Lecture Power Point Chemistry The Molecular Nature of



![The Reaction Quotient Q For the general reaction [C]c[D]d the reaction quotient Qc = The Reaction Quotient Q For the general reaction [C]c[D]d the reaction quotient Qc =](https://slidetodoc.com/presentation_image/42d2360d0b60c4070dd1db70cb8a1f6a/image-9.jpg)










![The Simplifying Assumption We assume that x([A]reacting) can be neglected if • Kc is The Simplifying Assumption We assume that x([A]reacting) can be neglected if • Kc is](https://slidetodoc.com/presentation_image/42d2360d0b60c4070dd1db70cb8a1f6a/image-32.jpg)













- Slides: 54

Lecture Power. Point Chemistry The Molecular Nature of Matter and Change Seventh Edition Martin S. Silberberg and Patricia G. Amateis 17 -1 Copyright Mc. Graw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of Mc. Graw-Hill Education.

Chapter 17 Equilibrium: The Extent of Chemical Reactions 17 -2

Equilibrium: The Extent of Chemical Reactions 17. 1 The Equilibrium State and the Equilibrium Constant 17. 2 The Reaction Quotient and the Equilibrium Constant 17. 3 Expressing Equilibria with Pressure Terms: Relation between Kc and Kp 17. 4 Comparing Q and K to Determine Reaction Direction 17. 5 How to Solve Equilibrium Problems 17. 6 Reaction Conditions and Equilibrium: Le Châtelier’s Principle 17 -3

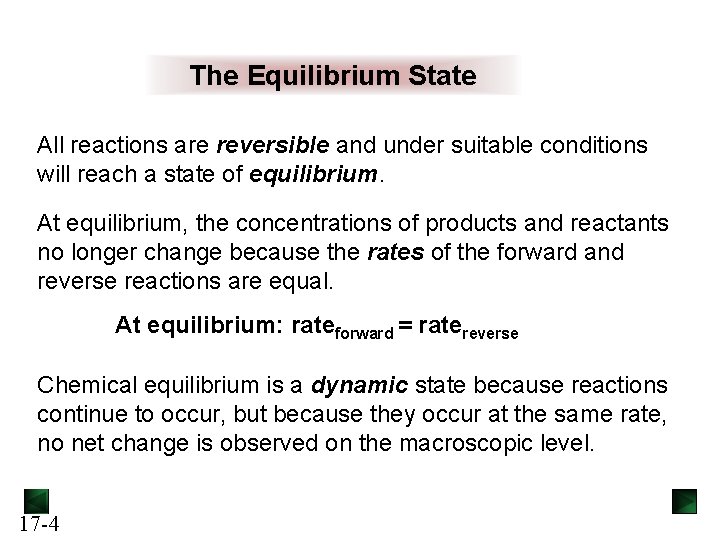
The Equilibrium State All reactions are reversible and under suitable conditions will reach a state of equilibrium. At equilibrium, the concentrations of products and reactants no longer change because the rates of the forward and reverse reactions are equal. At equilibrium: rateforward = ratereverse Chemical equilibrium is a dynamic state because reactions continue to occur, but because they occur at the same rate, no net change is observed on the macroscopic level. 17 -4

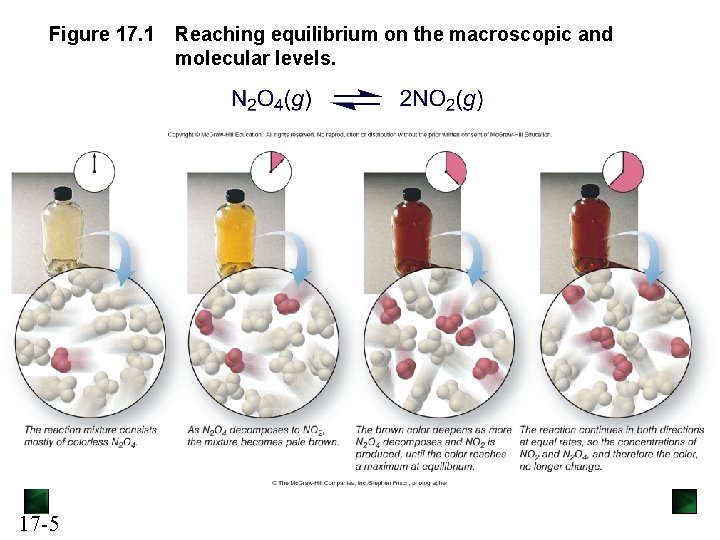
Figure 17. 1 17 -5 Reaching equilibrium on the macroscopic and molecular levels.

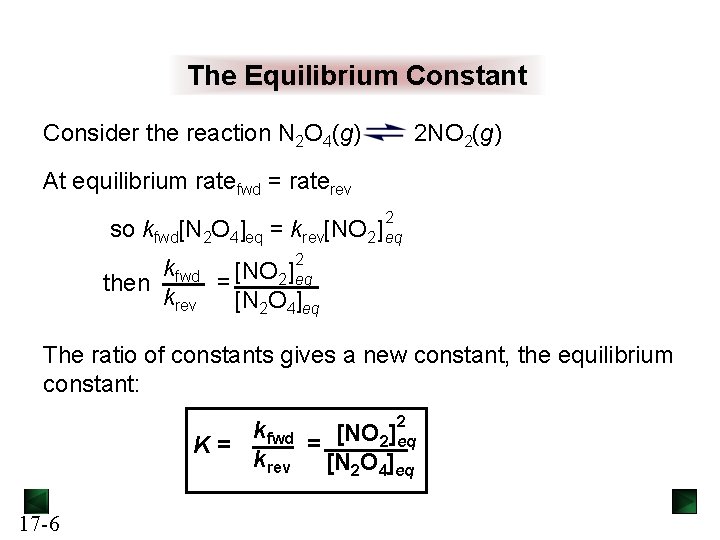
The Equilibrium Constant Consider the reaction N 2 O 4(g) 2 NO 2(g) At equilibrium ratefwd = raterev 2 so kfwd[N 2 O 4]eq = krev[NO 2] eq kfwd [NO 2] 2 eq = then krev [N 2 O 4]eq The ratio of constants gives a new constant, the equilibrium constant: 2 kfwd = [NO 2]eq K= krev [N 2 O 4]eq 17 -6

