Daily Science 1 List the diatomic elements 2




- Slides: 11

Daily Science 1. List the diatomic elements 2. Write the formula for the following ionic bonds: 1. Magnesium and chlorine 2. Calcium and phosphate

Chemical Reactions PG. 61

Evidence of chemical reactions �Atoms rearrange to form different substances �Just interactions of valence electrons. The nucleus does not change �Temperature change, energy in the form of heat or light, a color change, odor, gas bubbles, appearance of a solid (precipitate)

Representing chemical reactions �Use an arrow to show direction (its like an equal sign) �More than one reactant/product separate with + �Reactants Products yields

Symbols �After each reactant or product, the state of matter is listed in parentheses � If the product or reactant is dissolved in water, we say it is aqueous �(s) �(l) �(g) �(aq)

Word and skeleton equations �Checklist for writing Are there any diatomic elements BY THEMSELVES? Br. INCl. HOF (put a subscripted 2) Do you have ionic compounds? Check charges Do you have the state of matter after each substance?

Practice problems �Hydrogen gas and solid calcium phosphate produce liquid hydrogen phosphate and solid calcium �Carbon monoxide (g) reacts with oxygen (g) to form carbon dioxide (g)


Daily Science �Write a skeleton equation from the following word equation: Liquid mercury reacts with liquid hydrogen bromide to produce liquid mercury (II) bromide and hydrogen gas

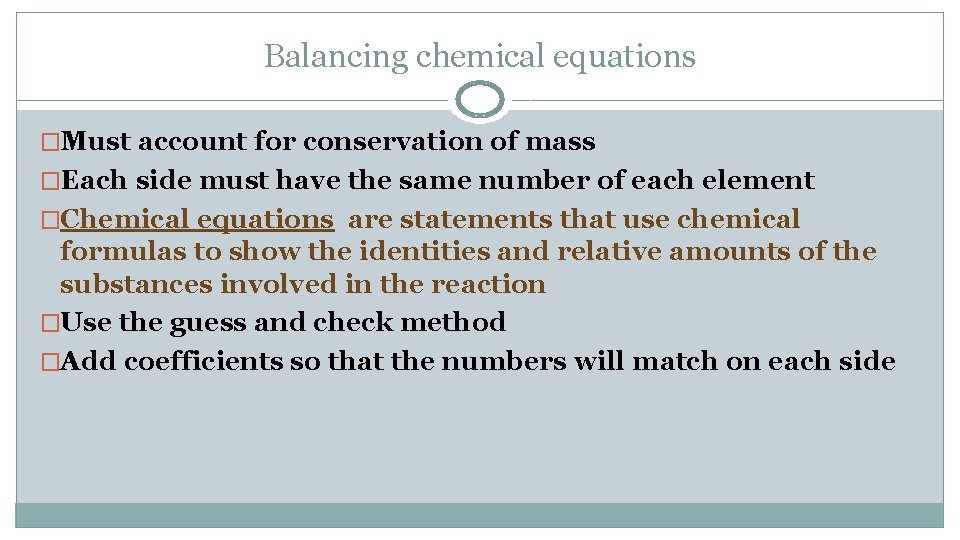
Balancing chemical equations �Must account for conservation of mass �Each side must have the same number of each element �Chemical equations are statements that use chemical formulas to show the identities and relative amounts of the substances involved in the reaction �Use the guess and check method �Add coefficients so that the numbers will match on each side

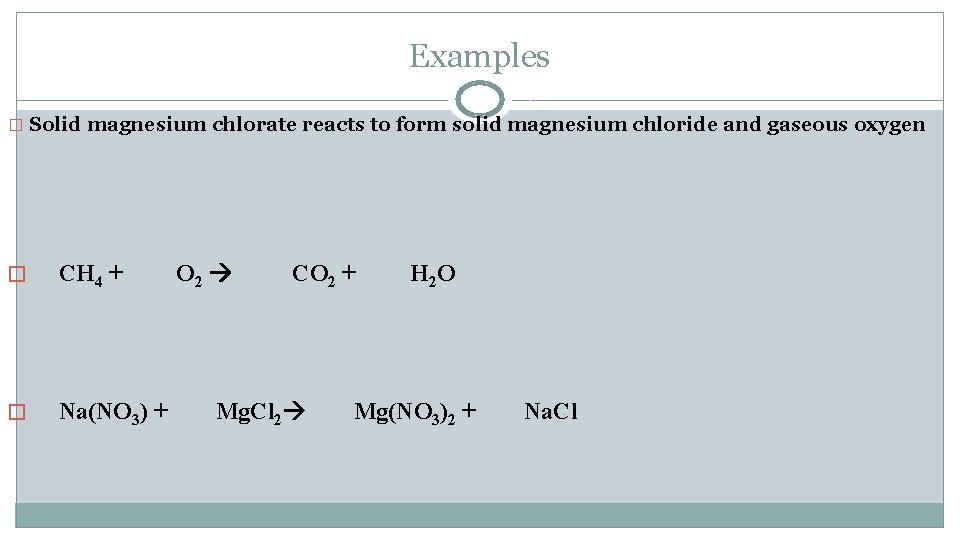
Examples � Solid magnesium chlorate reacts to form solid magnesium chloride and gaseous oxygen � CH 4 + � Na(NO 3) + O 2 CO 2 + Mg. Cl 2 H 2 O Mg(NO 3)2 + Na. Cl