Kinetic Molecular Theory Targets and Review Targets Review


























- Slides: 61

Kinetic Molecular Theory

Targets and Review Targets Review Use the Kinetic Molecular Theory to define & describe the behavior of an ideal gas. Kinetic Energy is the Energy of Motion

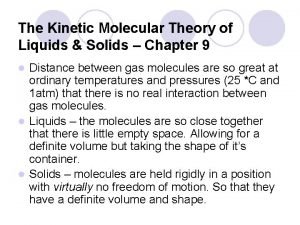
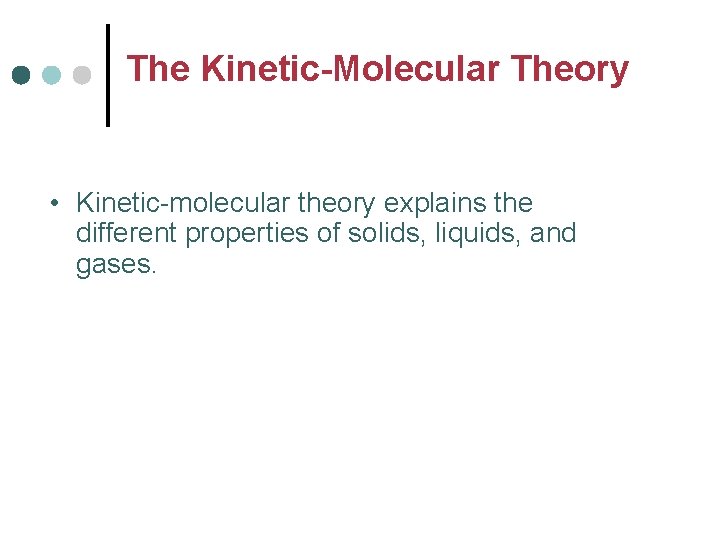
The Kinetic-Molecular Theory • Kinetic-molecular theory explains the different properties of solids, liquids, and gases.

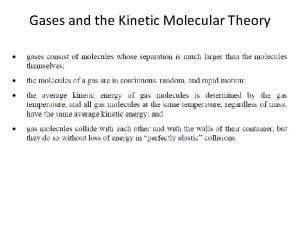
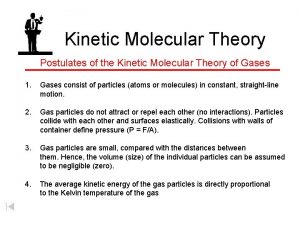
KMT 1. Particle Size l Gases consist of tiny particles (atoms or molecules) l These particles are so small, compared to the distance between them, that the volume of the individual particle is negligible (zero) l Gas particles are too far apart to experience significant attractive or repulsive forces.

KMT 2. Particle Motion l The particles are in constant, random motion and are colliding with the walls of the container. • l This creates pressure. When particles collide, the collision is said to be elastic. • An elastic collision is one in which no kinetic energy is lost.

KMT 3. Particle Energy l The average kinetic energy is directly proportional to the temperature of the gas. • Temperature is a measure of the average kinetic energy of the particles in a sample of matter

Explaining Behavior ¢ Low Density is a measure of mass for a given volume l The difference between a high density solid and a low density gas is due to the large amount of space in a gas. l

Explaining Behavior ¢ Compression and Expansion A gas will expand to fill its container l The density of a gas will change with a change in the volume of a container l The gas will become more dense as a gas is compressed and less dense as a gas expands l

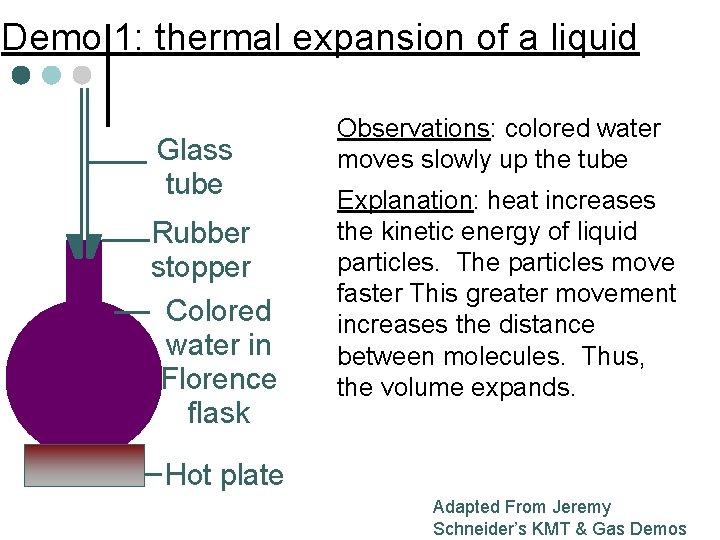
Demo 1: thermal expansion of a liquid Glass tube Rubber stopper Colored water in Florence flask Observations: colored water moves slowly up the tube Explanation: heat increases the kinetic energy of liquid particles. The particles move faster This greater movement increases the distance between molecules. Thus, the volume expands. Hot plate Adapted From Jeremy Schneider’s KMT & Gas Demos

Explaining Behavior ¢ Diffusion l l l Gas particles move past each other easily due to negligible attractive or repulsive forces When one gas flows into a space occupied by another gas, diffusion has occurred Rate of diffusion depends on the size of the particles • Lighter diffuses faster than Heavier ¢ Effusion is the escape of a gas through a small opening (flat tire)