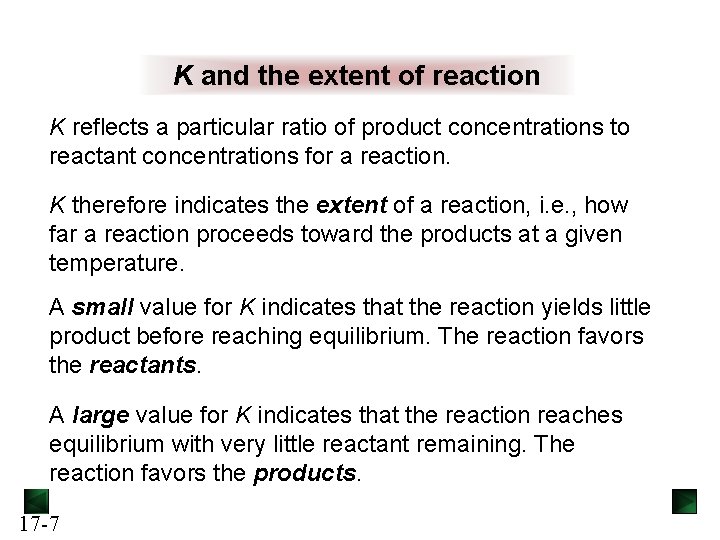
K and the extent of reaction K reflects a particular ratio of product concentrations to reactant concentrations for a reaction. K therefore indicates the extent of a reaction, i. e. , how far a reaction proceeds toward the products at a given temperature. A small value for K indicates that the reaction yields little product before reaching equilibrium. The reaction favors the reactants. A large value for K indicates that the reaction reaches equilibrium with very little reactant remaining. The reaction favors the products. 17 -7

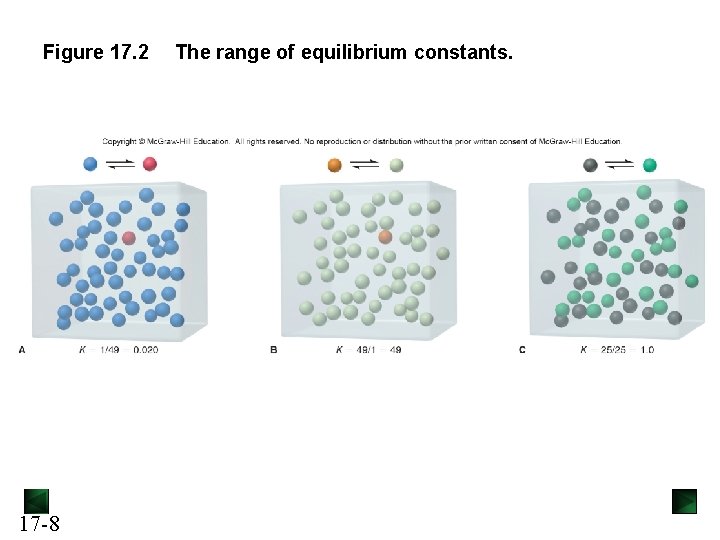
Figure 17. 2 17 -8 The range of equilibrium constants.
![The Reaction Quotient Q For the general reaction CcDd the reaction quotient Qc The Reaction Quotient Q For the general reaction [C]c[D]d the reaction quotient Qc =](https://slidetodoc.com/presentation_image/42d2360d0b60c4070dd1db70cb8a1f6a/image-9.jpg)
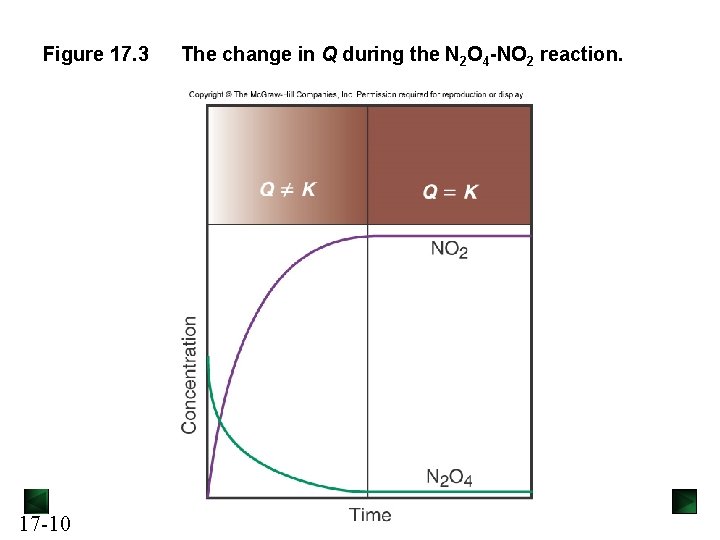
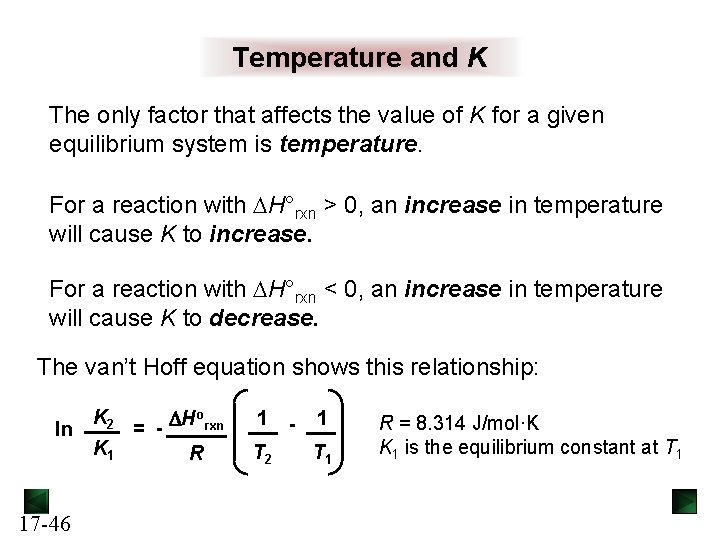
The Reaction Quotient Q For the general reaction [C]c[D]d the reaction quotient Qc = [A]a[B]b Q gives the ratio of product concentrations to reactant concentrations at any point in a reaction. At equilibrium: Q = K For a particular system and temperature, the same equilibrium state is attained regardless of starting concentrations. The value of Q indicates how close the reaction is to equilibrium, and in which direction it must proceed to reach equilibrium. 17 -9

Figure 17. 3 17 -10 The change in Q during the N 2 O 4 -NO 2 reaction.

