Lecture Power Point Chemistry The Molecular Nature of




![Sample Problem 19. 1 0. 020 mol = 0. 020 M OH– (b) [OH−]added Sample Problem 19. 1 0. 020 mol = 0. 020 M OH– (b) [OH−]added](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-14.jpg)
![The Henderson-Hasselbalch Equation HA(aq) + H 2 O(l) [H 3 O+][A–] Ka = [HA] The Henderson-Hasselbalch Equation HA(aq) + H 2 O(l) [H 3 O+][A–] Ka = [HA]](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-19.jpg)
![Sample Problem 19. 3 [CO 3 2–] = Ka[HCO 3–] [H 3 O+ ] Sample Problem 19. 3 [CO 3 2–] = Ka[HCO 3–] [H 3 O+ ]](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-28.jpg)
![[H 3 O+ ] 2. 000 x 10– 3 mol = = 0. 03333 [H 3 O+ ] 2. 000 x 10– 3 mol = = 0. 03333](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-37.jpg)

![Sample Problem 19. 8 [Ca 2+]init = 0. 10 M because Ca(NO 3)2 is Sample Problem 19. 8 [Ca 2+]init = 0. 10 M because Ca(NO 3)2 is](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-63.jpg)


![Sample Problem 19. 13 [Zn(H 2 O)42+]initial = 50. 0 L x 0. 0020 Sample Problem 19. 13 [Zn(H 2 O)42+]initial = 50. 0 L x 0. 0020](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-83.jpg)
![Sample Problem 19. 13 Kf = [Zn(NH 3)42+] [Zn(H 2 O)42+][NH 3]4 = 7. Sample Problem 19. 13 Kf = [Zn(NH 3)42+] [Zn(H 2 O)42+][NH 3]4 = 7.](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-84.jpg)
- Slides: 88

Lecture Power. Point Chemistry The Molecular Nature of Matter and Change Seventh Edition Martin S. Silberberg and Patricia G. Amateis 19 -1 Copyright Mc. Graw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of Mc. Graw-Hill Education.

Chapter 19 Ionic Equilibria in Aqueous Systems 19 -2

Ionic Equilibria in Aqueous Systems 19. 1 Equilibria of Acid-Base Buffers 19. 2 Acid-Base Titration Curves 19. 3 Equilibria of Slightly Soluble Ionic Compounds 19. 4 Equilibria Involving Complex Ions 19 -3

Acid-Base Buffers An acid-base buffer is a solution that lessens the impact of p. H from the addition of acid or base. An acid-base buffer usually consists of a conjugate acidbase pair where both species are present in appreciable quantities in solution. An acid-base buffer is therefore a solution of a weak acid and its conjugate base, or a weak base and its conjugate acid. 19 -4

Figure 19. 1 The effect of adding acid or base to an unbuffered solution. A 100 -m. L sample of dilute HCl is adjusted to p. H 5. 00. 19 -5 The addition of 1 m. L of strong acid (left) or strong base (right) changes the p. H by several units.

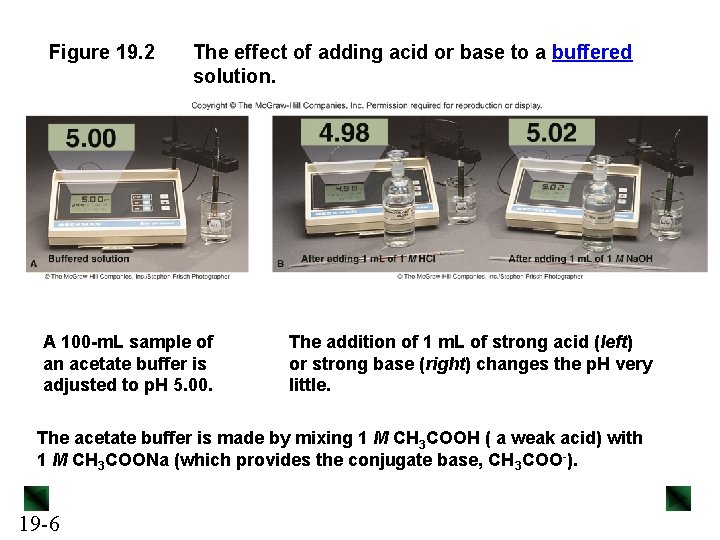
Figure 19. 2 The effect of adding acid or base to a buffered solution. A 100 -m. L sample of an acetate buffer is adjusted to p. H 5. 00. The addition of 1 m. L of strong acid (left) or strong base (right) changes the p. H very little. The acetate buffer is made by mixing 1 M CH 3 COOH ( a weak acid) with 1 M CH 3 COONa (which provides the conjugate base, CH 3 COO-). 19 -6