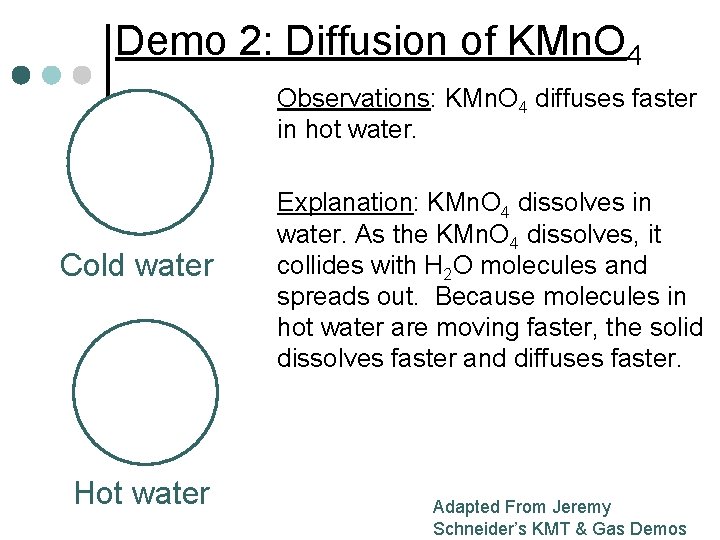
Demo 2: Diffusion of KMn. O 4 Observations: KMn. O 4 diffuses faster in hot water. Cold water Hot water Explanation: KMn. O 4 dissolves in water. As the KMn. O 4 dissolves, it collides with H 2 O molecules and spreads out. Because molecules in hot water are moving faster, the solid dissolves faster and diffuses faster. Adapted From Jeremy Schneider’s KMT & Gas Demos

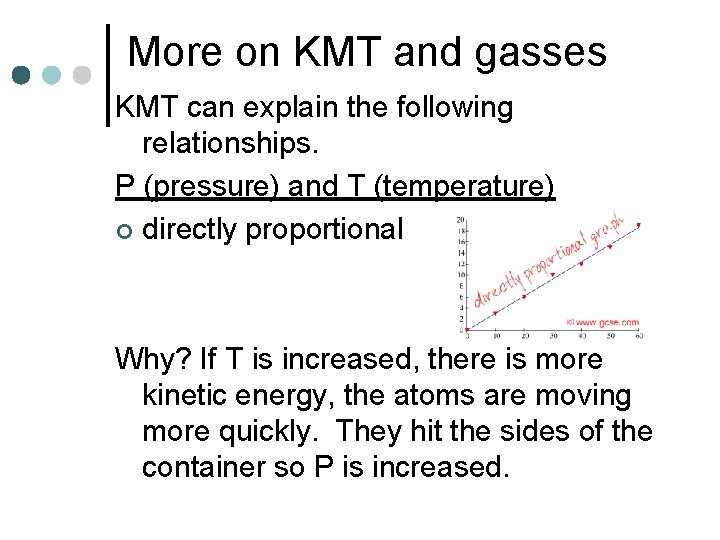
More on KMT and gasses KMT can explain the following relationships. P (pressure) and T (temperature) ¢ directly proportional Why? If T is increased, there is more kinetic energy, the atoms are moving more quickly. They hit the sides of the container so P is increased.

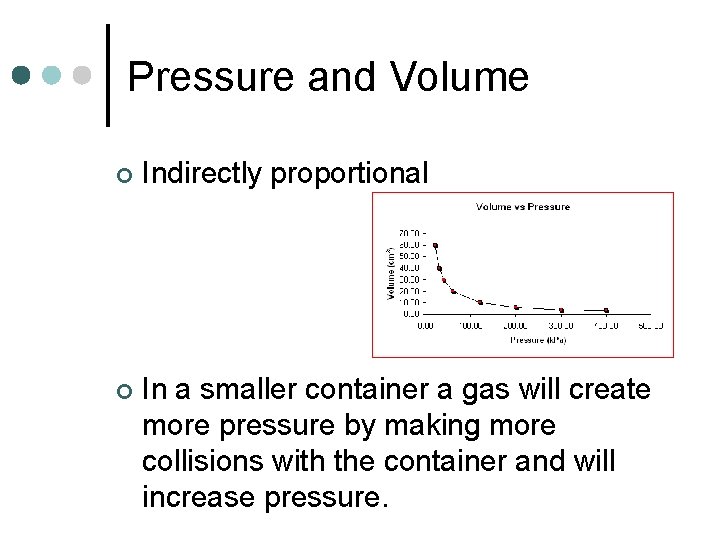
Pressure and Volume ¢ Indirectly proportional ¢ In a smaller container a gas will create more pressure by making more collisions with the container and will increase pressure.

