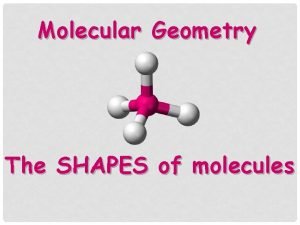
Shapes of molecules Level 3 To determine the

- Slides: 14

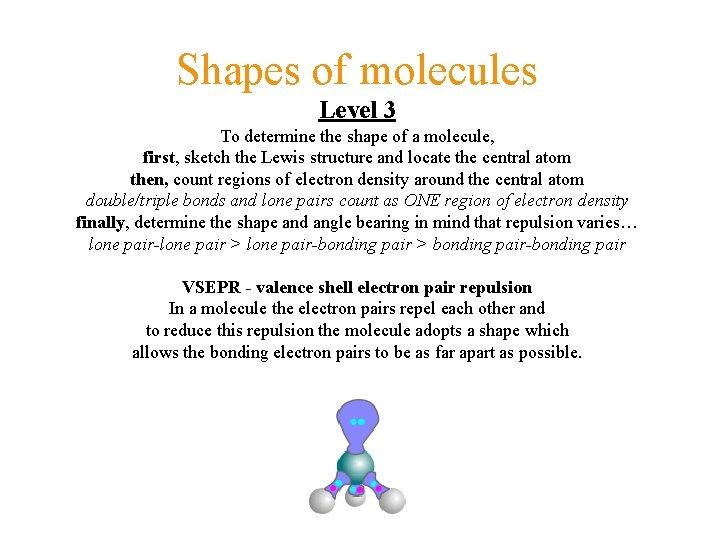
Shapes of molecules Level 3 To determine the shape of a molecule, first, sketch the Lewis structure and locate the central atom then, count regions of electron density around the central atom double/triple bonds and lone pairs count as ONE region of electron density finally, determine the shape and angle bearing in mind that repulsion varies… lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair VSEPR - valence shell electron pair repulsion In a molecule the electron pairs repel each other and to reduce this repulsion the molecule adopts a shape which allows the bonding electron pairs to be as far apart as possible.

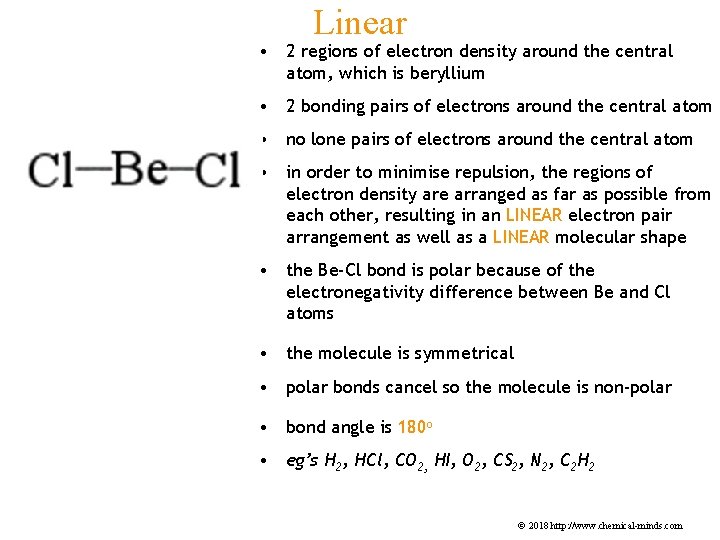
Linear • 2 regions of electron density around the central atom, which is beryllium • 2 bonding pairs of electrons around the central atom • no lone pairs of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an LINEAR electron pair arrangement as well as a LINEAR molecular shape • the Be-Cl bond is polar because of the electronegativity difference between Be and Cl atoms • the molecule is symmetrical • polar bonds cancel so the molecule is non-polar • bond angle is 180 o • eg’s H 2, HCl, CO 2, HI, O 2, CS 2, N 2, C 2 H 2 © 2018 http: //www. chemical-minds. com

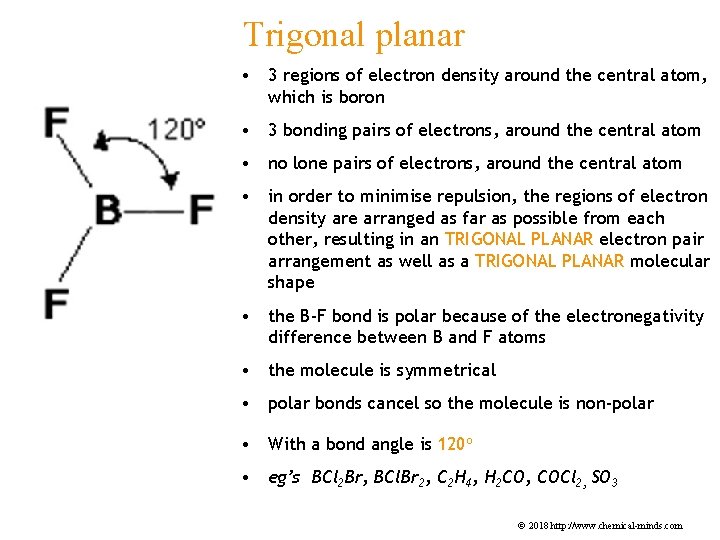
Trigonal planar • 3 regions of electron density around the central atom, which is boron • 3 bonding pairs of electrons, around the central atom • no lone pairs of electrons, around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an TRIGONAL PLANAR electron pair arrangement as well as a TRIGONAL PLANAR molecular shape • the B-F bond is polar because of the electronegativity difference between B and F atoms • the molecule is symmetrical • polar bonds cancel so the molecule is non-polar • With a bond angle is 120 o • eg’s BCl 2 Br, BCl. Br 2, C 2 H 4, H 2 CO, COCl 2, SO 3 © 2018 http: //www. chemical-minds. com

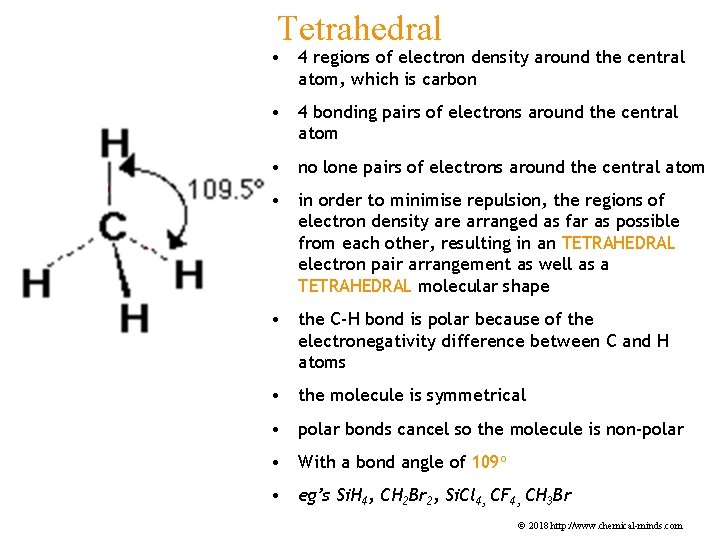
Tetrahedral • 4 regions of electron density around the central atom, which is carbon • 4 bonding pairs of electrons around the central atom • no lone pairs of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an TETRAHEDRAL electron pair arrangement as well as a TETRAHEDRAL molecular shape • the C-H bond is polar because of the electronegativity difference between C and H atoms • the molecule is symmetrical • polar bonds cancel so the molecule is non-polar • With a bond angle of 109 o • eg’s Si. H 4, CH 2 Br 2, Si. Cl 4, CF 4, CH 3 Br © 2018 http: //www. chemical-minds. com

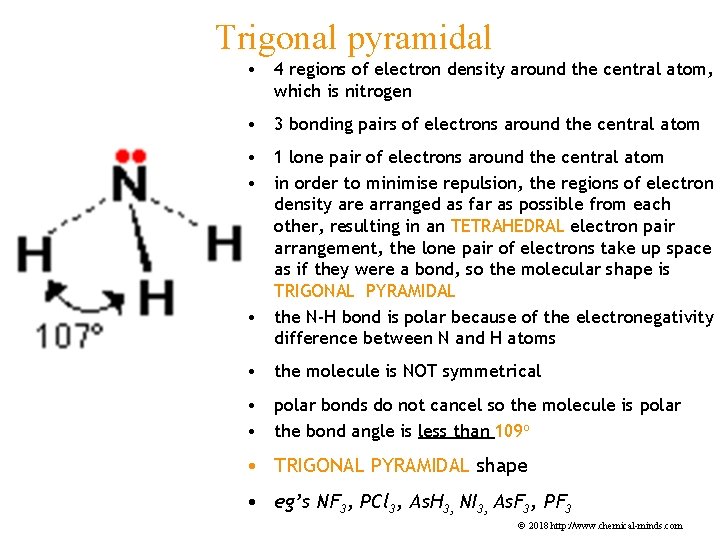
Trigonal pyramidal • 4 regions of electron density around the central atom, which is nitrogen • 3 bonding pairs of electrons around the central atom • 1 lone pair of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an TETRAHEDRAL electron pair arrangement, the lone pair of electrons take up space as if they were a bond, so the molecular shape is TRIGONAL PYRAMIDAL • the N-H bond is polar because of the electronegativity difference between N and H atoms • the molecule is NOT symmetrical • polar bonds do not cancel so the molecule is polar • the bond angle is less than 109 o • TRIGONAL PYRAMIDAL shape • eg’s NF 3, PCl 3, As. H 3, NI 3, As. F 3, PF 3 © 2018 http: //www. chemical-minds. com