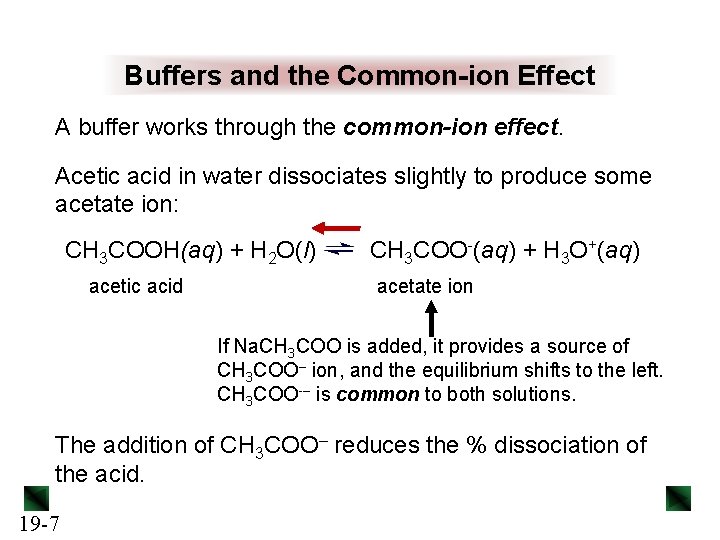
Buffers and the Common-ion Effect A buffer works through the common-ion effect. Acetic acid in water dissociates slightly to produce some acetate ion: CH 3 COOH(aq) + H 2 O(l) acetic acid CH 3 COO-(aq) + H 3 O+(aq) acetate ion If Na. CH 3 COO is added, it provides a source of CH 3 COO– ion, and the equilibrium shifts to the left. CH 3 COO-– is common to both solutions. The addition of CH 3 COO– reduces the % dissociation of the acid. 19 -7

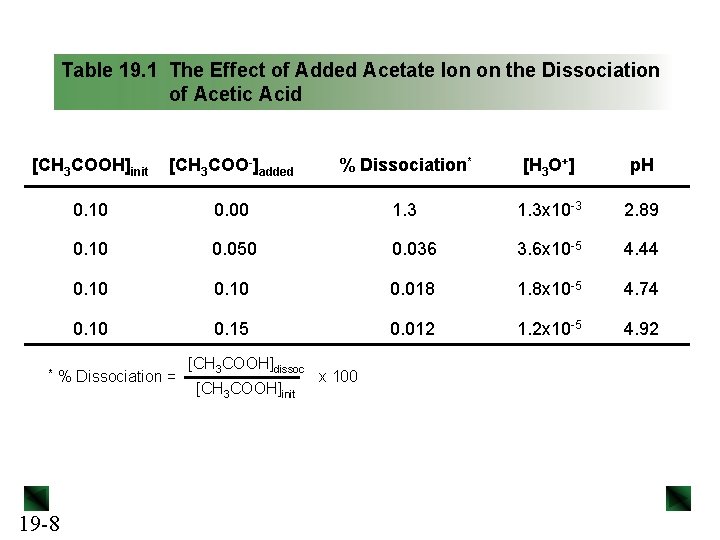
Table 19. 1 The Effect of Added Acetate Ion on the Dissociation of Acetic Acid [CH 3 COOH]init [CH 3 COO-]added % Dissociation* [H 3 O+] p. H 0. 10 0. 00 1. 3 x 10 -3 2. 89 0. 10 0. 050 0. 036 3. 6 x 10 -5 4. 44 0. 10 0. 018 1. 8 x 10 -5 4. 74 0. 10 0. 15 0. 012 1. 2 x 10 -5 4. 92 * % Dissociation = 19 -8 [CH 3 COOH]dissoc [CH 3 COOH]init x 100