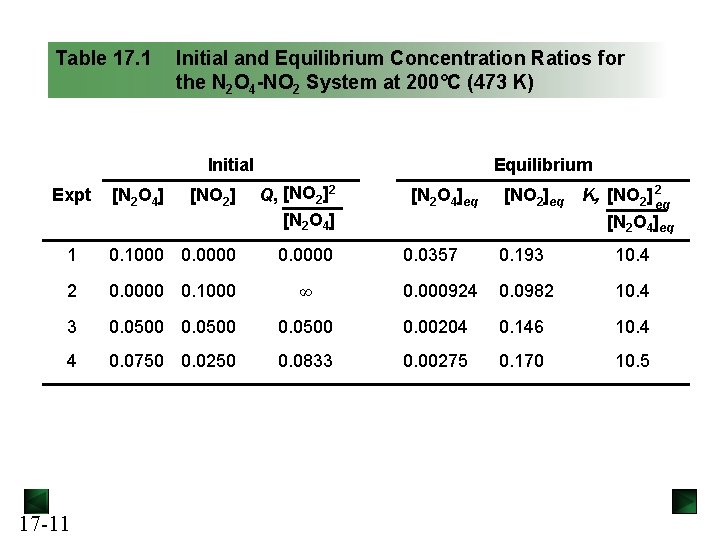
Table 17. 1 Initial and Equilibrium Concentration Ratios for the N 2 O 4 -NO 2 System at 200°C (473 K) Initial Equilibrium [NO 2] Q, [NO 2]2 [N 2 O 4] 1 0. 1000 0. 0000 2 0. 0000 0. 1000 3 4 Expt 17 -11 [N 2 O 4]eq [NO 2]eq K, [NO 2] 2 eq [N 2 O 4]eq 0. 0357 0. 193 10. 4 ∞ 0. 000924 0. 0982 10. 4 0. 0500 0. 00204 0. 146 10. 4 0. 0750 0. 0250 0. 0833 0. 00275 0. 170 10. 5

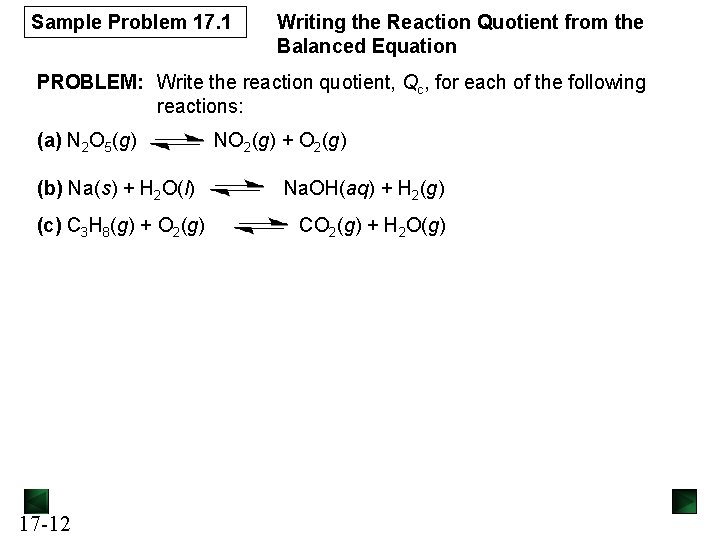
Sample Problem 17. 1 Writing the Reaction Quotient from the Balanced Equation PROBLEM: Write the reaction quotient, Qc, for each of the following reactions: (a) N 2 O 5(g) (b) Na(s) + H 2 O(l) (c) C 3 H 8(g) + O 2(g) 17 -12 NO 2(g) + O 2(g) Na. OH(aq) + H 2(g) CO 2(g) + H 2 O(g)

Forms of K and Q For an overall reaction that is the sum of two more individual reactions: Qoverall = Q 1 x Q 2 x Q 3 x …. . and Koverall = K 1 x K 2 x K 3 x …… The form of Q and K depend on the direction in which the balanced equation is written: Qc(rev) = 17 -13 1 Qc(fwd) Kc(rev) = 1 Kc(fwd)

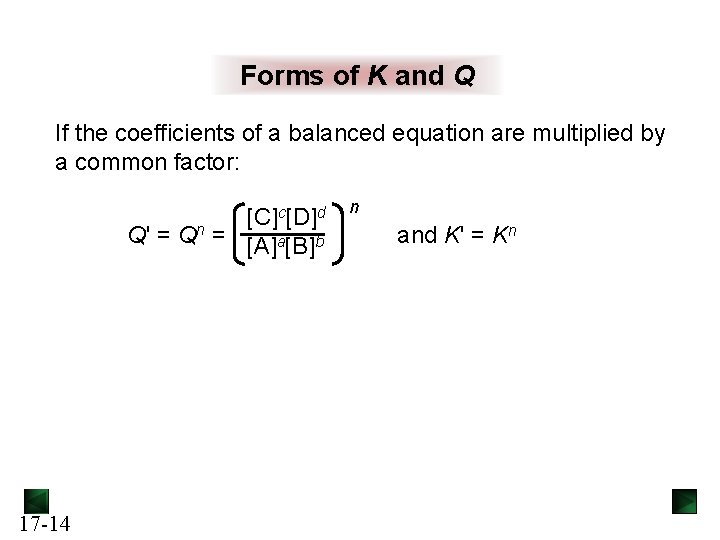
Forms of K and Q If the coefficients of a balanced equation are multiplied by a common factor: c[D]d [C] Q' = Qn = [A]a[B]b 17 -14 n and K' = Kn

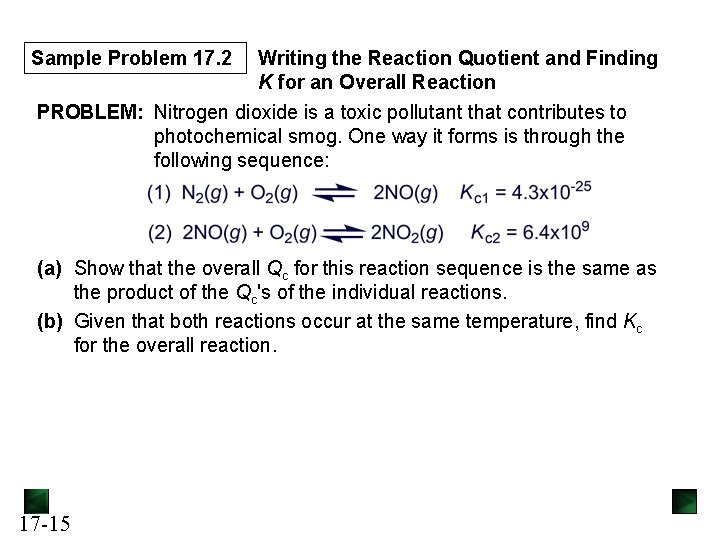
Sample Problem 17. 2 Writing the Reaction Quotient and Finding K for an Overall Reaction PROBLEM: Nitrogen dioxide is a toxic pollutant that contributes to photochemical smog. One way it forms is through the following sequence: (a) Show that the overall Qc for this reaction sequence is the same as the product of the Qc's of the individual reactions. (b) Given that both reactions occur at the same temperature, find Kc for the overall reaction. 17 -15

Sample Problem 17. 3 Finding the Equilibrium Constant for an Equation Multiplied by a Common Factor PROBLEM: For the ammonia formation reaction, the reference equation is Kc is 2. 4 x 10 -3 at 1000 K. What are the values of Kc for the following balanced equations? (a) 17 -16 (b)

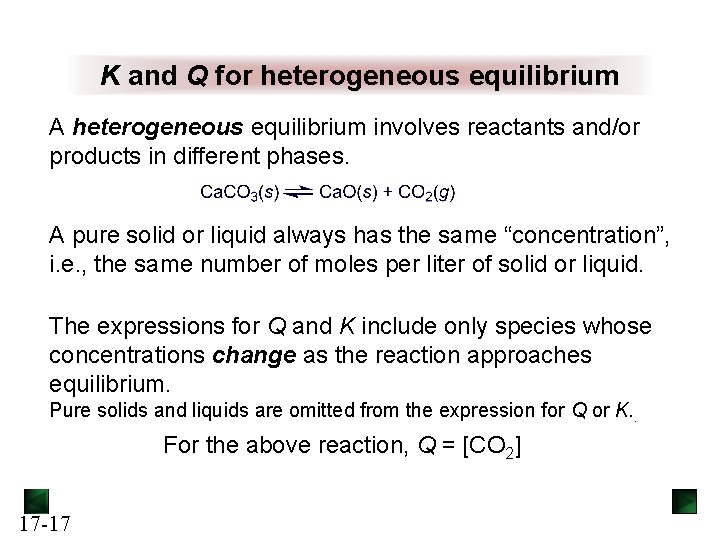
K and Q for heterogeneous equilibrium A heterogeneous equilibrium involves reactants and/or products in different phases. A pure solid or liquid always has the same “concentration”, i. e. , the same number of moles per liter of solid or liquid. The expressions for Q and K include only species whose concentrations change as the reaction approaches equilibrium. Pure solids and liquids are omitted from the expression for Q or K. . For the above reaction, Q = [CO 2] 17 -17

Figure 17. 4 The reaction quotient for a heterogeneous system depends only on concentrations that change. Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. 17 -18 solids do not change their concentrations

Table 17. 2 17 -19 Ways of Expressing Q and Calculating K

Expressing Equilibria with Pressure Terms Kc and Kp K for a reaction may be expressed using partial pressures of gaseous reactants instead of molarity. The partial pressure of each gas is directly proportional to its molarity. Qp = 2 P NO 2 P 2 NO x PO Qc = [NO 2]2 [NO]2 [O 2] 2 Kp = Kc (RT) n(gas) If the amount (mol) of gas does not change in the reaction, ngas = 0 and Kp = Kc. 17 -20

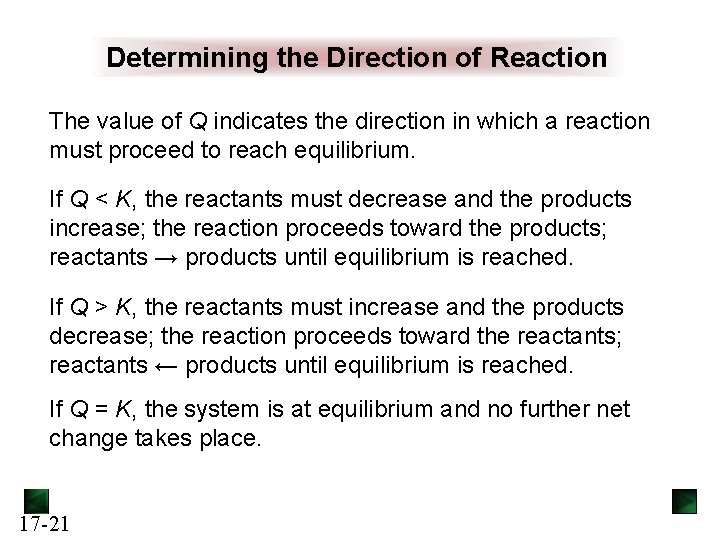
Determining the Direction of Reaction The value of Q indicates the direction in which a reaction must proceed to reach equilibrium. If Q < K, the reactants must decrease and the products increase; the reaction proceeds toward the products; reactants → products until equilibrium is reached. If Q > K, the reactants must increase and the products decrease; the reaction proceeds toward the reactants; reactants ← products until equilibrium is reached. If Q = K, the system is at equilibrium and no further net change takes place. 17 -21

Figure 17. 5 Reaction direction and the relative sizes of Q and K. Q>K Q<K 17 -22 Q=K

Sample Problem 17. 5 Using Molecular Scenes to Determine Reaction Direction B(g), the equilibrium mixture at PROBLEM: For the reaction A(g) 175°C is [A] = 2. 8 x 10– 4 M and [B] = 1. 2 x 10– 4 M. The molecular scenes below represent mixtures at various times during runs 1 -4 of this reaction (A is red; B is blue). Does the reaction progress to the right or left or not at all for each mixture to reach equilibrium? 17 -23

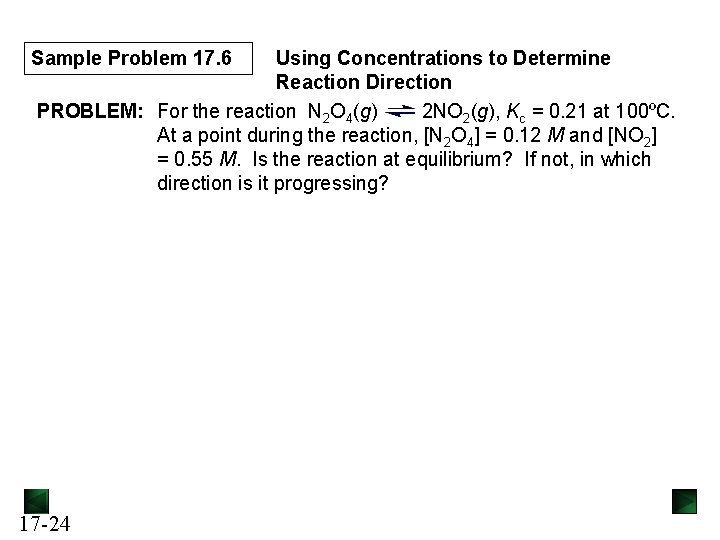
Sample Problem 17. 6 Using Concentrations to Determine Reaction Direction PROBLEM: For the reaction N 2 O 4(g) 2 NO 2(g), Kc = 0. 21 at 100ºC. At a point during the reaction, [N 2 O 4] = 0. 12 M and [NO 2] = 0. 55 M. Is the reaction at equilibrium? If not, in which direction is it progressing? 17 -24

Solving Equilibrium Problems If equilibrium quantities are given, we simply substitute these into the expression for Kc to calculate its value. If only some equilibrium quantities are given, we use a reaction table to calculate them and find Kc. A reaction table shows • the balanced equation, • the initial quantities of reactants and products, • the changes in these quantities during the reaction, and • the equilibrium quantities. 17 -25