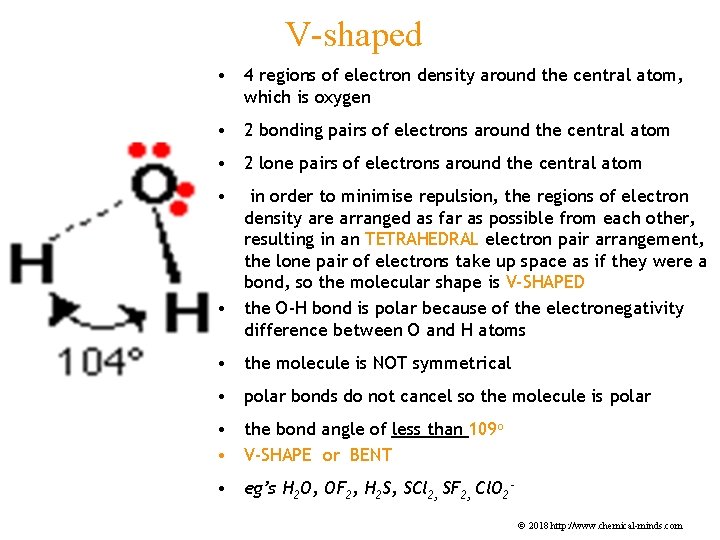
V-shaped • 4 regions of electron density around the central atom, which is oxygen • 2 bonding pairs of electrons around the central atom • 2 lone pairs of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an TETRAHEDRAL electron pair arrangement, the lone pair of electrons take up space as if they were a bond, so the molecular shape is V-SHAPED • the O-H bond is polar because of the electronegativity difference between O and H atoms • the molecule is NOT symmetrical • polar bonds do not cancel so the molecule is polar • the bond angle of less than 109 o • V-SHAPE or BENT • eg’s H 2 O, OF 2, H 2 S, SCl 2, SF 2, Cl. O 2© 2018 http: //www. chemical-minds. com

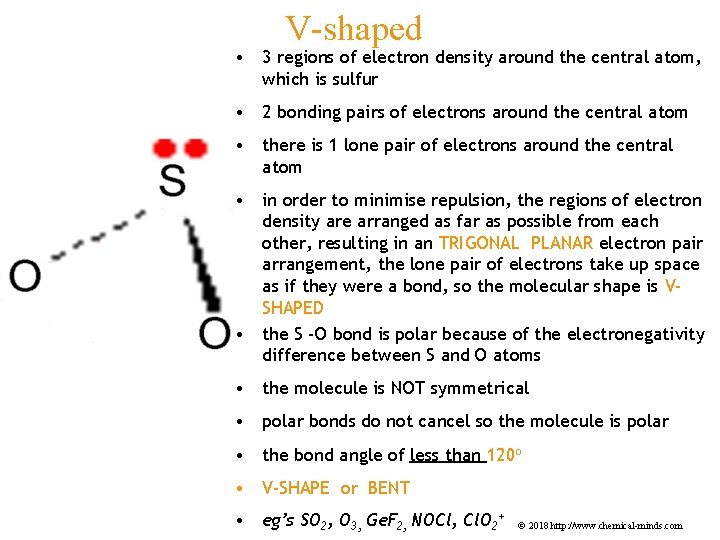
V-shaped • 3 regions of electron density around the central atom, which is sulfur • 2 bonding pairs of electrons around the central atom • there is 1 lone pair of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an TRIGONAL PLANAR electron pair arrangement, the lone pair of electrons take up space as if they were a bond, so the molecular shape is VSHAPED • the S -O bond is polar because of the electronegativity difference between S and O atoms • the molecule is NOT symmetrical • polar bonds do not cancel so the molecule is polar • the bond angle of less than 120 o • V-SHAPE or BENT • eg’s SO 2, O 3, Ge. F 2, NOCl, Cl. O 2+ © 2018 http: //www. chemical-minds. com

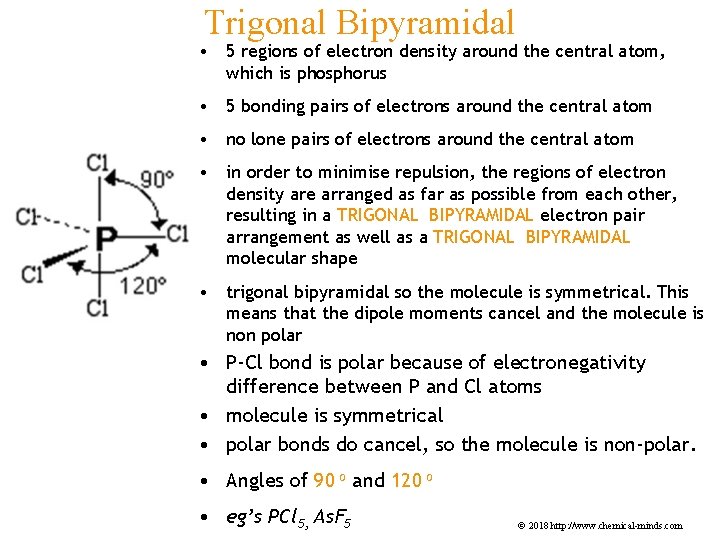
Trigonal Bipyramidal • 5 regions of electron density around the central atom, which is phosphorus • 5 bonding pairs of electrons around the central atom • no lone pairs of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in a TRIGONAL BIPYRAMIDAL electron pair arrangement as well as a TRIGONAL BIPYRAMIDAL molecular shape • trigonal bipyramidal so the molecule is symmetrical. This means that the dipole moments cancel and the molecule is non polar • P-Cl bond is polar because of electronegativity difference between P and Cl atoms • molecule is symmetrical • polar bonds do cancel, so the molecule is non-polar. • Angles of 90 o and 120 o • eg’s PCl 5, As. F 5 © 2018 http: //www. chemical-minds. com

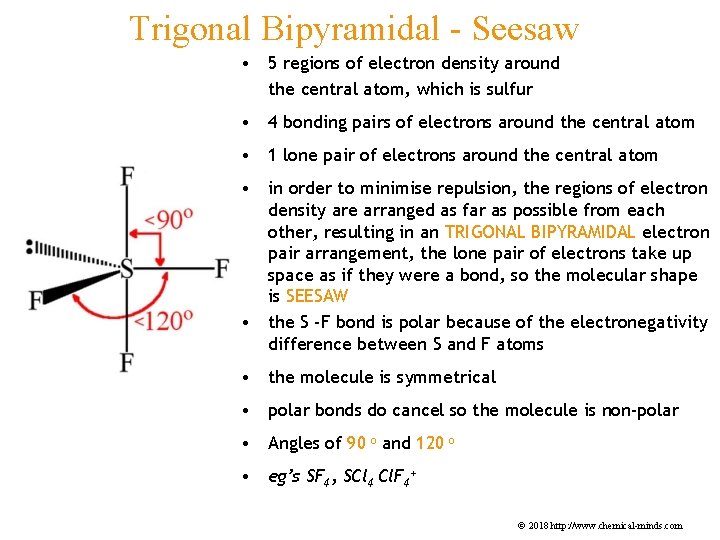
Trigonal Bipyramidal - Seesaw • 5 regions of electron density around the central atom, which is sulfur • 4 bonding pairs of electrons around the central atom • 1 lone pair of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an TRIGONAL BIPYRAMIDAL electron pair arrangement, the lone pair of electrons take up space as if they were a bond, so the molecular shape is SEESAW • the S -F bond is polar because of the electronegativity difference between S and F atoms • the molecule is symmetrical • polar bonds do cancel so the molecule is non-polar • Angles of 90 o and 120 o • eg’s SF 4, SCl 4 Cl. F 4+ © 2018 http: //www. chemical-minds. com