Pressure and density Directly proportional ¢ Since D=M/V, increased densities will have small volume. Since there is less volume; there will be more pressure. ¢

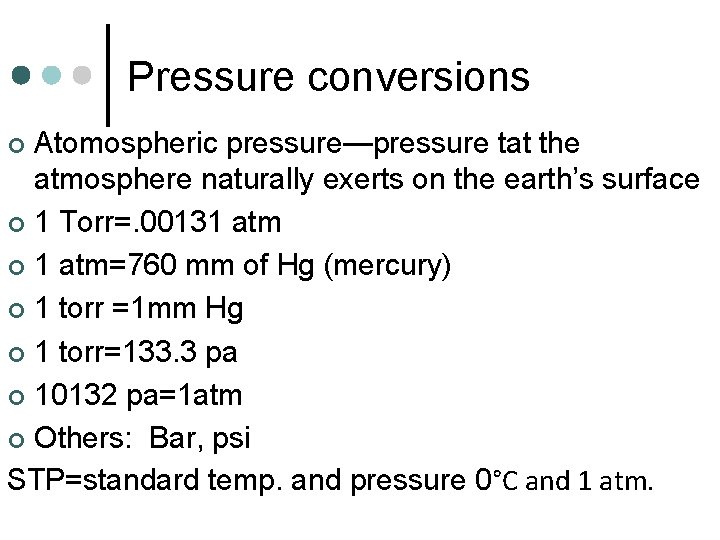
Pressure conversions Atomospheric pressure—pressure tat the atmosphere naturally exerts on the earth’s surface ¢ 1 Torr=. 00131 atm ¢ 1 atm=760 mm of Hg (mercury) ¢ 1 torr =1 mm Hg ¢ 1 torr=133. 3 pa ¢ 10132 pa=1 atm ¢ Others: Bar, psi STP=standard temp. and pressure 0°C and 1 atm. ¢


Intermolecular Forces

Targets and Review Targets Review Describe how Different intermolecular forces affect the behavior of liquids and solids. Chemical Bond Attractive forces between atoms

Forces of Attraction ¢ Why are some materials gasses, others liquids, and others solids at room temperature? ¢ Answer - Attractive forces within and between particles hold those particles together. l Some forces are stronger than others

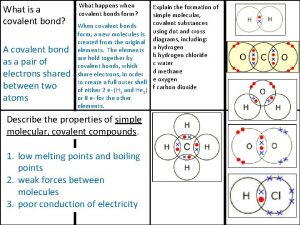
Forces of Attraction ¢ Forces of attraction between molecules are called INTERMOLECULAR FORCES l These forces can hold together identical particles or two different particles l We will discuss three different intermolecular forces

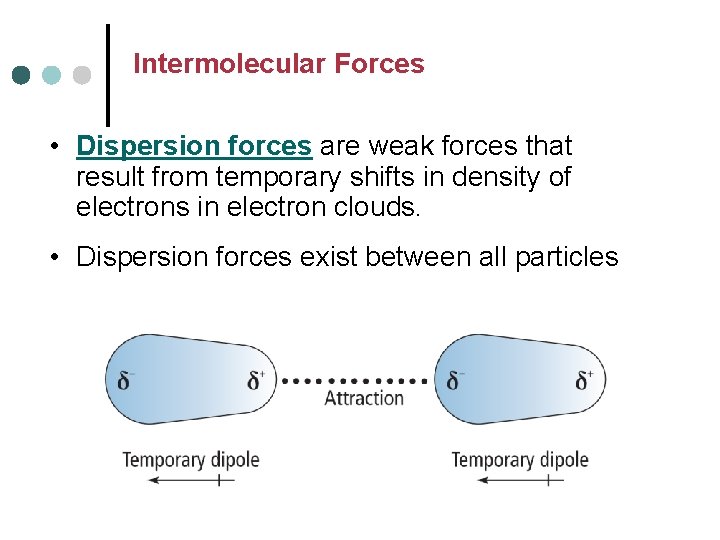
Intermolecular Forces • Dispersion forces are weak forces that result from temporary shifts in density of electrons in electron clouds. • Dispersion forces exist between all particles

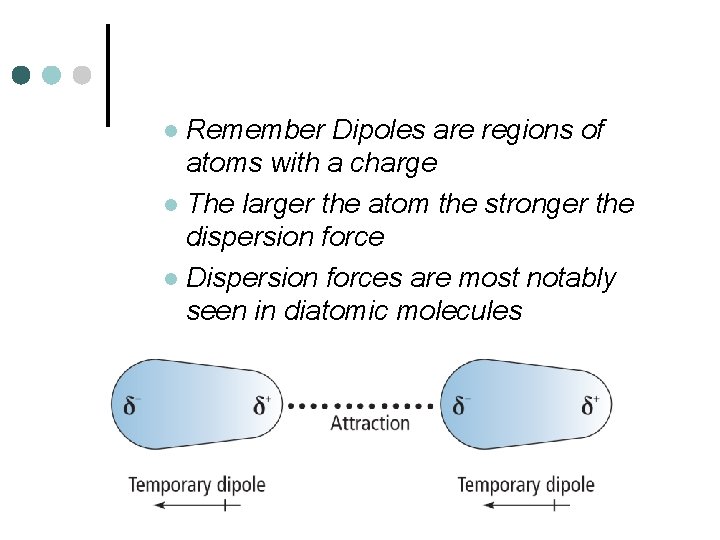
Remember Dipoles are regions of atoms with a charge l The larger the atom the stronger the dispersion force l Dispersion forces are most notably seen in diatomic molecules l

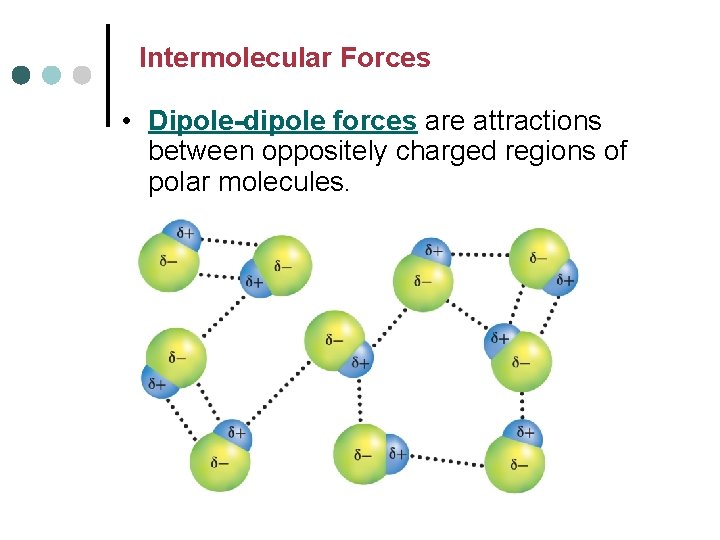
Intermolecular Forces • Dipole-dipole forces are attractions between oppositely charged regions of polar molecules.

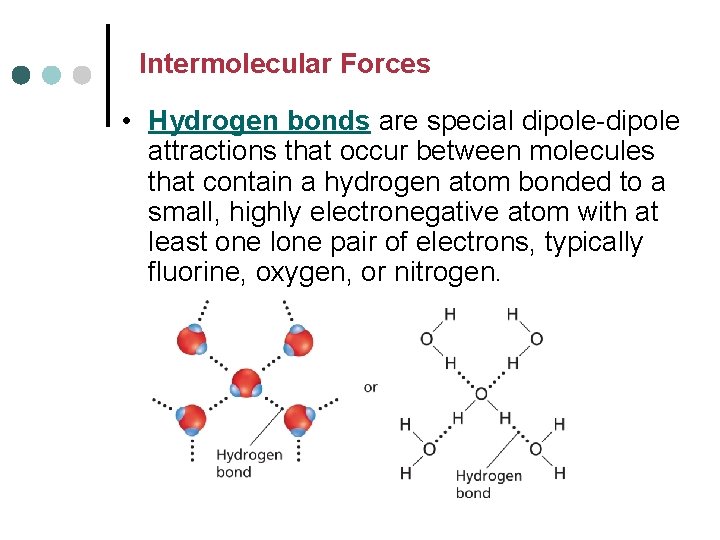
Intermolecular Forces • Hydrogen bonds are special dipole-dipole attractions that occur between molecules that contain a hydrogen atom bonded to a small, highly electronegative atom with at least one lone pair of electrons, typically fluorine, oxygen, or nitrogen.

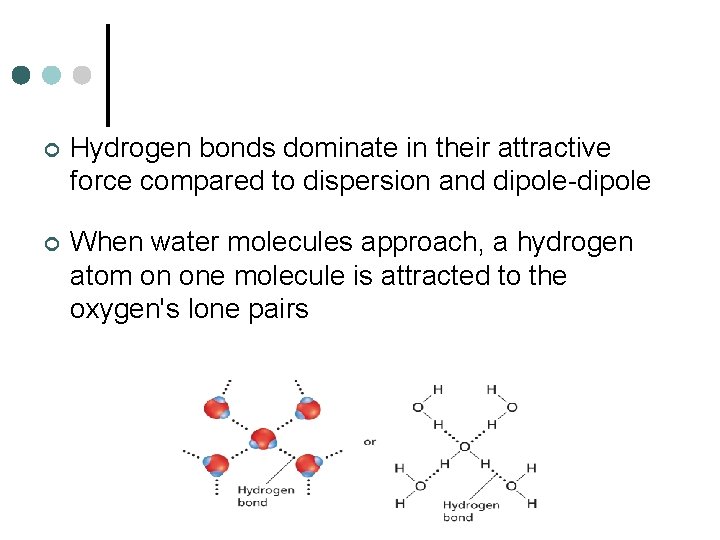
¢ Hydrogen bonds dominate in their attractive force compared to dispersion and dipole-dipole ¢ When water molecules approach, a hydrogen atom on one molecule is attracted to the oxygen's lone pairs


Liquids • Forces of attraction keep molecules closely packed in a fixed volume, but not in a fixed position. • Liquids are much denser than gases because of the stronger intermolecular forces holding the particles together. • Large amounts of pressure must be applied to compress liquids to very small amounts.

Liquids (cont. ) • Surface tension is the energy required to increase the surface area of a liquid by a given amount. • Surfactants are compounds that lower the surface tension of water.

Solids • Solids contain particles with strong attractive intermolecular forces. • Particles in a solid vibrate in a fixed position. • Most solids are more dense than liquids. • Ice is not more dense than water due to the air pockets that form from Hydrogen bonding during freezing. • Molecules are less compact = less dense

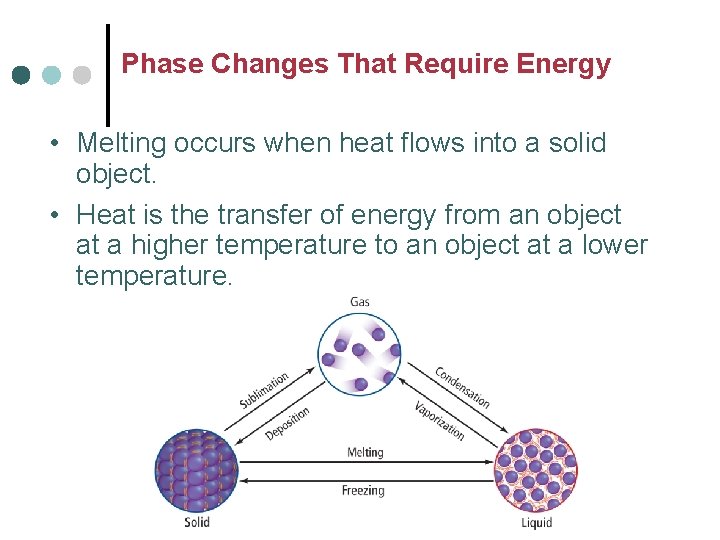
Phase Changes That Require Energy • Melting occurs when heat flows into a solid object. • Heat is the transfer of energy from an object at a higher temperature to an object at a lower temperature.

Phase Changes That Require Energy (cont. ) • When ice is heated, the ice eventually absorbs enough energy to break the hydrogen bonds that hold the water molecules together. • When the bonds break, the particles move apart and ice melts into water. • The melting point of a solid is the temperature at which the forces holding the particles together are broken and it becomes a liquid.

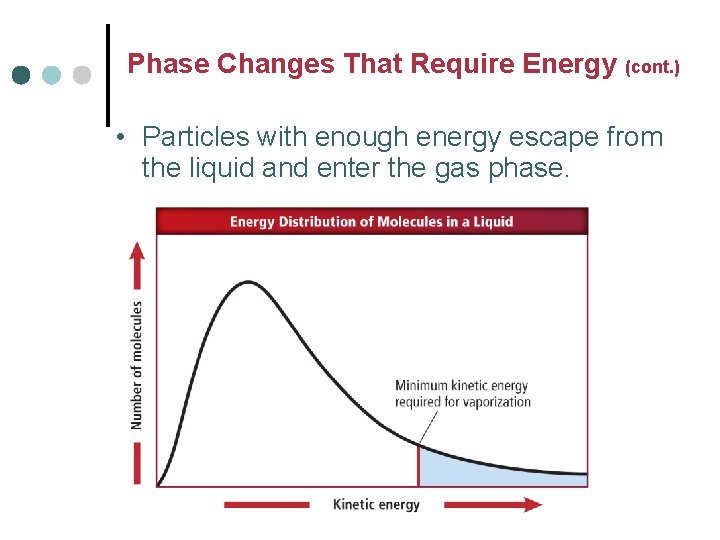
Phase Changes That Require Energy (cont. ) • Particles with enough energy escape from the liquid and enter the gas phase.

Phase Changes That Require Energy (cont. ) • Vaporization is the process by which a liquid changes to a gas or vapor. • Evaporation is vaporization only at the surface of a liquid.

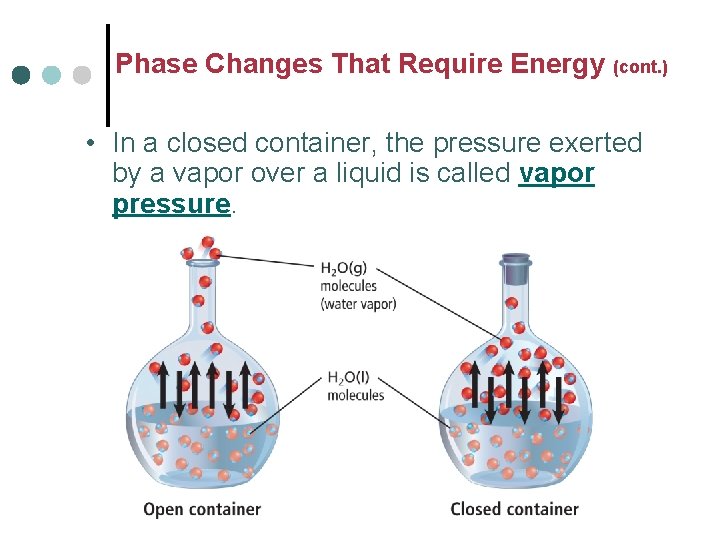
Phase Changes That Require Energy (cont. ) • In a closed container, the pressure exerted by a vapor over a liquid is called vapor pressure.

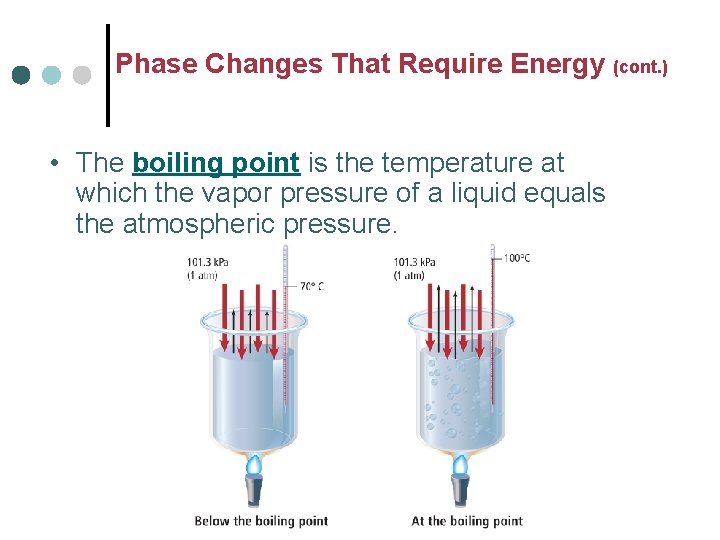
Phase Changes That Require Energy (cont. ) • The boiling point is the temperature at which the vapor pressure of a liquid equals the atmospheric pressure.

Phase Changes That Require Energy (cont. ) • Sublimation is the process by which a solid changes into a gas without becoming a liquid.

Phase Changes That Release Energy • As heat flows from water to the surroundings, the particles lose energy. • The freezing point is the temperature at which a liquid is converted into a solid.

Phase Changes That Release Energy (cont. ) • As energy flows from water vapor, the velocity of the particles decrease. • The process by which a gas or vapor becomes a liquid is called condensation. • Deposition is the process by which a gas or vapor changes directly to a solid, and is the reverse of sublimation.


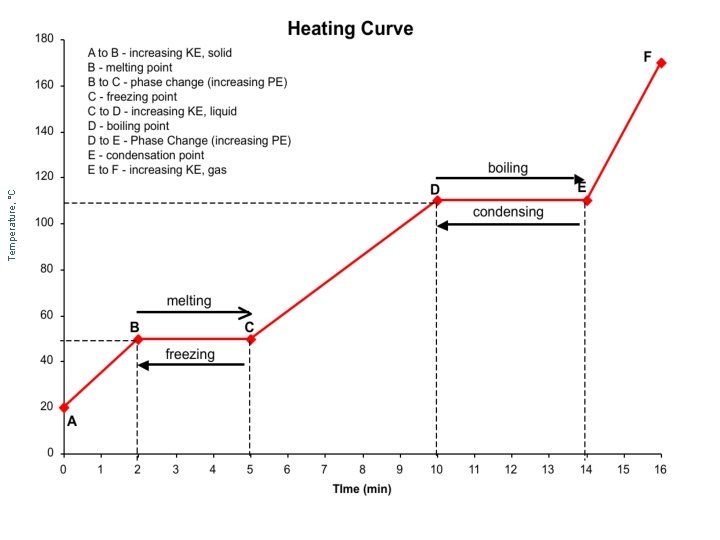
¢ Graphical representation of phase changes ¢ Heating curves start in the solid phase ¢ Cooling curves start in the gaseous phase

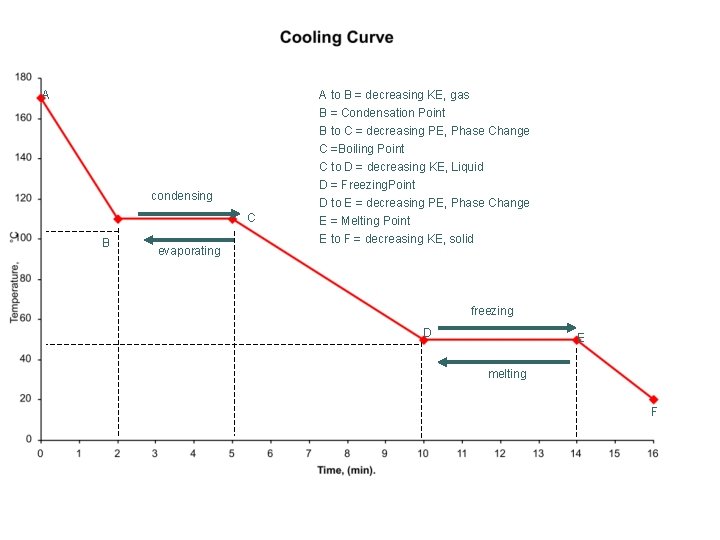
A A to B = decreasing KE, gas B = Condensation Point B to C = decreasing PE, Phase Change condensing C B evaporating C =Boiling Point C to D = decreasing KE, Liquid D = Freezing. Point D to E = decreasing PE, Phase Change E = Melting Point E to F = decreasing KE, solid freezing D E melting F

Temperature, °C

Example Heating and Cooling Curves ¢ Melting and Boiling ¢

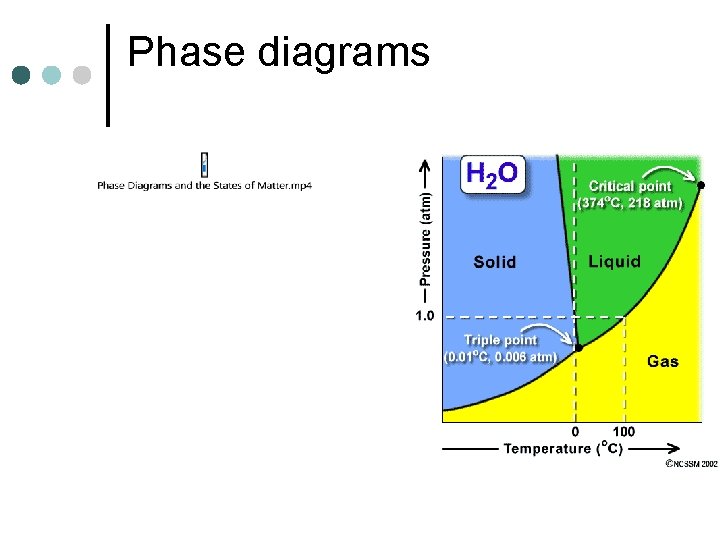
Phase diagrams



Uses of critical point ¢ Drying biological samples ¢ Dry cleaning

super

Energy and Matter Sections 15. 1 and 15. 2

Energy The capacity to do work or produce heat ¢ Kinetic Energy (KE) ¢ Energy of motion l Anything in motion has KE l ¢ Potential Energy (PE) l Stored energy due to position or chemical nature

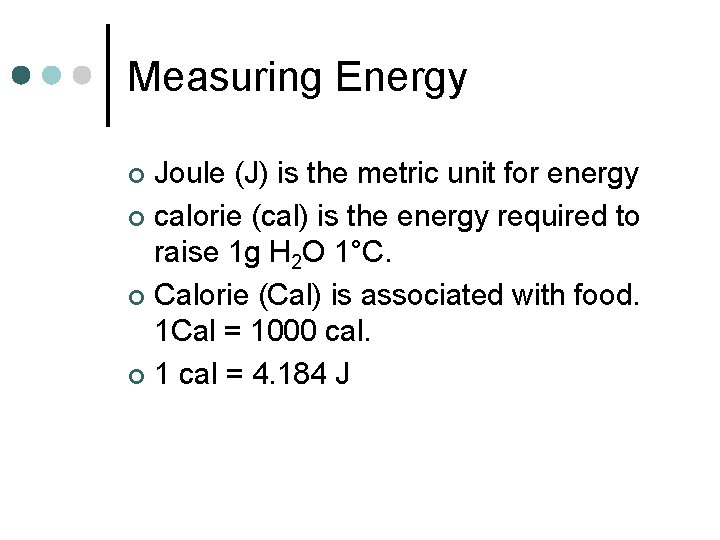
Measuring Energy Joule (J) is the metric unit for energy ¢ calorie (cal) is the energy required to raise 1 g H 2 O 1°C. ¢ Calorie (Cal) is associated with food. 1 Cal = 1000 cal. ¢ 1 cal = 4. 184 J ¢

Temperature (Remember? !) ¢ Heat is a form of KE ¢ Temperature is the average measure of KE

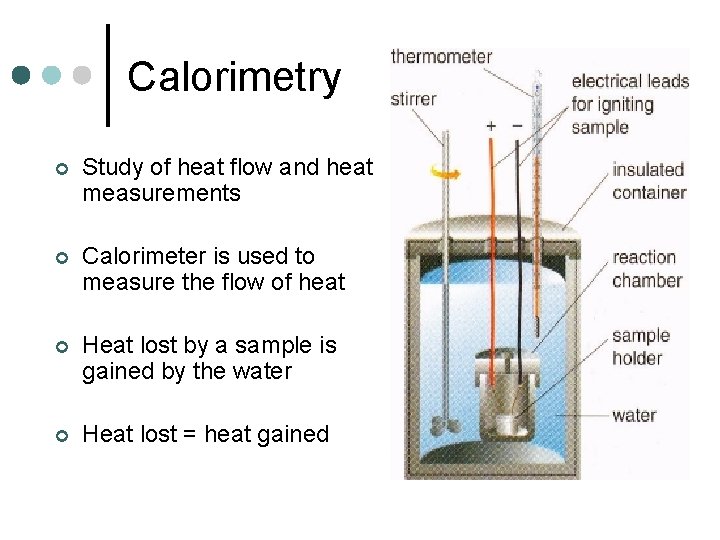
Calorimetry ¢ Study of heat flow and heat measurements ¢ Calorimeter is used to measure the flow of heat ¢ Heat lost by a sample is gained by the water ¢ Heat lost = heat gained

Heat Capacity The ability of a substance or object to absorb heat (KE) ¢ The amount of energy needed to raise the temperature of an object by 1°C ¢

Specific Heat capacity of 1 g of a substance ¢ The amount of energy needed to raise the temperature of 1 g of a substance by 1°C. ¢ Units = J / g°C or cal / g°C ¢

Specific Heat Values Substance H 2 O (liquid) Specific Heat, J/g°C 4. 184 H 2 O (solid) 2. 03 Al (solid) 0. 89 C (solid) 0. 71 Fe (solid) 0. 45 Hg (liquid) 0. 14

Specific Heat Calculations ¢ q = m c ∆T ¢ q = amt. of energy or heat needed or released (cal or J) ¢ m = mass (g) ¢ c = specific heat of the substance (J/g°C or cal/g°C) ¢ ∆T = Temperature change (|Tf – Ti|) l Tf is the final temp. l Ti is the initial temp.