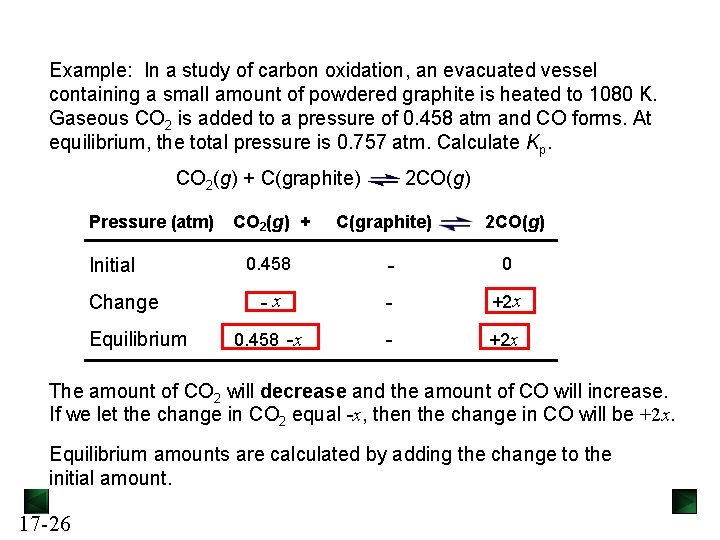
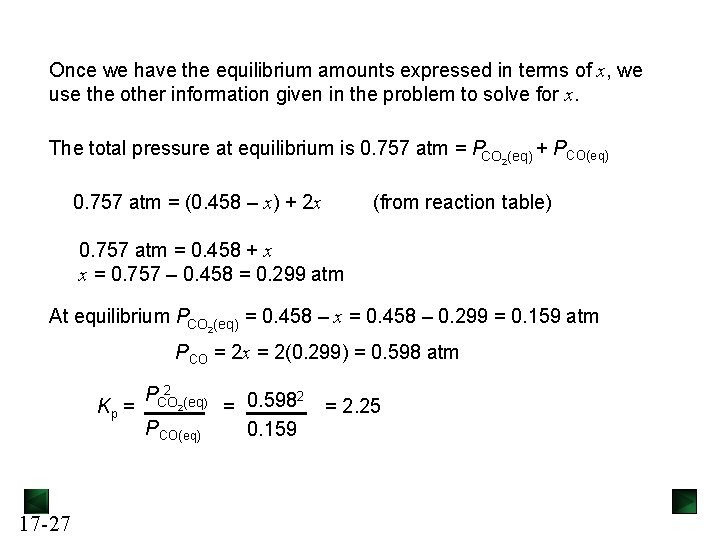
Example: In a study of carbon oxidation, an evacuated vessel containing a small amount of powdered graphite is heated to 1080 K. Gaseous CO 2 is added to a pressure of 0. 458 atm and CO forms. At equilibrium, the total pressure is 0. 757 atm. Calculate Kp. CO 2(g) + C(graphite) Pressure (atm) Initial Change Equilibrium CO 2(g) + 2 CO(g) C(graphite) 2 CO(g) 0. 458 - 0 -x - +2 x 0. 458 -x - +2 x The amount of CO 2 will decrease and the amount of CO will increase. If we let the change in CO 2 equal -x, then the change in CO will be +2 x. Equilibrium amounts are calculated by adding the change to the initial amount. 17 -26

Once we have the equilibrium amounts expressed in terms of x, we use the other information given in the problem to solve for x. The total pressure at equilibrium is 0. 757 atm = PCO 2(eq) + PCO(eq) 0. 757 atm = (0. 458 – x) + 2 x (from reaction table) 0. 757 atm = 0. 458 + x x = 0. 757 – 0. 458 = 0. 299 atm At equilibrium PCO 2(eq) = 0. 458 – x = 0. 458 – 0. 299 = 0. 159 atm PCO = 2 x = 2(0. 299) = 0. 598 atm Kp = 17 -27 2 PCO 2(eq) PCO(eq) 2 = 0. 598 0. 159 = 2. 25

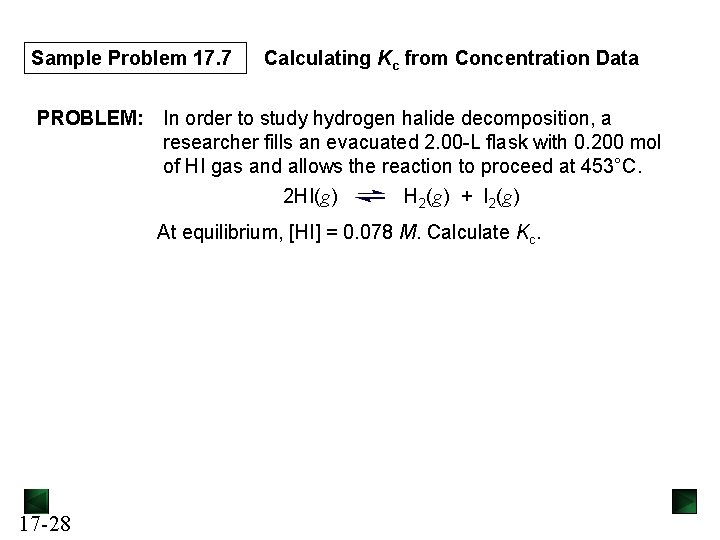
Sample Problem 17. 7 Calculating Kc from Concentration Data PROBLEM: In order to study hydrogen halide decomposition, a researcher fills an evacuated 2. 00 -L flask with 0. 200 mol of HI gas and allows the reaction to proceed at 453°C. 2 HI(g) H 2(g) + I 2(g) At equilibrium, [HI] = 0. 078 M. Calculate Kc. 17 -28

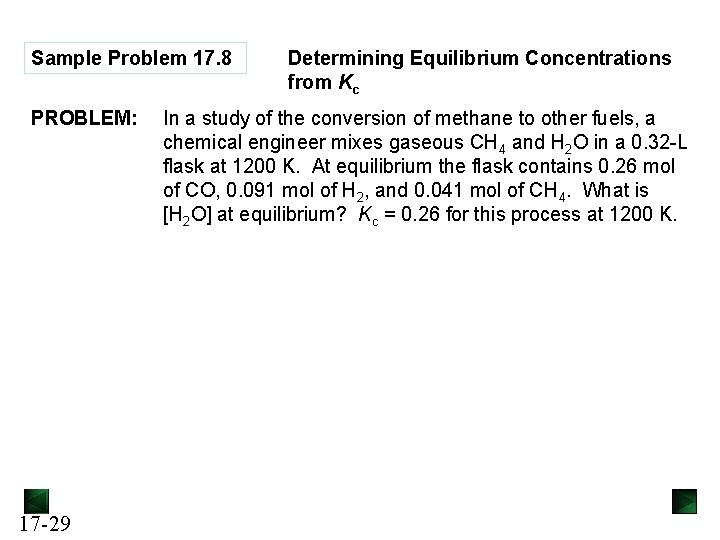
Sample Problem 17. 8 PROBLEM: 17 -29 Determining Equilibrium Concentrations from Kc In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous CH 4 and H 2 O in a 0. 32 -L flask at 1200 K. At equilibrium the flask contains 0. 26 mol of CO, 0. 091 mol of H 2, and 0. 041 mol of CH 4. What is [H 2 O] at equilibrium? Kc = 0. 26 for this process at 1200 K.

Sample Problem 17. 9 Determining Equilibrium Concentrations from Initial Concentrations and Kc PROBLEM: Fuel engineers use the extent of the change from CO and H 2 O to CO 2 and H 2 to regulate the proportions of synthetic fuel mixtures. If 0. 250 mol of CO and 0. 250 mol of H 2 O gases are placed in a 125 -m. L flask at 900 K, what is the composition of the equilibrium mixture? At this temperature, Kc is 1. 56. 17 -30

Sample Problem 17. 10 Making a Simplifying Assumption to Calculate Equilibrium Concentrations PROBLEM: Phosgene is a potent chemical warfare agent that is now outlawed by international agreement. It decomposes by the reaction COCl 2(g) CO(g) + Cl 2(g); Kc = 8. 3 x 10– 4 at 360ºC. Calculate [CO], [Cl 2], and [COCl 2] when the following amounts of phosgene decompose and reach equilibrium in a 10. 0 -L flask. (a) 5. 00 mol COCl 2 (b) 0. 100 mol COCl 2 17 -31
![The Simplifying Assumption We assume that xAreacting can be neglected if Kc is The Simplifying Assumption We assume that x([A]reacting) can be neglected if • Kc is](https://slidetodoc.com/presentation_image/42d2360d0b60c4070dd1db70cb8a1f6a/image-32.jpg)
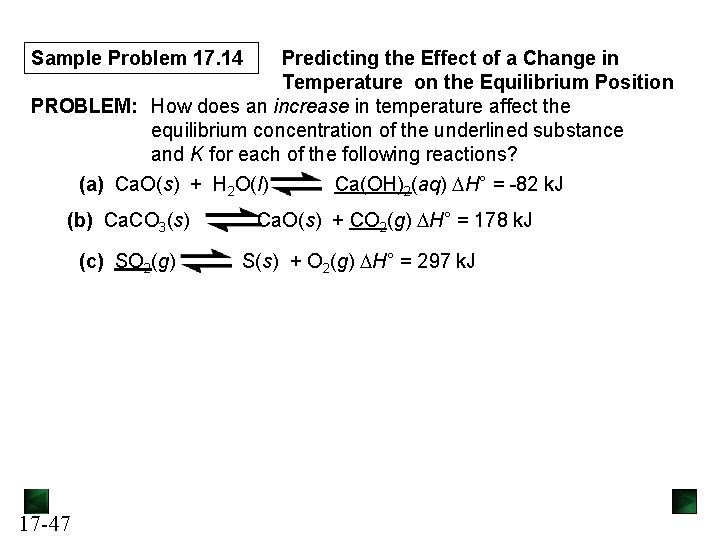
The Simplifying Assumption We assume that x([A]reacting) can be neglected if • Kc is relatively small and/or • [A]init is relatively large. If [A]init > 400, the assumption is justified; neglecting x Kc introduces an error < 5%. If [A]init < 400, the assumption is not justified; neglecting x Kc introduces an error > 5%. 17 -32

Sample Problem 17. 11 Predicting Reaction Direction and Calculating Equilibrium Concentrations PROBLEM: The research and development unit of a chemical company is studying the reaction of CH 4 and H 2 S, two components of natural gas: CH 4(g) + 2 H 2 S(g) CS 2(g) + 4 H 2(g) In one experiment, 1. 00 mol of CH 4, 1. 00 mol of CS 2, 2. 00 mol of H 2 S, and 2. 00 mol of H 2 are mixed in a 250 -m. L vessel at 960ºC. At this temperature, Kc = 0. 036. (a) In which direction will the reaction proceed to reach equilibrium? (b) If [CH 4] = 5. 56 M at equilibrium, what are the equilibrium concentrations of the other substances? 17 -33

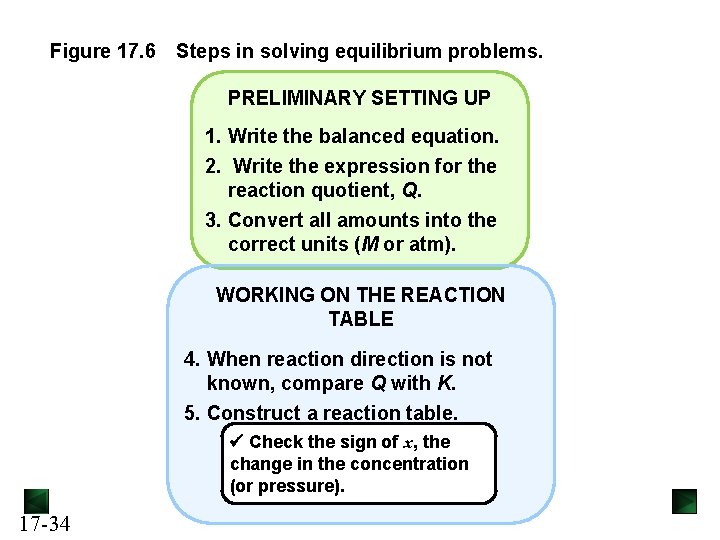
Figure 17. 6 Steps in solving equilibrium problems. PRELIMINARY SETTING UP 1. Write the balanced equation. 2. Write the expression for the reaction quotient, Q. 3. Convert all amounts into the correct units (M or atm). WORKING ON THE REACTION TABLE 4. When reaction direction is not known, compare Q with K. 5. Construct a reaction table. Check the sign of x, the change in the concentration (or pressure). 17 -34

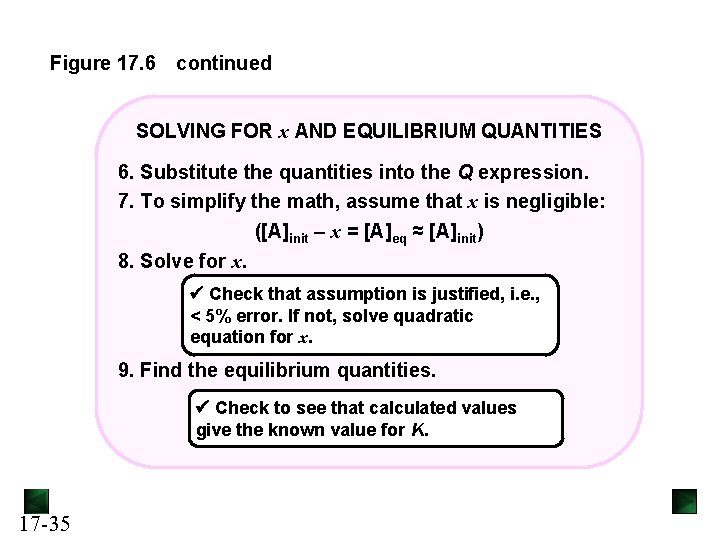
Figure 17. 6 continued SOLVING FOR x AND EQUILIBRIUM QUANTITIES 6. Substitute the quantities into the Q expression. 7. To simplify the math, assume that x is negligible: ([A]init – x = [A]eq ≈ [A]init) 8. Solve for x. Check that assumption is justified, i. e. , < 5% error. If not, solve quadratic equation for x. 9. Find the equilibrium quantities. Check to see that calculated values give the known value for K. 17 -35