Trigonal Bipyramidal – T-shaped • 5 regions of electron density around the central atom, which is chlorine • 3 bonding pairs of electrons around the central atom • 2 lone pairs of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an OCTAHEDRAL electron pair arrangement, the 2 lone pairs of electrons take up space as if they were a bond, so the molecular shape is T-SHAPED • the Cl-F bond is polar because of the electronegativity difference between F and Cl atoms • the molecule is NOT symmetrical • polar bonds do not cancel so the molecule is polar • angles of 90 o • eg’s Cl. F 3, IF 3, Br. F 3 © 2018 http: //www. chemical-minds. com

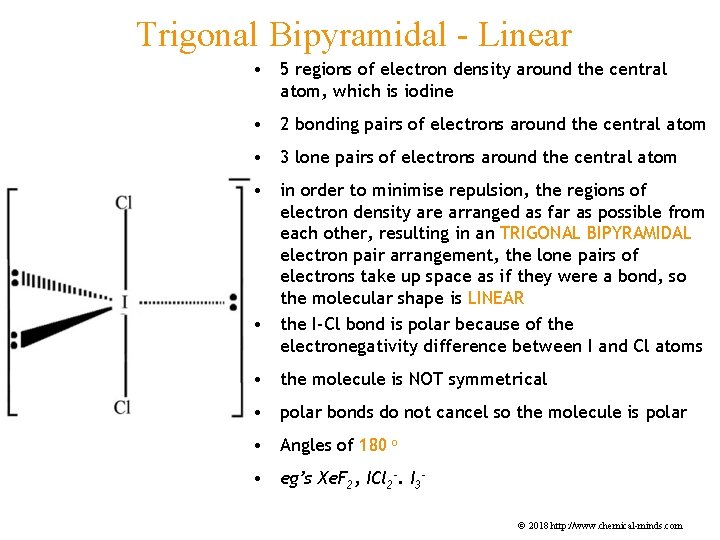
Trigonal Bipyramidal - Linear • 5 regions of electron density around the central atom, which is iodine • 2 bonding pairs of electrons around the central atom • 3 lone pairs of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an TRIGONAL BIPYRAMIDAL electron pair arrangement, the lone pairs of electrons take up space as if they were a bond, so the molecular shape is LINEAR • the I-Cl bond is polar because of the electronegativity difference between I and Cl atoms • the molecule is NOT symmetrical • polar bonds do not cancel so the molecule is polar • Angles of 180 o • eg’s Xe. F 2, ICl 2 -. I 3© 2018 http: //www. chemical-minds. com

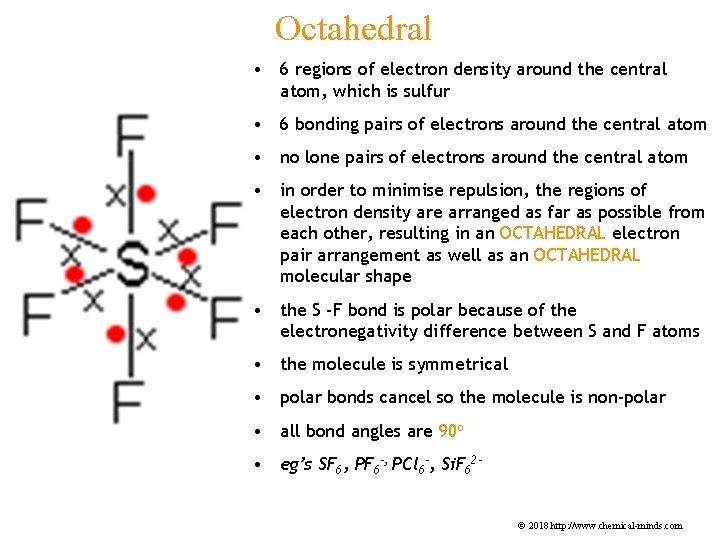
Octahedral • 6 regions of electron density around the central atom, which is sulfur • 6 bonding pairs of electrons around the central atom • no lone pairs of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an OCTAHEDRAL electron pair arrangement as well as an OCTAHEDRAL molecular shape • the S -F bond is polar because of the electronegativity difference between S and F atoms • the molecule is symmetrical • polar bonds cancel so the molecule is non-polar • all bond angles are 90 o • eg’s SF 6, PF 6 -, PCl 6 -, Si. F 62© 2018 http: //www. chemical-minds. com