Example: How much energy, (J) is needed to heat 50. 0 g of H 2 O from 0. 0°C to 100. 0°C if the specific heat of H 2 O is 4. 184 J/g°C? Convert your answer to k. J. (1000. J = 1. 000 k. J)

Example: What mass, (g), of H 2 O can be warmed from 10. 5°C to 75. 0°C by absorbing 4. 56 k. J? (Specific heat for water is 4. 184 J/g°) (1000 J = 1. 000 k. J)
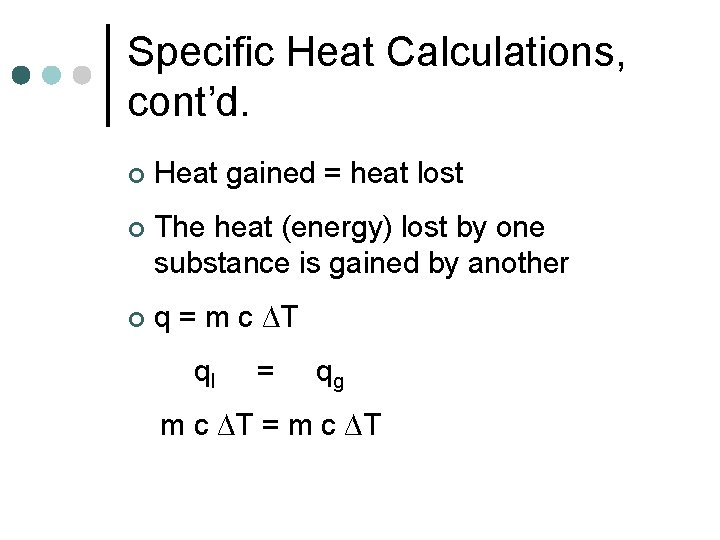
Specific Heat Calculations, cont’d. ¢ Heat gained = heat lost ¢ The heat (energy) lost by one substance is gained by another ¢ q = m c ∆T ql = qg m c ∆T = m c ∆T

Example: A 565 g cube of iron is cooled from 100. 0°C to 25. 0°C by dropping it in water at 10. 0°C. What mass (g) of water is needed. cwater = 4. 184 J/g°C and ciron = 0. 45 J/g°C.
 Kinetic molecular theory of solids
Kinetic molecular theory of solids Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic theory for ideal gases
Kinetic theory for ideal gases Kinetic molecular theory volume
Kinetic molecular theory volume Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic theory def
Kinetic theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulate of kinetic theory of gases
Postulate of kinetic theory of gases Avogadro's law
Avogadro's law Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Equation of state of real gas
Equation of state of real gas Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Valence bond vs molecular orbital theory
Valence bond vs molecular orbital theory Dxz and dxz overlap
Dxz and dxz overlap Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Theories of covalent bonding
Theories of covalent bonding Valence bond theory hybridization
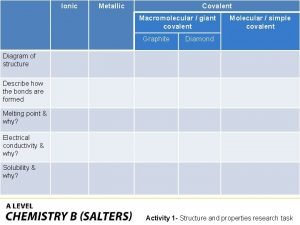
Valence bond theory hybridization Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Molecular theory of gases and liquids
Molecular theory of gases and liquids Objectives of warehouse management
Objectives of warehouse management Identifying market segments and targets chapter 9
Identifying market segments and targets chapter 9 Identifying market segments and targets chapter 9
Identifying market segments and targets chapter 9 Identifying market segments and targets chapter 9
Identifying market segments and targets chapter 9 Five patterns of target market selection
Five patterns of target market selection Segment by segment invasion
Segment by segment invasion Parathyroid gland chief cell
Parathyroid gland chief cell Smarterbalanced.alohahsap.org
Smarterbalanced.alohahsap.org Identifying market segments and targets
Identifying market segments and targets Kinetic attraction review
Kinetic attraction review The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter Buoyancyability
Buoyancyability Kinetic particle model
Kinetic particle model The attraction between particles gives solids a definite
The attraction between particles gives solids a definite Kinetic theory of gases
Kinetic theory of gases Kinetic theory
Kinetic theory What is a kinetic theory of matter
What is a kinetic theory of matter Postulates of kinetic theory of gases
Postulates of kinetic theory of gases General gas equation is
General gas equation is Molecular clock theory
Molecular clock theory Co2 molecular orbital diagram
Co2 molecular orbital diagram Neutral theory of molecular evolution notes
Neutral theory of molecular evolution notes Atomic molecular theory
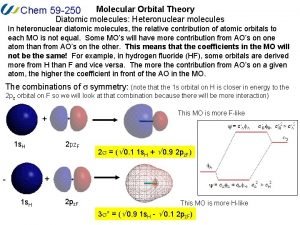
Atomic molecular theory Heteronuclear molecular orbital diagram
Heteronuclear molecular orbital diagram How to memorize vsepr theory
How to memorize vsepr theory Molecular orbital theory
Molecular orbital theory Frontier molecular orbital theory
Frontier molecular orbital theory Writing learning targets
Writing learning targets Jim crow laws in what region or regions did it exist
Jim crow laws in what region or regions did it exist Brides magazine targets consumers who are in
Brides magazine targets consumers who are in Targets of change
Targets of change What does smart stand for in pe
What does smart stand for in pe Learning targets
Learning targets Final sketch
Final sketch Product learning target example
Product learning target example Learning targets knowledge, reasoning, skill product
Learning targets knowledge, reasoning, skill product