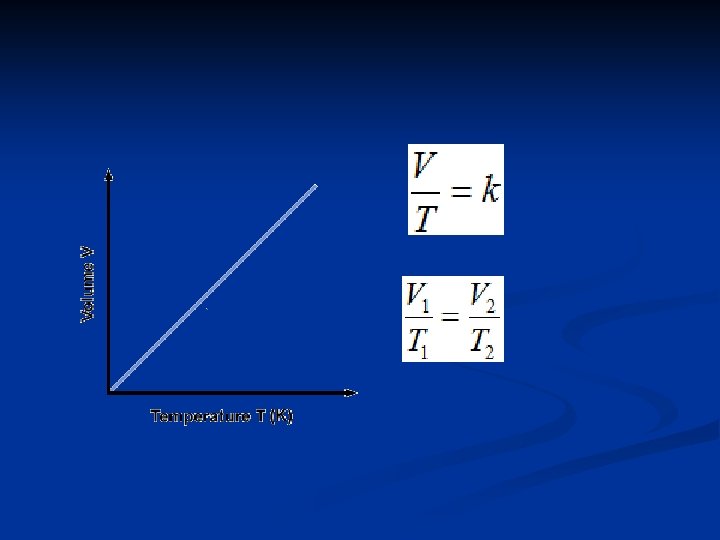
Charles’s Law (temperature – volume) Charles’s Law : the volume of a fixed amount of gas maintained at constant pressure is directly proportional to its absolute temperature. n French Scientist Jacques Charles (1746 -1823): found that volume of fixed quantity of gas at constant pressure increases linearly with temperature. n


In 1848 William Thomson(1824 -1907) proposed an absolute temperature scale, Kelvin. n Absolute zero = -273. 15°C. n

Avogadro’s Law n In 1808, Gay Lussac(1778 -1823) observed the law of combining volumes; at a given pressure and temperature, the volumes of gases that react with one another are in the ratios of small whole numbers.

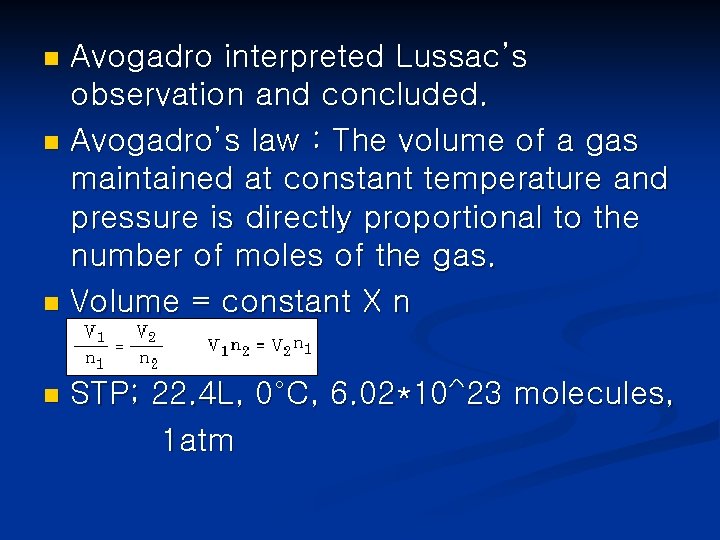
Avogadro interpreted Lussac’s observation and concluded. n Avogadro’s law : The volume of a gas maintained at constant temperature and pressure is directly proportional to the number of moles of the gas. n Volume = constant X n n n STP; 22. 4 L, 0°C, 6. 02*10^23 molecules, 1 atm

10. 4 The Ideal-Gas Equation Ideal-gas equation: Pressure*Volume= #of moles* R(0. 0821 L-atm/mol-K)* temperature n Ideal gas is STP. n The ideal-gas equation does not always accurately describe real gas. n P 1/T 1=P 2 T 2, P/T=n. R / V, n P 1 V 1/T 1 = P 2 V 2/T 2 n

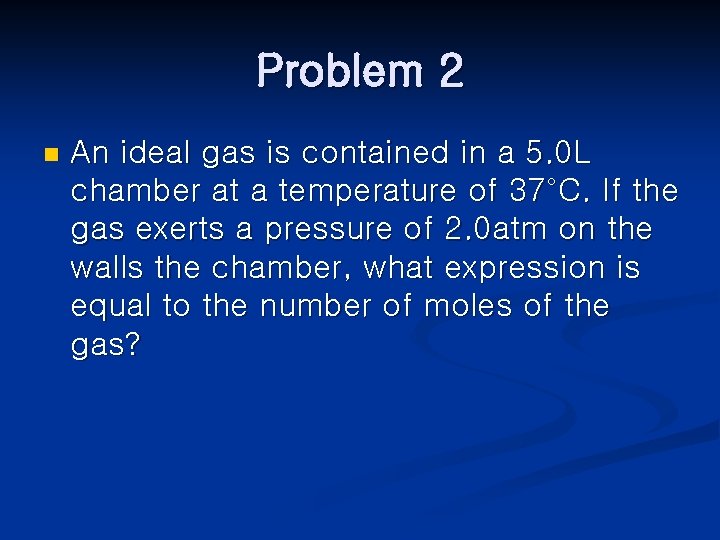
Problem 2 n An ideal gas is contained in a 5. 0 L chamber at a temperature of 37°C. If the gas exerts a pressure of 2. 0 atm on the walls the chamber, what expression is equal to the number of moles of the gas?

Solution 2 n = PV/RT = (2. 0 atm)(5. 0 L)/(0. 0821 L-atm)(310 K) moles

Problem 3 n A 0. 5 mol sample of oxygen gas is confined at 0°C in a cylinder with a movable piston. The gas has an initial pressure of 1. 0 atm. The gas is then compressed by the piston so that its final volume is half the initial volume. The final pressure of gas is 2. 2 atm. What is the final temperature?

Solution 3 n = 1 atm* XL/ 273 K = 2. 2 atm *. 5 XL/temperature X/273 = 1. 1 X/temperature = 300. 3 K = 27°C

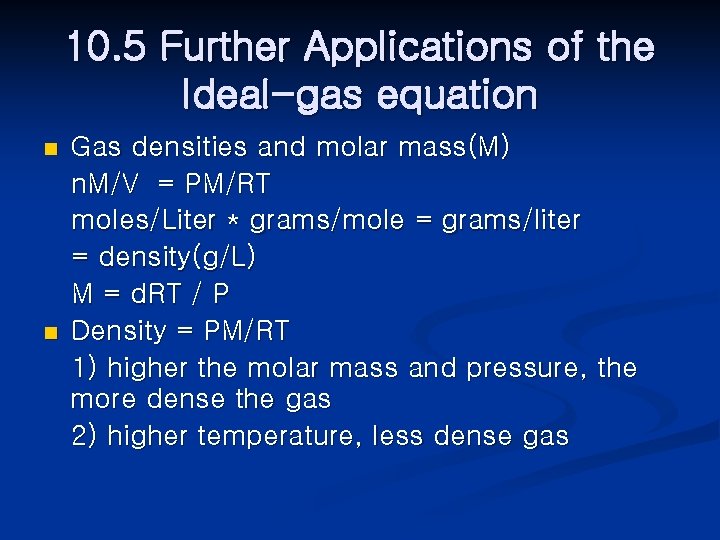
10. 5 Further Applications of the Ideal-gas equation n n Gas densities and molar mass(M) n. M/V = PM/RT moles/Liter * grams/mole = grams/liter = density(g/L) M = d. RT / P Density = PM/RT 1) higher the molar mass and pressure, the more dense the gas 2) higher temperature, less dense gas

Problem 4 n Molar mass is 28. 6 g/mol, temperature is 95 K, and the pressure is 1. 6 atm. Calculate the density

Solution 4 n D = PM/RT = (28. 6 g/mol)(1. 6 atm)/(0. 0821 L-atm/mol -k)(95 K) = 5. 9 g/L

Problem 5 n Find the molar mass of an unknown gas. First, a large flask is evacuated and found to weigh 134. 567 g. It’s then filled with the gas to a pressure of 735 torr at 31° and reweighed. Its mass is now 137. 456 g. Finally, the flask is filled with water at 31°C and found to weigh 1067. 9 g. (the density of the water at this temperature is 0. 977 g/ml. ) calculate the molar mass of unknown gas.

Solution 5 The mass of the gas : 137. 456 g – 134. 567 g = 2. 889 g The mass of water : 1067. 9 g – 134. 567 g = 933. 3 g Volume of flask = m/d = (933. 3 g)/(0. 997 g/ml) = 936 ml So, the density of the gas = 2. 889 g/0. 936 L = 3. 09 g/L Molar mass of the gas = d. RT/ P = (3. 09 g/L)(0. 0821 L-atm/mol-K)(304 K)/(735/760)atm = 79. 7 g/mol n

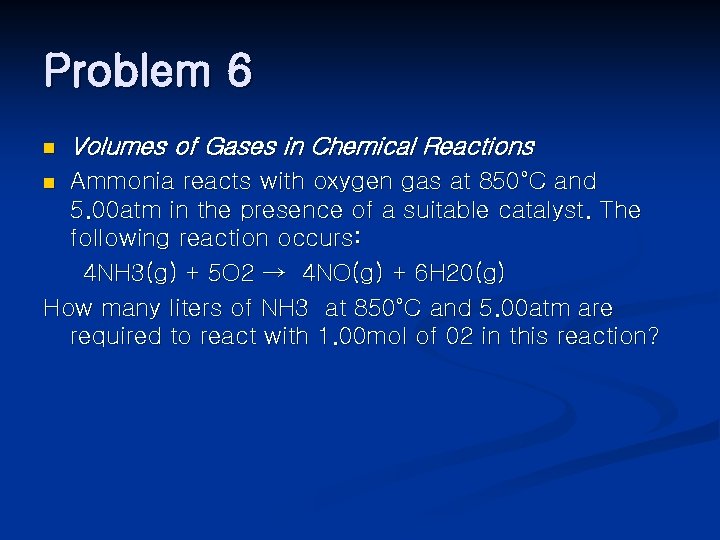
Problem 6 n Volumes of Gases in Chemical Reactions Ammonia reacts with oxygen gas at 850°C and 5. 00 atm in the presence of a suitable catalyst. The following reaction occurs: 4 NH 3(g) + 5 O 2 → 4 NO(g) + 6 H 20(g) How many liters of NH 3 at 850°C and 5. 00 atm are required to react with 1. 00 mol of 02 in this reaction? n

Solution 6 850°C = 1123 K, 5 atm, 1 mol of O 2 n V= n. RT/P = (1 mol of O 2)(0. 0821 L-atm/mol-)(1123 K)/ (5 atm) = 18. 4 L 18. 4 L of O 2 * (4 mol NH 3)/(5 mol O 2) = 14. 8 L of NH 3 n

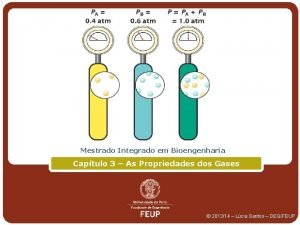
10. 6 Gas Mixtures and Partial Pressures John Dalton(1766 -1844), English chemist, tells us that the total pressure of mixture of gases is just the sum of all the partial pressures of the individual gases in the mixture. n Dalton’s Law : Ptotal = Pa + Pb …. . Ptotal = # of toal moles(RT/V) n

Problem 7 n A gaseous mixture made from 6. 00 g O 2 and 9. 00 g CH 4 is placed 15 L. What is partial pressure of each gas and total pressure?

Solution 7 n. O 2 = (6. 00 g O 2)(1 mol. O 2/32. 0 g O 2)=0. 188 mol O 2 n. CH 4 =(9. 00 g. CH 4)(1 mol. CH 4/16 g CH 4)= 0. 563 mol PO 2= no 2 RT/ V = (0. 188 mol)(0. 0821)(273 K)/(15 L) = 0. 281 atm PCH 4 = (0. 563 mol)(0. 0821)(273 K)/(15 L) = 0. 841 atm Pt = 0. 281 atm + 0. 841 atm = 1. 122 atm

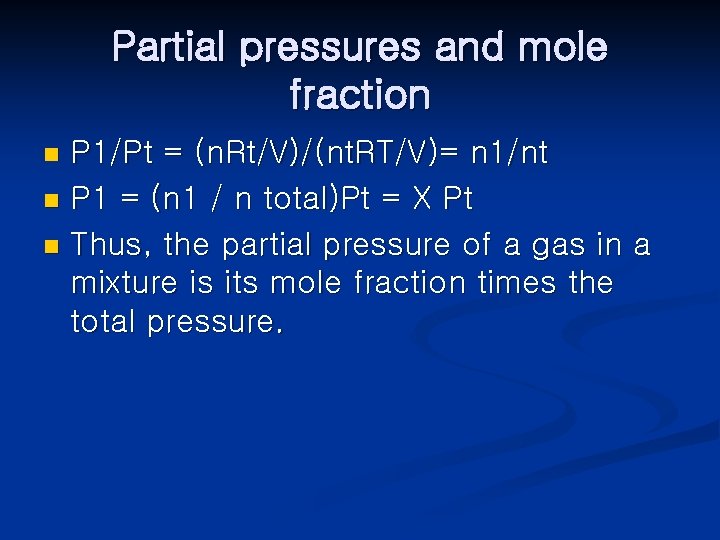
Partial pressures and mole fraction P 1/Pt = (n. Rt/V)/(nt. RT/V)= n 1/nt n P 1 = (n 1 / n total)Pt = X Pt n Thus, the partial pressure of a gas in a mixture is its mole fraction times the total pressure. n

Problem 8 n The atmosphere is composed of 1. 5 mol percent CO 2, 18. 0 mol percent O 2, and 80. 5 mol percent Ar. a) calculate the partial pressure of O 2 in the mixture if the total pressure is 745 torr. b) if this atmosphere is to be held in a 120 L space at 295 K, how many moles of O 2 are needed?

Solution 8 a) PO 2 = (0. 180(745 torr)= 134 torr n b) PO 2 = (134 torr)(1 atm/760 torr)=0. 176 atm V = 120 L, T = 295 K n. O 2 = PO 2(V/RT)= (0. 176 atm)(120 L)/(0. 0821 L-atm/Kmol)(295 K) = 0. 872 mol n

Collecting gases over water The volume of gas collected is measured until the inside water levels and outside the bottle are same. n The total pressure inside is the sum of the pressure of gas collected and pressure of water vapor in equilibrium with liquid water. n Ptotal = Pgas + Pwater n

Problem 9 Ammonium nitrate, NH 4 NO 2, decomposes upon heating to form N 2 gas. NH 4 NO 2→ N 2 + 2 H 2 O When a sample of NH 4 NO 2 is decomposed in a test tube, 511 ml of N 2 gas is collected over water at 26°C and 745 torr total pressure. How many grams of NH 4 NO 2 were decomposed? n

Solution 9 Pressure of water vapor = 25 torr n 745 torr – 25 torr= 720 torr n Number of moles N 2 = PV/RT = (720 torr*(1 atm/760 torr)(0. 511 L) /(0. 0821)(299 K) =0. 0197 mol N 2 n 0. 0197 mol. N 2(1 mol. NH 4 NO 2/1 Mol N 2) *(64. 04 g NH 4 NO 2/1 mol. NH 4 NO 2) = 1. 26 g NH 4 NO 2

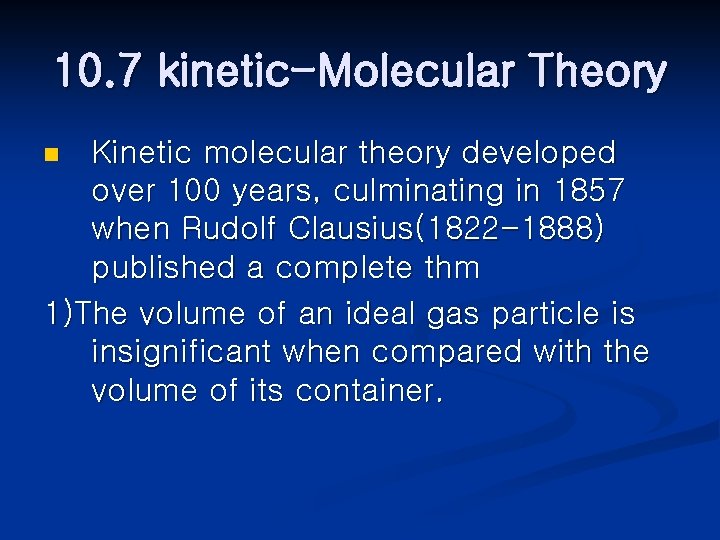
10. 7 kinetic-Molecular Theory Kinetic molecular theory developed over 100 years, culminating in 1857 when Rudolf Clausius(1822 -1888) published a complete thm 1)The volume of an ideal gas particle is insignificant when compared with the volume of its container. n

2) Attractive and repulsive forces between gases molecules are negligible. 3) Energy can be transferred during collision, but the average kinetic energy does not change. 4) The average kinetic energy of molecules is proportional to the absolute temperature.

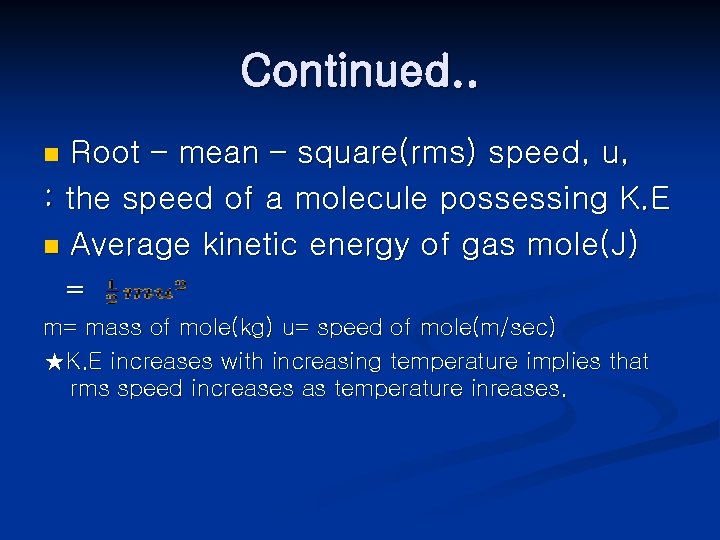
Continued. . Root – mean – square(rms) speed, u, : the speed of a molecule possessing K. E n Average kinetic energy of gas mole(J) = n m= mass of mole(kg) u= speed of mole(m/sec) ★K. E increases with increasing temperature implies that rms speed increases as temperature inreases.

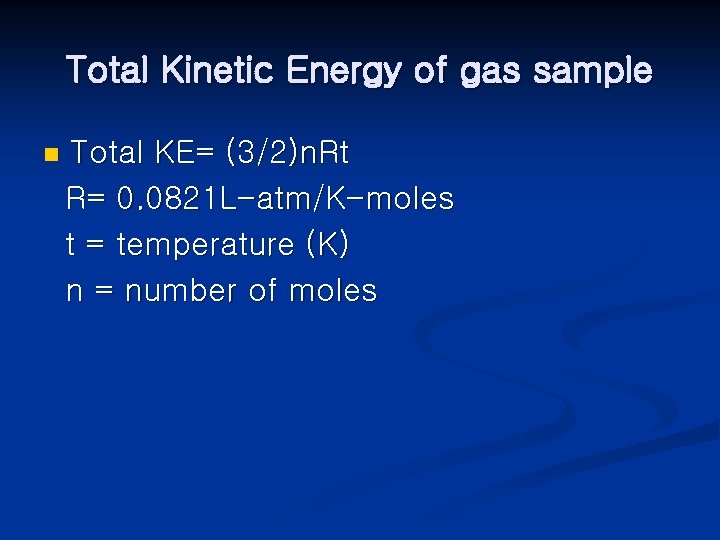
Total Kinetic Energy of gas sample n Total KE= (3/2)n. Rt R= 0. 0821 L-atm/K-moles t = temperature (K) n = number of moles

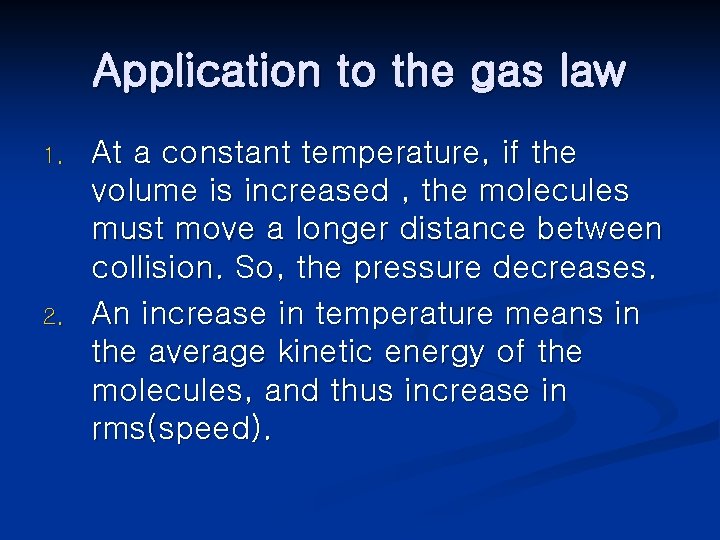
Application to the gas law 1. 2. At a constant temperature, if the volume is increased , the molecules must move a longer distance between collision. So, the pressure decreases. An increase in temperature means in the average kinetic energy of the molecules, and thus increase in rms(speed).

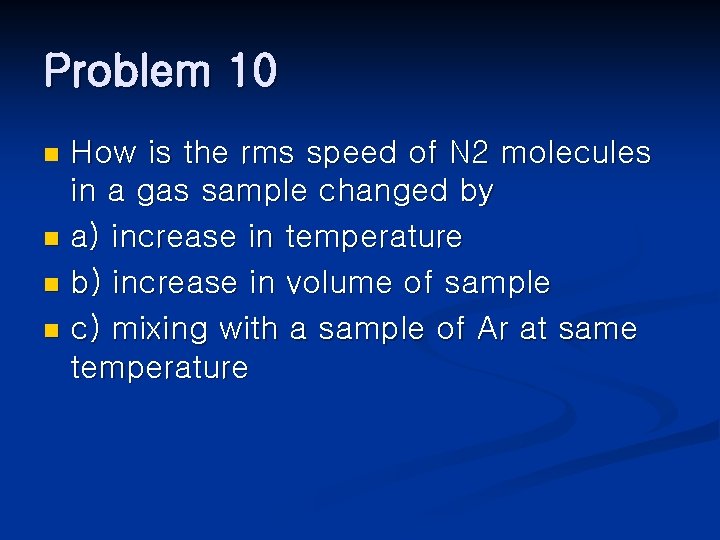
Problem 10 How is the rms speed of N 2 molecules in a gas sample changed by n a) increase in temperature n b) increase in volume of sample n c) mixing with a sample of Ar at same temperature n

Solution 10 A) increase n B) no change n C) no change n

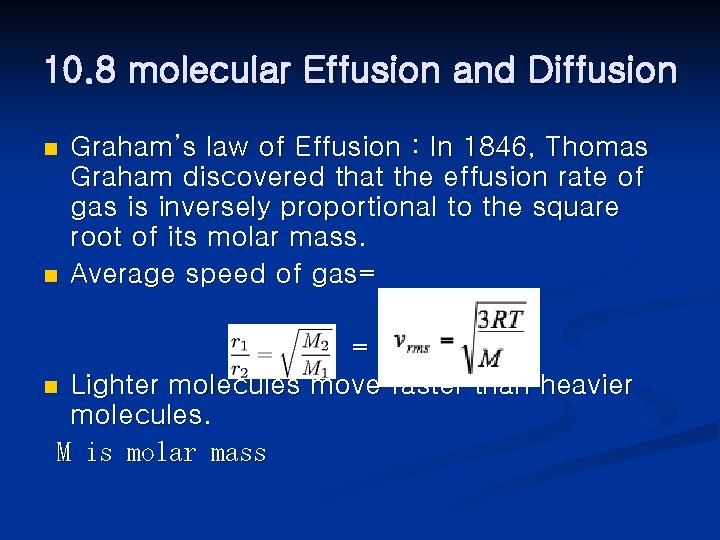
10. 8 molecular Effusion and Diffusion n n Graham’s law of Effusion : In 1846, Thomas Graham discovered that the effusion rate of gas is inversely proportional to the square root of its molar mass. Average speed of gas= = n Lighter molecules move faster than heavier molecules. M is molar mass

Problem 11 n An unknown gas composed of homonuclear diatomic molecules effuses at a rate that is only 0. 355 times that of O 2 at the same temperature what is the gas?

Solution 11 n n r 1 = 0. 355 * r 2 n r 1/r 2= 0. 355= square root of(32 g/mol)/M 1 (32 g/mol)/M=(0. 355)^2=0. 126 M 1 = (32 g/mol)/(0. 126)= 254 g/mol Only di iodine has this atomic weight. n n n

Diffusion and mean free path n n Diffusion: spread of one substance throughout a space or a second substance. Diffusion of gas is slower than molecular speed, because of molecular collision. Mean free path : the average distance traveled by a molecule between collisions. the mean free path for air molecules at sea level is about 60 nm.

10. 9 Real Gases: deviation from ideal behavior Real molecules do have finite volumes and they do attract one another. n The difference that remains at high temperature stems mainly from the effect of the finite volume of the molecuels. n

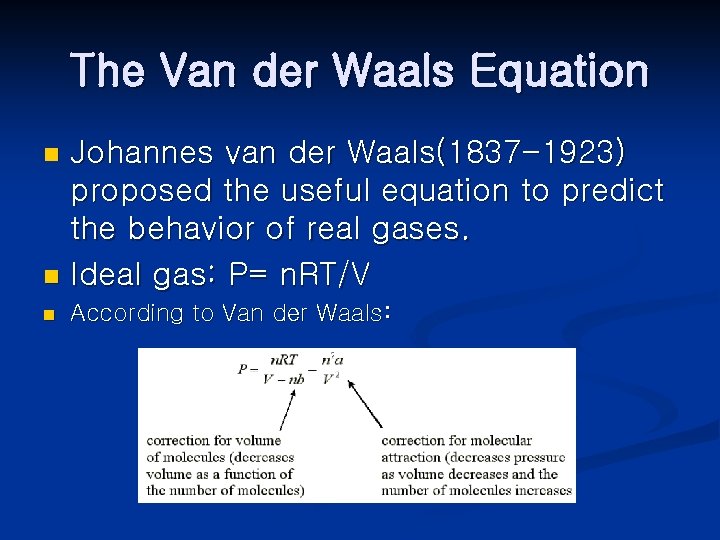
The Van der Waals Equation Johannes van der Waals(1837 -1923) proposed the useful equation to predict the behavior of real gases. n Ideal gas: P= n. RT/V n n According to Van der Waals:

Both a and b are given. A has units of L^2 -atm/mol^2. n Van der Waals equation: n

Problem 12 n If 1. 00 mol of an ideal gas were confined to 22. 41 L at 0℃, it would exert a pressure of 1. 00 atm. Use Van der Waals equation to estimate the pressure exerted by 1. 00 mol of Cl 2 in 22. 4 L at 0℃.

Solution 12 n n = 1 mol, R= 0. 0821, T=273 K, V=22. 4 L a= 6. 49 L^2 atm/mol, b= 0. 0562 L^2 atm/mol P = (1 mol)(0. 0821)(273. 2)/22. 4 -(1)(0. 0562) -(1^2)(6. 49)/(22. 4)^2 = 1. 003 atm – 0. 013 atm = 0. 990 atm

WE CAN GET 5!

Work cite www. google. com n http: //www. molecularsoft. com/help/Gas _Laws-Real_Gas. htm n www. yahoo. com n
 Myeongjae jeon
Myeongjae jeon Language p
Language p Irene jeon
Irene jeon Ethan soo
Ethan soo Tan soo suan
Tan soo suan Soo-siang lim
Soo-siang lim Dr ida soo
Dr ida soo Park bong
Park bong Vrf cisco configuration
Vrf cisco configuration Lady macbeth important quotes
Lady macbeth important quotes Soo
Soo Teo soo hwang
Teo soo hwang Browards sso
Browards sso Are gasses highly compressible
Are gasses highly compressible Properties of nobel gases
Properties of nobel gases Physical properties of gases
Physical properties of gases Gases characteristics
Gases characteristics Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Characteristics of ideal gases
Characteristics of ideal gases Characteristics of ideal gas
Characteristics of ideal gas Chemistry chapter 11 gases test
Chemistry chapter 11 gases test Chapter 11 review gases section 1
Chapter 11 review gases section 1 Combined gas law practice worksheet answers
Combined gas law practice worksheet answers 14 the behavior of gases
14 the behavior of gases Which gas law relates pressure and temperature
Which gas law relates pressure and temperature Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of solid
Kinetic molecular theory of solid Trabajo neto ciclo de carnot
Trabajo neto ciclo de carnot Where are the noble gases on the periodic table
Where are the noble gases on the periodic table Thermal expansion and contraction examples
Thermal expansion and contraction examples Periodic table metals nonmetals metalloids noble gases
Periodic table metals nonmetals metalloids noble gases Gases on the periodic table
Gases on the periodic table Greenhouse gases are good or bad
Greenhouse gases are good or bad Atmosphere it is a mixture of
Atmosphere it is a mixture of Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Stoichiometry of gases
Stoichiometry of gases Diagram of the states of matter
Diagram of the states of matter The actual exchange of gases occurs at the site of the
The actual exchange of gases occurs at the site of the Degree of freedom of gases
Degree of freedom of gases 2ª série
2ª série Lei de dalton
Lei de dalton Properties of gases
Properties of gases Diffusion of gases across respiratory membranes:
Diffusion of gases across respiratory membranes: Mendeleev
Mendeleev Noble gas
Noble gas Fraccion molar gases
Fraccion molar gases Ley de dalton de las presiones parciales
Ley de dalton de las presiones parciales Constante dos gases perfeitos
Constante dos gases perfeitos Properties of solid, liquid and gas
Properties of solid, liquid and gas Los gases y su comportamiento
Los gases y su comportamiento Globo aerostatico ley de los gases
Globo aerostatico ley de los gases Constante r de los gases
Constante r de los gases