Le Châtelier’s Principle When a chemical system at equilibrium is disturbed, it reattains equilibrium by undergoing a net reaction that reduces the effect of the disturbance. A system is disturbed when a change in conditions forces it temporarily out of equilibrium. The system responds to a disturbance by a shift in the equilibrium position. A shift to the left is a net reaction from product to reactant. A shift to the right is a net reaction from reactant to product. 17 -36

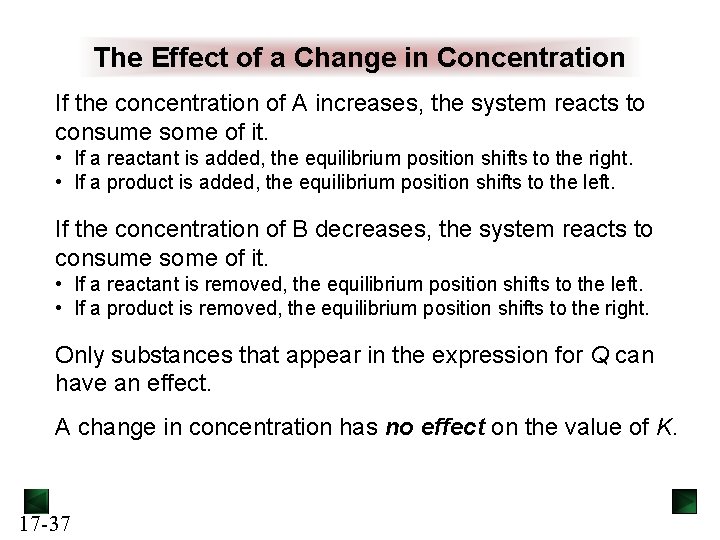
The Effect of a Change in Concentration If the concentration of A increases, the system reacts to consume some of it. • If a reactant is added, the equilibrium position shifts to the right. • If a product is added, the equilibrium position shifts to the left. If the concentration of B decreases, the system reacts to consume some of it. • If a reactant is removed, the equilibrium position shifts to the left. • If a product is removed, the equilibrium position shifts to the right. Only substances that appear in the expression for Q can have an effect. A change in concentration has no effect on the value of K. 17 -37

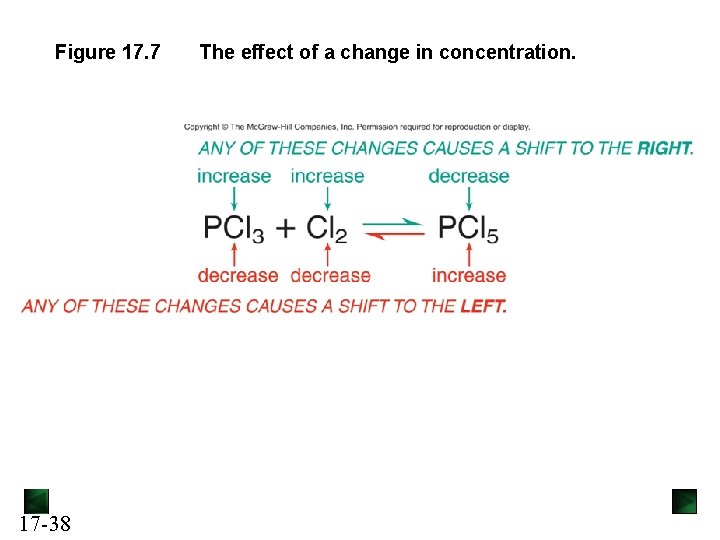
Figure 17. 7 17 -38 The effect of a change in concentration.

Table 17. 3 The Effect of Added Cl 2 on the PCl 3 -Cl 2 -PCl 5 System Concentration (M) Original equilibrium PCl 3(g) + 0. 200 Disturbance Change New equilibrium 17 -39 0. 125 PCl 3(g) 0. 600 +0. 075 New initial *Experimentally Cl 2(g) 0. 200 0. 600 –x –x +x 0. 200 – x 0. 600 + x (0. 637)* determined value

Figure 17. 8 The effect of added Cl 2 on the PCl 3 -Cl 2 -PCl 5 system. Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. PCl 3(g) + Cl 2(g) PCl 5(g) When Cl 2 (yellow curve) is added, its concentration increases instantly (vertical part of yellow curve) and then falls gradually as it reacts with PCl 3 to form more PCl 5. Equilibrium is re-established at new concentrations but with the same value of K. 17 -40

Sample Problem 17. 12 Predicting the Effect of a Change in Concentration on the Equilibrium Position PROBLEM: To improve air quality and obtain a useful product, chemists often remove sulfur from coal and natural gas by treating the contaminant hydrogen sulfide with O 2: 2 H 2 S(g) + O 2(g) 2 S(s) + 2 H 2 O(g) What happens to (a) [H 2 O] if O 2 is added? (b) [H 2 S] if O 2 is added? (c) [O 2] if H 2 S is removed? (d) [H 2 S] if sulfur is added? 17 -41

The Effect of a Change in Pressure (Volume) Changes in pressure affect equilibrium systems containing gaseous components. • Changing the concentration of a gaseous component causes the equilibrium to shift accordingly. • Adding an inert gas has no effect on the equilibrium position, as long as the volume does not change. – This is because all concentrations and partial pressures remain unchanged. • Changing the volume of the reaction vessel will cause equilibrium to shift if ngas ≠ 0. • Changes in pressure (volume) have no effect on the value of K. 17 -42

Figure 17. 9 17 -43 The effect of a change in pressure (volume) on a system at equilibrium.

Sample Problem 17. 13 Predicting the Effect of a Change in Volume (Pressure) on the Equilibrium Position PROBLEM: How would you change the volume of each of the following reactions to increase the yield of the products? (a) Ca. CO 3(s) 17 -44 Ca. O(s) + CO 2(g) (b) S(s) + 3 F 2(g) SF 6(g) (c) Cl 2(g) + I 2(g) 2 ICl(g)

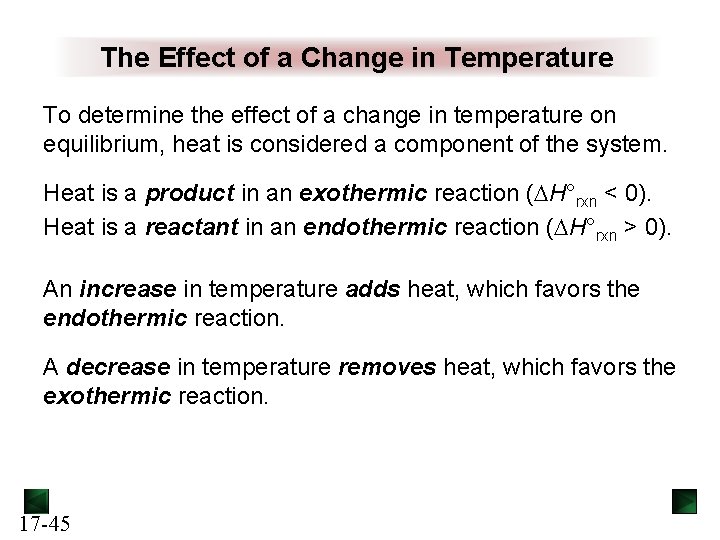
The Effect of a Change in Temperature To determine the effect of a change in temperature on equilibrium, heat is considered a component of the system. Heat is a product in an exothermic reaction ( H°rxn < 0). Heat is a reactant in an endothermic reaction ( H°rxn > 0). An increase in temperature adds heat, which favors the endothermic reaction. A decrease in temperature removes heat, which favors the exothermic reaction. 17 -45

Temperature and K The only factor that affects the value of K for a given equilibrium system is temperature. For a reaction with H°rxn > 0, an increase in temperature will cause K to increase. For a reaction with H°rxn < 0, an increase in temperature will cause K to decrease. The van’t Hoff equation shows this relationship: ln 17 -46 K 2 K 1 o H rxn = R 1 T 2 - 1 T 1 R = 8. 314 J/mol·K K 1 is the equilibrium constant at T 1

Sample Problem 17. 14 Predicting the Effect of a Change in Temperature on the Equilibrium Position PROBLEM: How does an increase in temperature affect the equilibrium concentration of the underlined substance and K for each of the following reactions? (a) Ca. O(s) + H 2 O(l) (b) Ca. CO 3(s) (c) SO 2(g) 17 -47 Ca(OH)2(aq) H° = -82 k. J Ca. O(s) + CO 2(g) H° = 178 k. J S(s) + O 2(g) H° = 297 k. J

Catalysts and Equilibrium A catalyst speeds up a reaction by lowering its activation energy. A catalyst therefore speeds up the forward and reverse reactions to the same extent. A catalyst causes a reaction to reach equilibrium more quickly, but has no effect on the equilibrium position. 17 -48

Table 17. 4 Effects of Various Disturbances on a System at Equilibrium 17 -49

Sample Problem 17. 15 Determining Equilibrium Parameters from Molecular Scenes PROBLEM: For the reaction X(g) + Y 2(g) XY(g) + Y(g) H > 0 the following molecular scenes depict different reaction mixtures. (X = green, Y = purple): (a) If K = 2 at the temperature of the reaction, which scene represents the mixture at equilibrium? (b) Will the reaction mixtures in the other two scenes proceed toward reactant or toward products to reach equilibrium? (c) For the mixture at equilibrium, how will a rise in temperature affect [Y 2]? 17 -50

The Synthesis of Ammonia is synthesized industrially via the Haber process: N 2(g) + 3 H 2(g) 2 NH 3(g) H°rxn = – 91. 8 k. J There are three ways to maximize the yield of NH 3: • Decrease [NH 3] by removing NH 3 as it forms. • Decrease the volume (increase the pressure). • Decrease the temperature. 17 -51

Table 17. 5 Effect of Temperature on Kc for Ammonia Synthesis N 2(g) + 3 H 2(g) 2 NH 3(g) H°rxn = – 91. 8 k. J T (K) Kc 200. 7. 17 x 1015 300. 2. 69 x 108 400. 3. 94 x 104 500. 1. 72 x 102 600. 4. 53 x 100 700. 2. 96 x 10– 1 800. 3. 96 x 10– 2 An increase in temperature causes the equilibrium to shift toward the reactants, since the reaction is exothermic. 17 -52

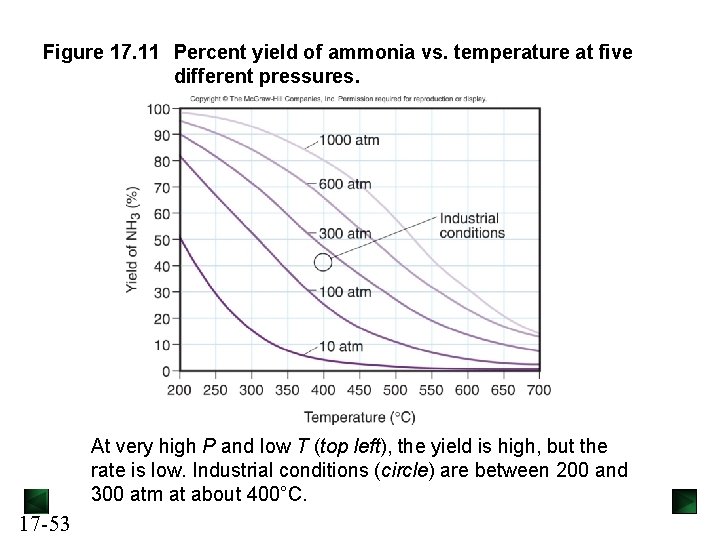
Figure 17. 11 Percent yield of ammonia vs. temperature at five different pressures. At very high P and low T (top left), the yield is high, but the rate is low. Industrial conditions (circle) are between 200 and 300 atm at about 400°C. 17 -53

Figure 17. 12 Key stages in the Haber process for synthesizing ammonia. 17 -54
 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Cell and molecular biology lectures
Cell and molecular biology lectures Dense regular ct
Dense regular ct Molecular cell biology lecture
Molecular cell biology lecture Covalent bond melting point
Covalent bond melting point Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Power traiangle
Power traiangle Power bi power point
Power bi power point Point point power
Point point power Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Lightning elves
Lightning elves Democritus atomic model diagram
Democritus atomic model diagram Ap chemistry unit 9 notes
Ap chemistry unit 9 notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Power system analysis lecture notes
Power system analysis lecture notes Power semiconductor devices lecture notes
Power semiconductor devices lecture notes Switch mode power supply lecture notes
Switch mode power supply lecture notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes What is the point of uchendu’s lecture to okonkwo?
What is the point of uchendu’s lecture to okonkwo? Nature and nature's laws lay hid in night meaning
Nature and nature's laws lay hid in night meaning Nature nature controversy
Nature nature controversy Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Foods that are heterogeneous mixtures
Foods that are heterogeneous mixtures Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Glasgow thang điểm
Glasgow thang điểm Hát lên người ơi
Hát lên người ơi Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế