States of Matter Kinetic Molecular Theory Kinetic Energy











- Slides: 15

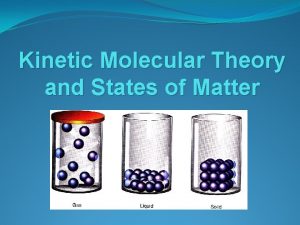
States of Matter

Kinetic Molecular Theory: § Kinetic Energy – The energy an object has because of its motion. Think about a bowing ball rolling down the lane, it has energy that it transfers to the pins because its moving. § Kinetic Molecular Theory- The tiny particles in all forms of matter are in constant motion. Even in solid objects, the atoms and molecules are moving.

Certain assumptions are made about the kinetic molecular theory: § A gas is composed of particles, usually molecules or atoms. a. the tiny particles are considered to be indivisible and have insignificant volume. The volume occupied by the gas is mainly the empty space between the particles. b. there are no attractive or repulsive forces between the particles. § The particles in a gas move rapidly and are in constant motion. a. The molecules move in strait paths, independent of each other, allowing them to mix by diffusion. b. This allows the molecules to fill any container in which they are placed. § All collisions are perfectly elastic.

Gas Pressure § Gas pressure – The force exerted by a gas per unit surface area of an object. § Vacuum – The absence of any pressure due to the lack of gas particles. § Atmospheric pressure – Pressure that results from the collisions of air molecules with objects. Atmospheric pressure is greatest at low elevations. Atmospheric pressure is least at high elevations. § Barometers – instruments used to measure atmospheric pressure.

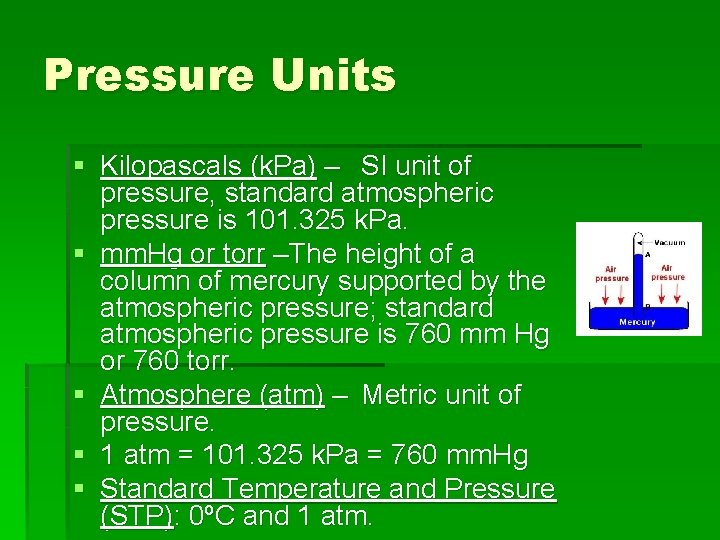
Pressure Units § Kilopascals (k. Pa) – SI unit of pressure, standard atmospheric pressure is 101. 325 k. Pa. § mm. Hg or torr –The height of a column of mercury supported by the atmospheric pressure; standard atmospheric pressure is 760 mm Hg or 760 torr. § Atmosphere (atm) – Metric unit of pressure. § 1 atm = 101. 325 k. Pa = 760 mm. Hg § Standard Temperature and Pressure (STP): 0ºC and 1 atm.

§ EXAMPLE 1: Convert 3. 25 atm to k. Pa and mm Hg. 3. 25 atm x 101. 325 k. Pa = 1 atm 3. 25 atm x 760 mm. Hg = 1 atm

§ EXAMPLE 1: Convert 3. 25 atm to k. Pa and mm Hg. 3. 25 atm x 101. 325 k. Pa = 329. 31 k. Pa 1 atm 3. 25 atm x 760 mm. Hg = 2470 mm. Hg 1 atm

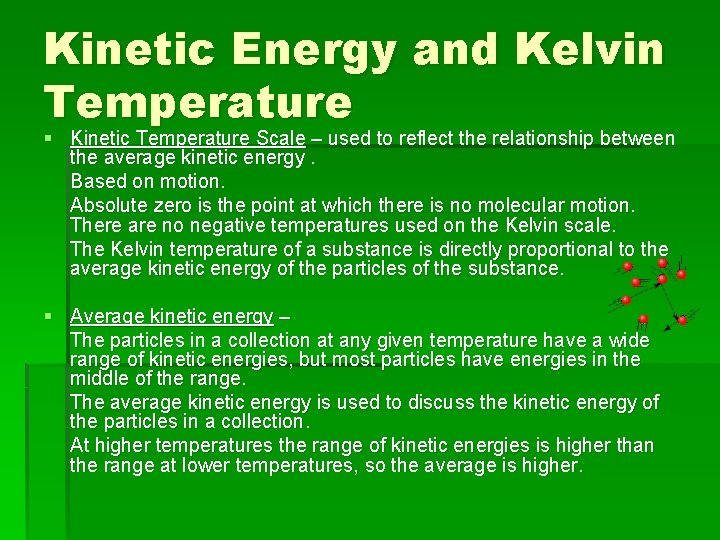
Kinetic Energy and Kelvin Temperature § Kinetic Temperature Scale – used to reflect the relationship between the average kinetic energy. Based on motion. Absolute zero is the point at which there is no molecular motion. There are no negative temperatures used on the Kelvin scale. The Kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance. § Average kinetic energy – The particles in a collection at any given temperature have a wide range of kinetic energies, but most particles have energies in the middle of the range. The average kinetic energy is used to discuss the kinetic energy of the particles in a collection. At higher temperatures the range of kinetic energies is higher than the range at lower temperatures, so the average is higher.

Condensed States of Matter – Solids and Liquids § The attractive forces (intermolecular forces) that occur between the particles of a solid or a liquid decrease the space between the particles. § An increase in pressure has very little effect on the volume of a solid or liquid

Liquids § Liquids – Particles are free to flow and take the shape of their container § Vaporization – The conversion of a liquid to a gas or vapor § Condensation – The reverse of vaporization

Liquids Cont… Evaporation –The conversion of a liquid to a gas or vapor at the surface of the liquid when the liquid is not boiling. Evaporation takes place more quickly at higher temperatures due to the increased kinetic energy of the particles which allows the particles to overcome the intermolecular forces and escape as gas or vapor. Since the higher energy particles are leaving and the lower energy particles remain behind the temperature of the liquid drops.

Liquids Cont… § Vapor Pressure – When gas molecules escape from a liquid in a sealed container, they collide with the walls of the container and produce a pressure called the vapor pressure. In time, the gas molecules will build up enough pressure that they will start condensing back into a liquid. Eventually a dynamic equilibrium will be formed so that the same numbers of molecules are vaporizing and condensing at the same time and the vapor pressure remains constant. § Boiling Point – The temperature at which the vapor pressure of the liquid is just equal to the external pressure. § Normal Boiling Point – The boiling point of a substance at standard pressure.

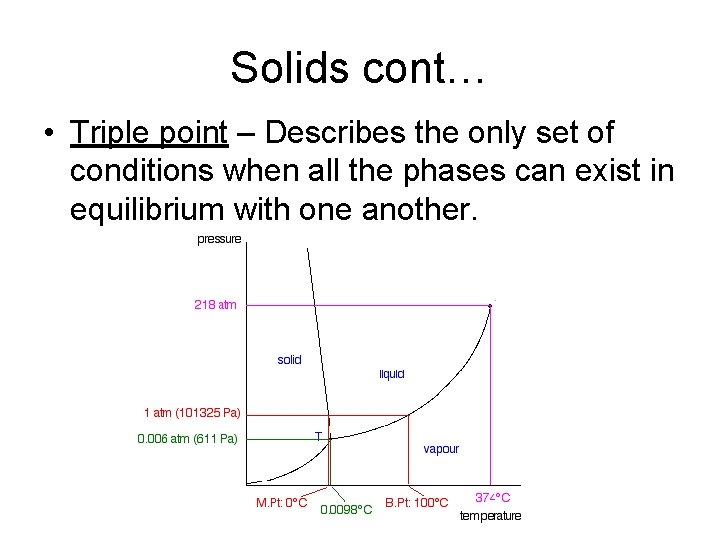
Solids § Melting Points –The temperature at which the vibrations of the solid molecules become strong enough to break the attractions holding them rigidly in place and allow a liquid to form. § Freezing – The reverse of melting. § Phase diagrams –Gives the conditions of temperature and pressure at which a substance exists as solid, liquid, & gas (example on next slide).

Solids cont… • Triple point – Describes the only set of conditions when all the phases can exist in equilibrium with one another.

Solids cont… § Sublimation – The change of a substance from a solid to a vapor without passing through a liquid phase. § Deposition – The change of a substance from a vapor to a solid without passing through a liquid phase.
 The kinetic theory of matter states that
The kinetic theory of matter states that Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic molecular theory of solid
Kinetic molecular theory of solid Kinetic molecular theory of gases
Kinetic molecular theory of gases Kinetic molecular theory volume
Kinetic molecular theory volume Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory def
Kinetic molecular theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kinetic postulates of gases
Kinetic postulates of gases Kenitic molecular theory
Kenitic molecular theory Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory