Kinetic Molecular Theory and States of Matter Kinetic




- Slides: 20

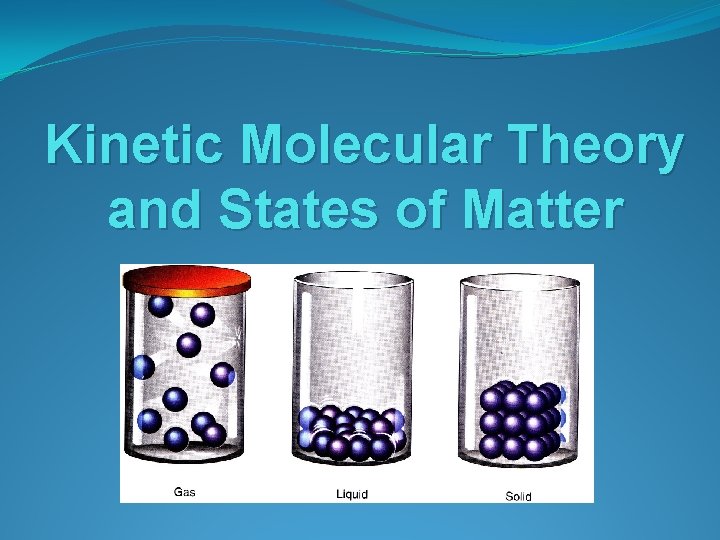
Kinetic Molecular Theory and States of Matter

Kinetic Theory of Matter Particles (molecules) are in constant motion The amount of kinetic energy determines the phase of the sample Temperature is a measure of average kinetic energy High kinetic energy = faster movement and bigger movement of particles Large distances between gas particles makes attractions between particles unimportant Strong attractions in solid and liquid phases make samples dense, not compressible

The Kinetic Theory 1. All matter is composed of small particles (atoms, molecules, or ions). 2. They are in constant, random motion. 3. They constantly collide with each other and with the walls of their container.

Thermal n E x p a n s i o Particles in any state expand when heated Expansion joints (solid) Mercury is a liquid that expands in thermometers Air expands when heated (becoming less dense)

STATES OF MATTER ØDefined by particle arrangement ØDefined by kinetic energy of particles ØDefined by distance between particles

Solids Fixed Volume Fixed Shape Not Compressible Very Dense Particles are tightly packed in an orderly arrangement, little space between particles • Very strong forces of attraction between particles • Particles vibrate about a fixed position • Lowest energy particles • • •

Liquids Fixed Volume Take the shape of the container Not Compressible Dense Particles are closely packed in a disorderly arrangement, spacing is far enough to slide past • Strong forces of attraction between particles • Particles can move past one another; Liquids flow • Middle energy particles • • •

Gases Not Fixed Volume Take the shape of the container Easily Compressible Not Dense Particles are far apart in random arrangement Forces of attraction between particles are negligible • Particles move about randomly at high speed • Highest energy particles • • •

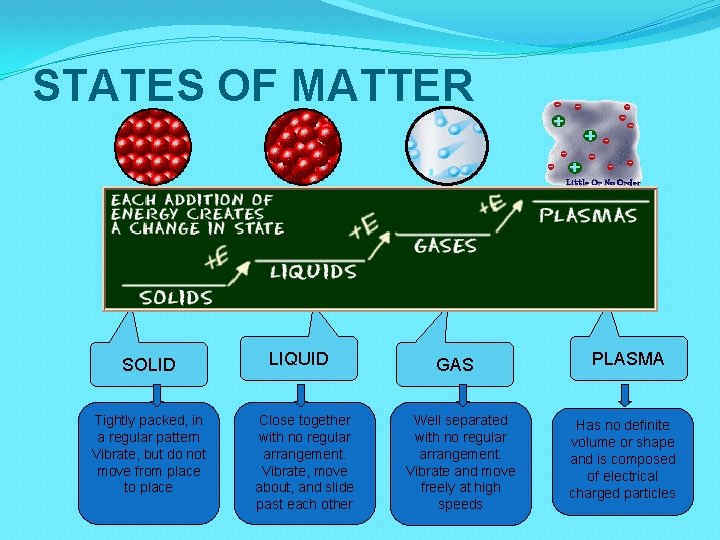
STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

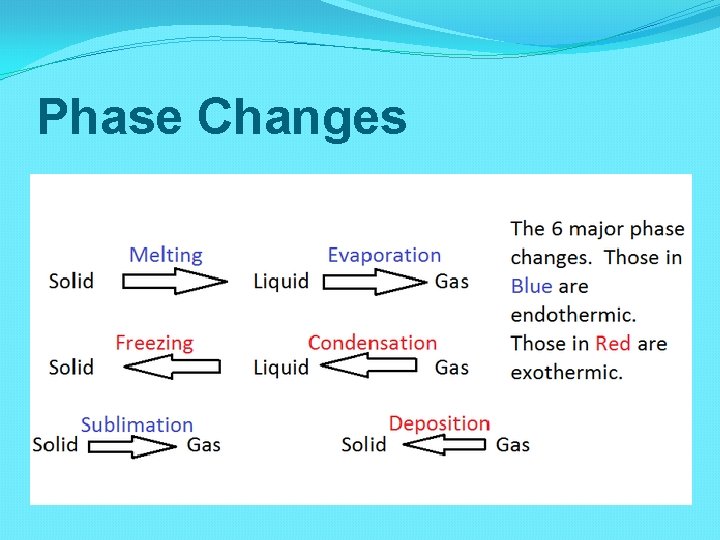
Phase Changes

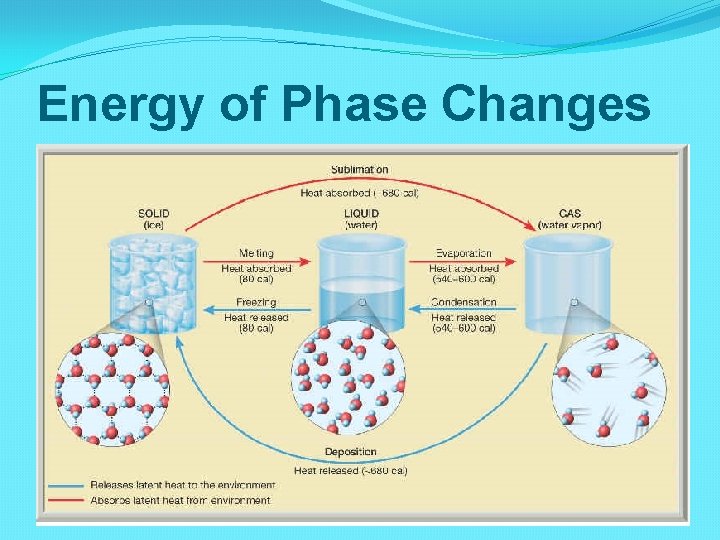
Energy of Phase Changes

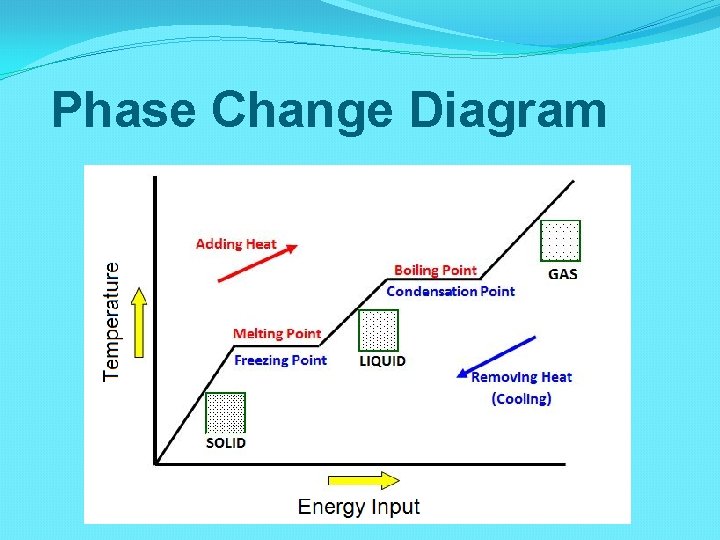
Phase Change Diagram

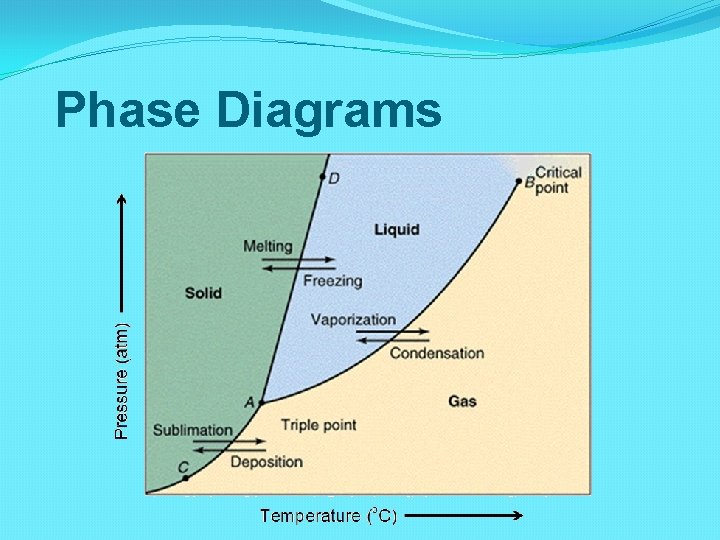
Phase Diagrams

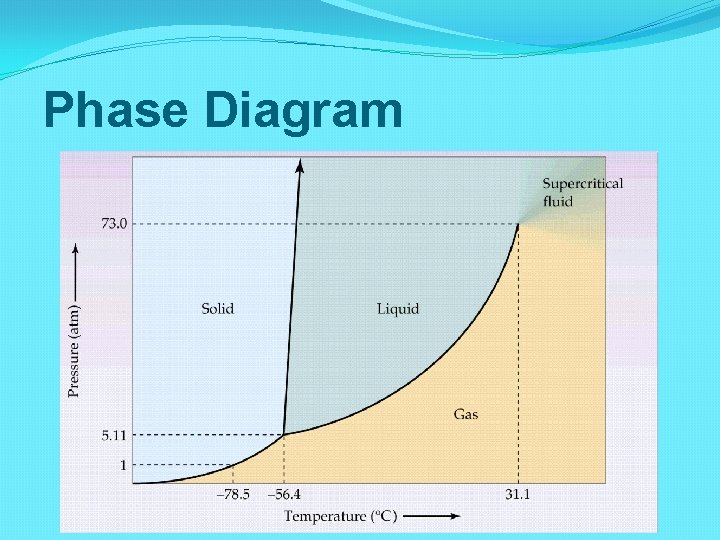
Phase Diagram

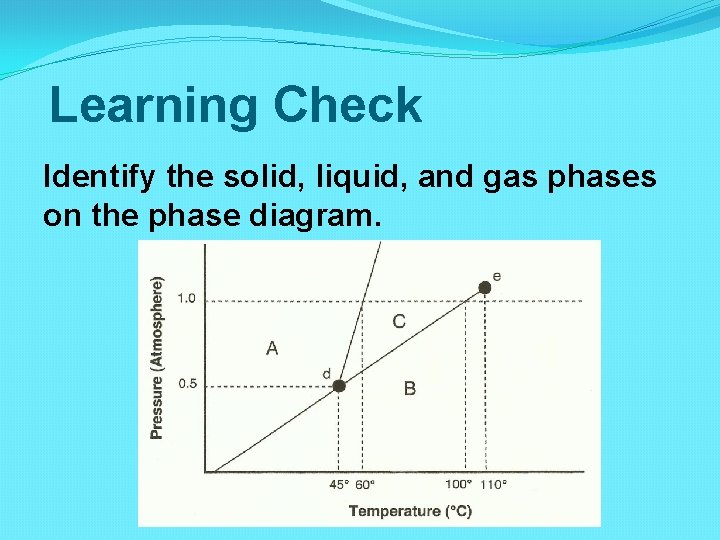
Learning Check Identify the solid, liquid, and gas phases on the phase diagram.

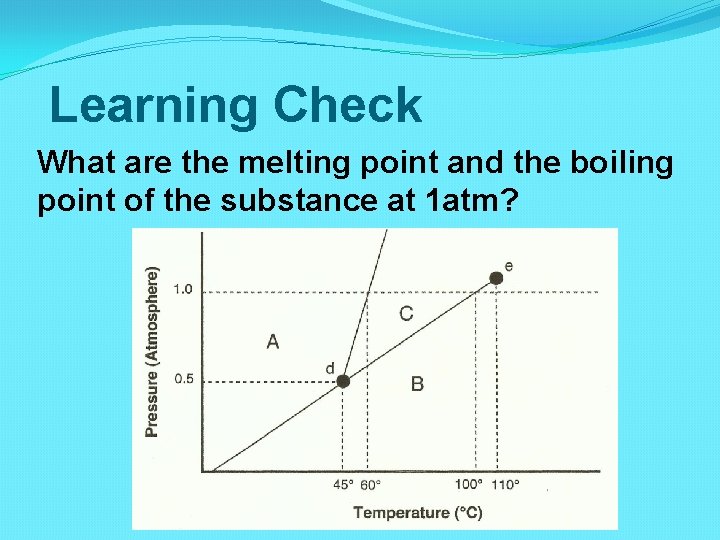
Learning Check What are the melting point and the boiling point of the substance at 1 atm?

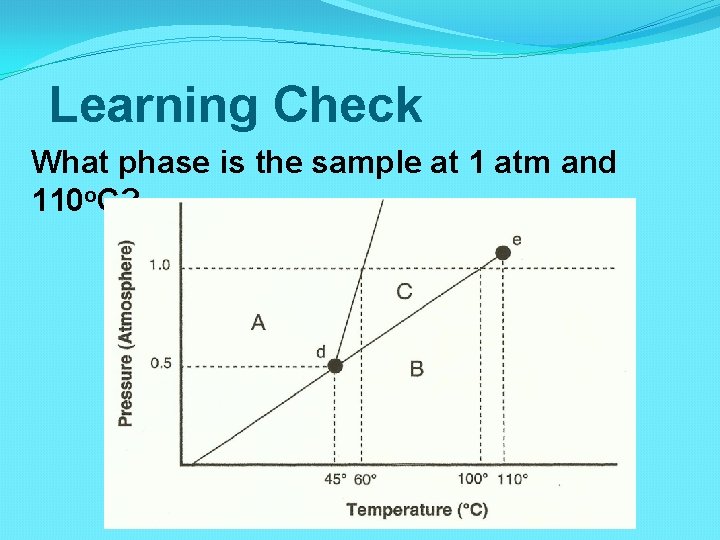
Learning Check What phase is the sample at 1 atm and 110 o. C?