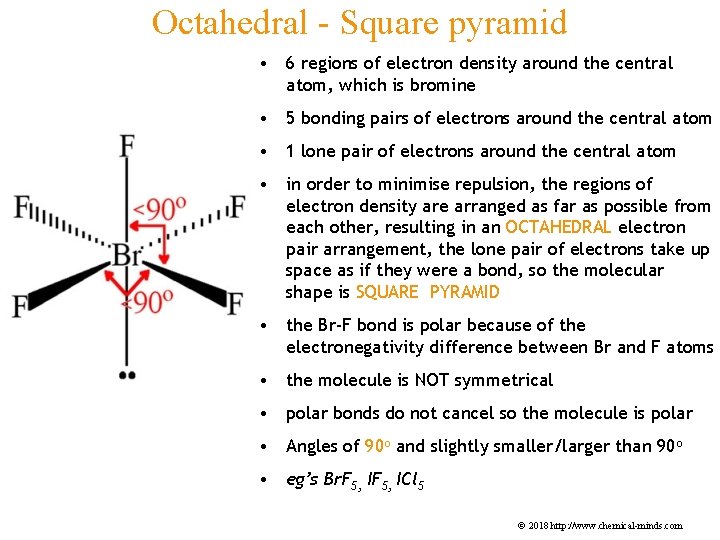
Octahedral - Square pyramid • 6 regions of electron density around the central atom, which is bromine • 5 bonding pairs of electrons around the central atom • 1 lone pair of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an OCTAHEDRAL electron pair arrangement, the lone pair of electrons take up space as if they were a bond, so the molecular shape is SQUARE PYRAMID • the Br-F bond is polar because of the electronegativity difference between Br and F atoms • the molecule is NOT symmetrical • polar bonds do not cancel so the molecule is polar • Angles of 90 o and slightly smaller/larger than 90 o • eg’s Br. F 5, ICl 5 © 2018 http: //www. chemical-minds. com

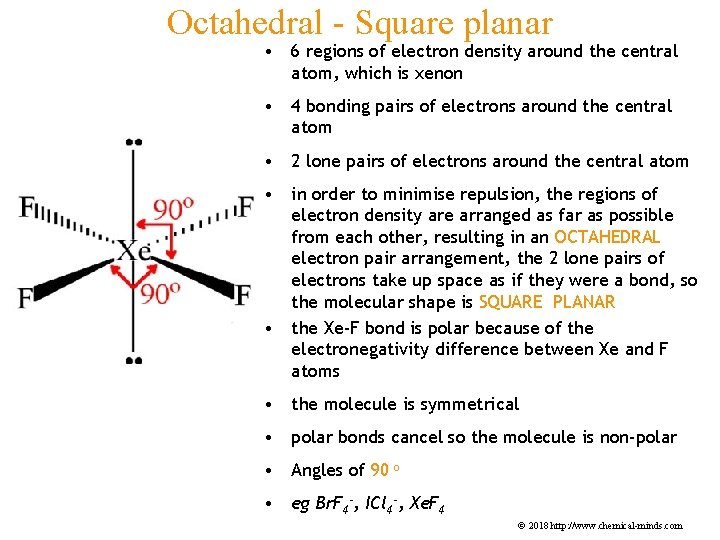
Octahedral - Square planar • 6 regions of electron density around the central atom, which is xenon • 4 bonding pairs of electrons around the central atom • 2 lone pairs of electrons around the central atom • in order to minimise repulsion, the regions of electron density are arranged as far as possible from each other, resulting in an OCTAHEDRAL electron pair arrangement, the 2 lone pairs of electrons take up space as if they were a bond, so the molecular shape is SQUARE PLANAR • the Xe-F bond is polar because of the electronegativity difference between Xe and F atoms • the molecule is symmetrical • polar bonds cancel so the molecule is non-polar • Angles of 90 o • eg Br. F 4 -, ICl 4 -, Xe. F 4 © 2018 http: //www. chemical-minds. com
 Shape of sf2cl +
Shape of sf2cl + Shapes of molecules a level
Shapes of molecules a level Shapes of molecules a level
Shapes of molecules a level Chemsheets as 1017 shapes of molecules
Chemsheets as 1017 shapes of molecules Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Iof4- shape
Iof4- shape Chemsheets shapes of molecules
Chemsheets shapes of molecules C2h4 vsper
C2h4 vsper Io2f lewis structure
Io2f lewis structure Chemsheets
Chemsheets Different molecular structures
Different molecular structures Nh3 polar or nonpolar
Nh3 polar or nonpolar These are shapes that seem to follow no rules.
These are shapes that seem to follow no rules. Importance of the level of product availability
Importance of the level of product availability Picolata crossing elementary school
Picolata crossing elementary school