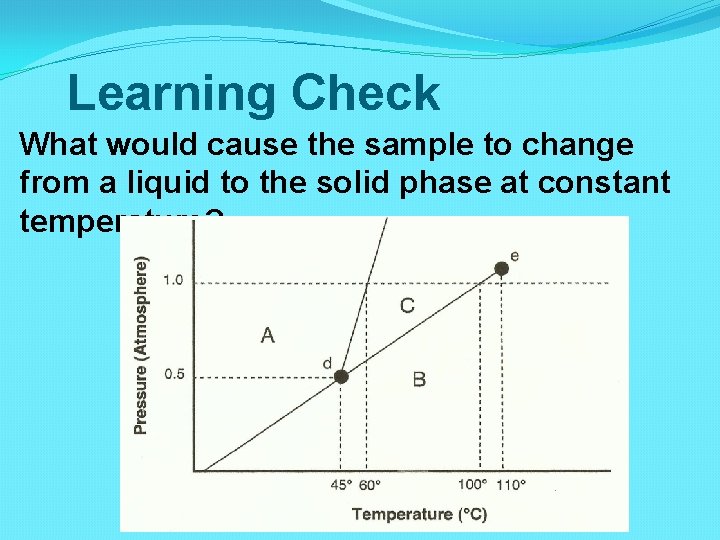
Learning Check What would cause the sample to change from a liquid to the solid phase at constant temperature?

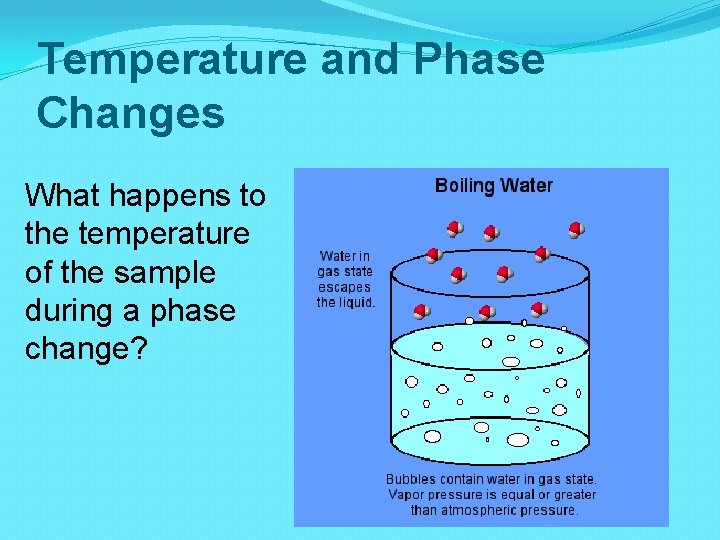
Temperature and Phase Changes What happens to the temperature of the sample during a phase change?

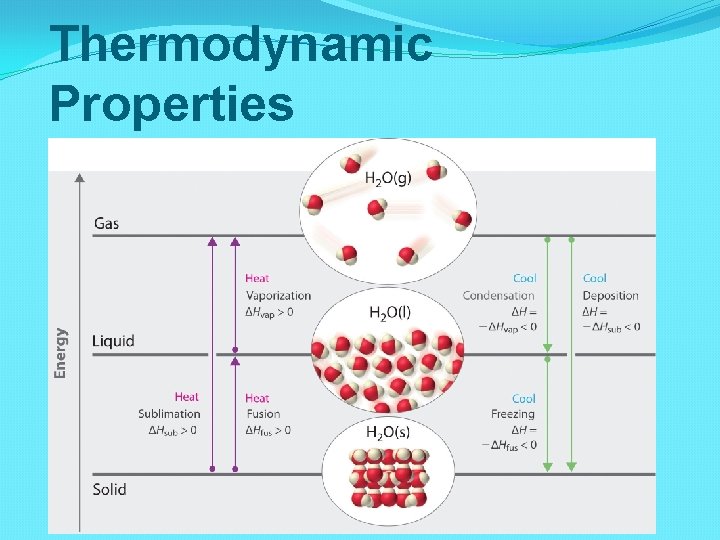
Thermodynamic Properties
 The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Adhesive force
Adhesive force Kinetic molecular theory
Kinetic molecular theory The kinetic molecular theory
The kinetic molecular theory Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic molecular theory def
Kinetic molecular theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kinetic theory of gases postulates
Kinetic theory of gases postulates Ideal gas law examples
Ideal gas law examples Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter Buoyancyability
Buoyancyability Particle theory melting
Particle theory melting What is a kinetic theory of matter
What is a kinetic theory of matter