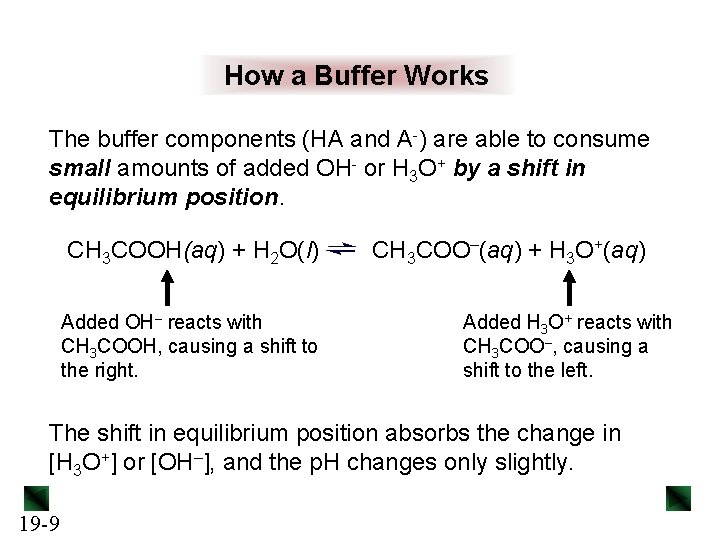
How a Buffer Works The buffer components (HA and A-) are able to consume small amounts of added OH- or H 3 O+ by a shift in equilibrium position. CH 3 COOH(aq) + H 2 O(l) Added OH– reacts with CH 3 COOH, causing a shift to the right. CH 3 COO–(aq) + H 3 O+(aq) Added H 3 O+ reacts with CH 3 COO–, causing a shift to the left. The shift in equilibrium position absorbs the change in [H 3 O+] or [OH–], and the p. H changes only slightly. 19 -9

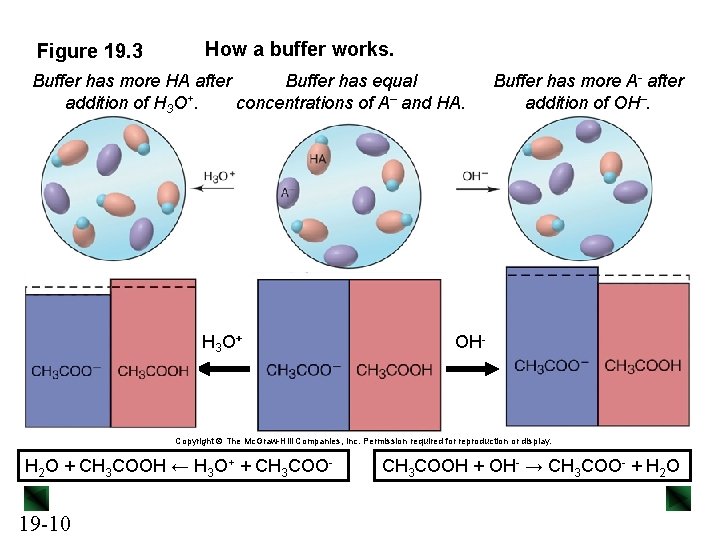
Figure 19. 3 How a buffer works. Buffer has more HA after Buffer has equal addition of H 3 O+. concentrations of A– and HA. H 3 O + Buffer has more A- after addition of OH–. OH- Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H 2 O + CH 3 COOH ← H 3 O+ + CH 3 COO- 19 -10 CH 3 COOH + OH- → CH 3 COO- + H 2 O

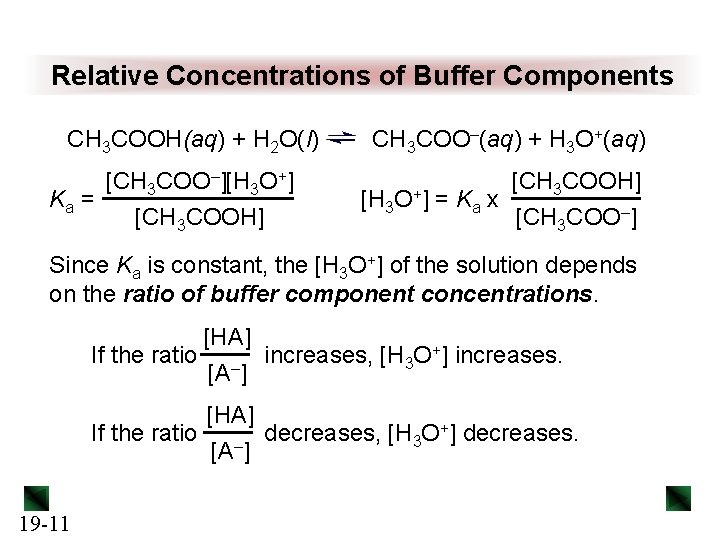
Relative Concentrations of Buffer Components CH 3 COOH(aq) + H 2 O(l) [CH 3 COO–][H 3 O+] Ka = [CH 3 COOH] CH 3 COO–(aq) + H 3 O+(aq) [H 3 O+] [CH 3 COOH] = Ka x [CH 3 COO–] Since Ka is constant, the [H 3 O+] of the solution depends on the ratio of buffer component concentrations. [HA] If the ratio – increases, [H 3 O+] increases. [A ] [HA] +] decreases. If the ratio decreases, [H O 3 [A–] 19 -11

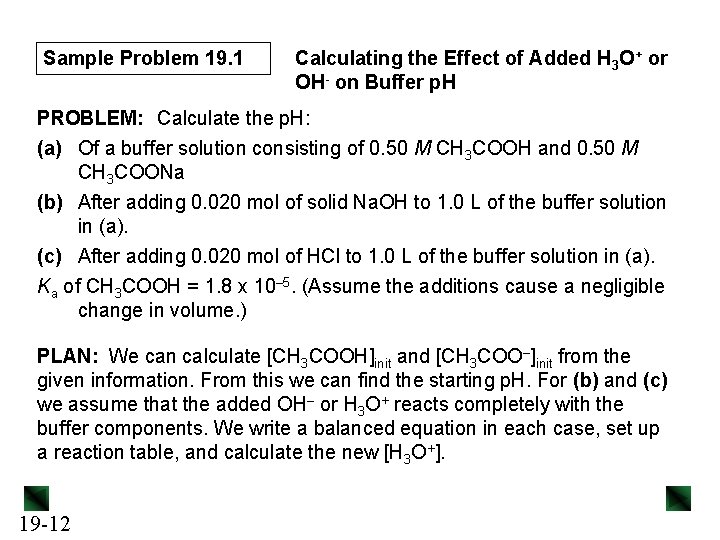
Sample Problem 19. 1 Calculating the Effect of Added H 3 O+ or OH- on Buffer p. H PROBLEM: Calculate the p. H: (a) Of a buffer solution consisting of 0. 50 M CH 3 COOH and 0. 50 M CH 3 COONa (b) After adding 0. 020 mol of solid Na. OH to 1. 0 L of the buffer solution in (a). (c) After adding 0. 020 mol of HCl to 1. 0 L of the buffer solution in (a). Ka of CH 3 COOH = 1. 8 x 10– 5. (Assume the additions cause a negligible change in volume. ) PLAN: We can calculate [CH 3 COOH]init and [CH 3 COO–]init from the given information. From this we can find the starting p. H. For (b) and (c) we assume that the added OH– or H 3 O+ reacts completely with the buffer components. We write a balanced equation in each case, set up a reaction table, and calculate the new [H 3 O+]. 19 -12

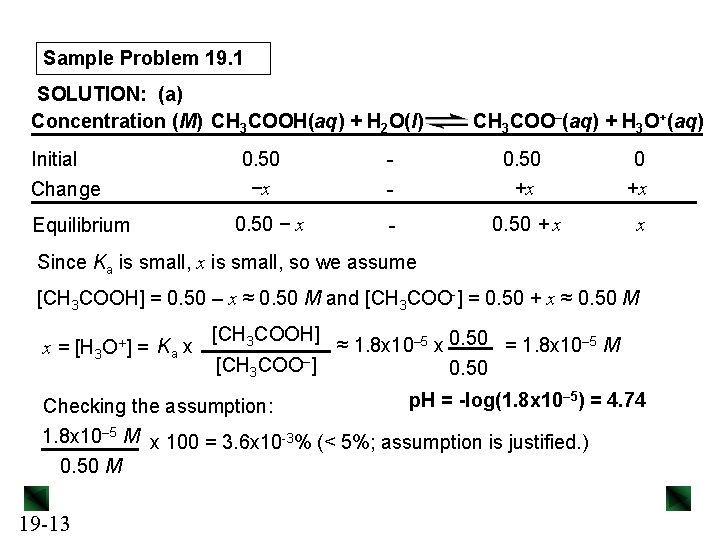
Sample Problem 19. 1 SOLUTION: (a) Concentration (M) CH 3 COOH(aq) + H 2 O(l) Initial Change Equilibrium 0. 50 −x 0. 50 − x - CH 3 COO–(aq) + H 3 O+(aq) - 0. 50 +x - 0. 50 + x x Since Ka is small, x is small, so we assume [CH 3 COOH] = 0. 50 – x ≈ 0. 50 M and [CH 3 COO-] = 0. 50 + x ≈ 0. 50 M x = [H 3 O+] = Ka x [CH 3 COOH] [CH 3 COO–] ≈ 1. 8 x 10– 5 x 0. 50 = 1. 8 x 10– 5 M 0. 50 p. H = -log(1. 8 x 10– 5) = 4. 74 Checking the assumption: 1. 8 x 10– 5 M x 100 = 3. 6 x 10 -3% (< 5%; assumption is justified. ) 0. 50 M 19 -13
![Sample Problem 19 1 0 020 mol 0 020 M OH b OHadded Sample Problem 19. 1 0. 020 mol = 0. 020 M OH– (b) [OH−]added](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-14.jpg)
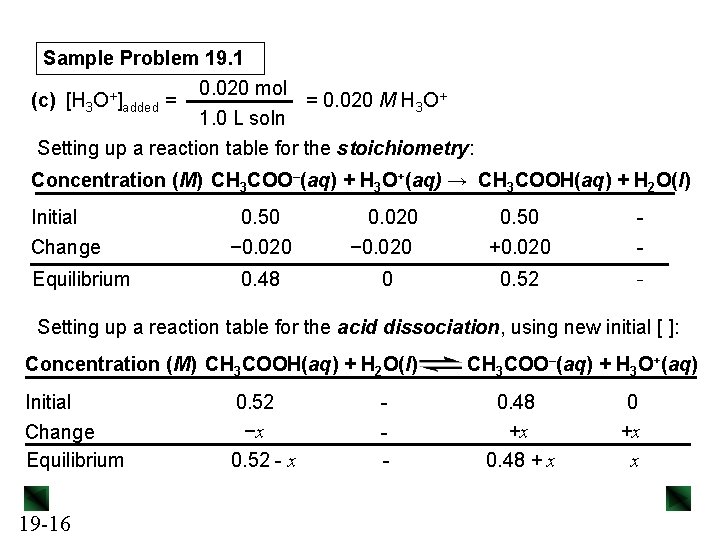
Sample Problem 19. 1 0. 020 mol = 0. 020 M OH– (b) [OH−]added = 1. 0 L soln Setting up a reaction table for the stoichiometry: Concentration (M) CH 3 COOH(aq) + OH–(aq) → CH 3 COO–(aq) + H 2 O(l) Initial Change Equilibrium 0. 50 − 0. 020 0. 50 +0. 020 - 0. 48 0 0. 52 - Setting up a reaction table for the acid dissociation, using new initial [ ]: Concentration (M) CH 3 COOH(aq) + H 2 O(l) Initial Change Equilibrium 19 -14 0. 48 −x 0. 48 − x - CH 3 COO–(aq) + H 3 O+(aq) 0. 52 +x 0. 52 + x 0 +x x

Sample Problem 19. 1 Since Ka is small, x is small, so we assume [CH 3 COOH] = 0. 48 – x ≈ 0. 48 M and [CH 3 COO–] = 0. 52 + x ≈ 0. 52 M x = [H 3 O+] = Ka x [CH 3 COOH] [CH 3 COO–] ≈ 1. 8 x 10– 5 x 0. 48 = 1. 7 x 10– 5 M 0. 52 p. H = −log(1. 7 x 10 -5) = 4. 77 Addition of a small amount of base caused the p. H to rise only slightly, from 4. 74 to 4. 77. 19 -15

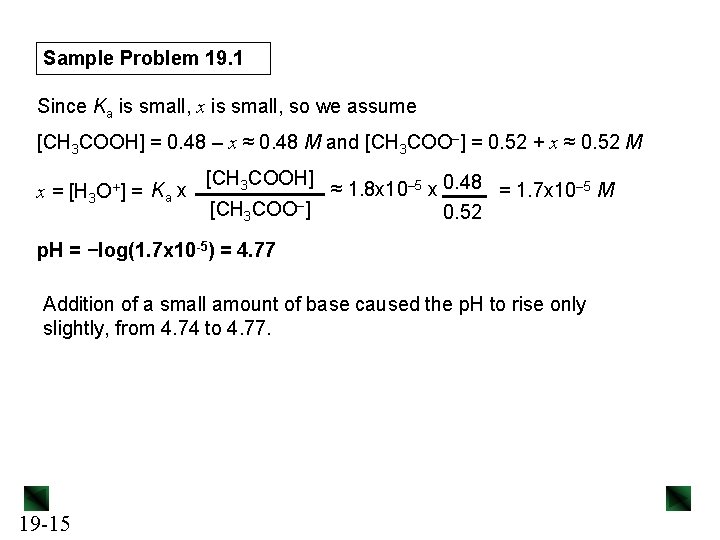
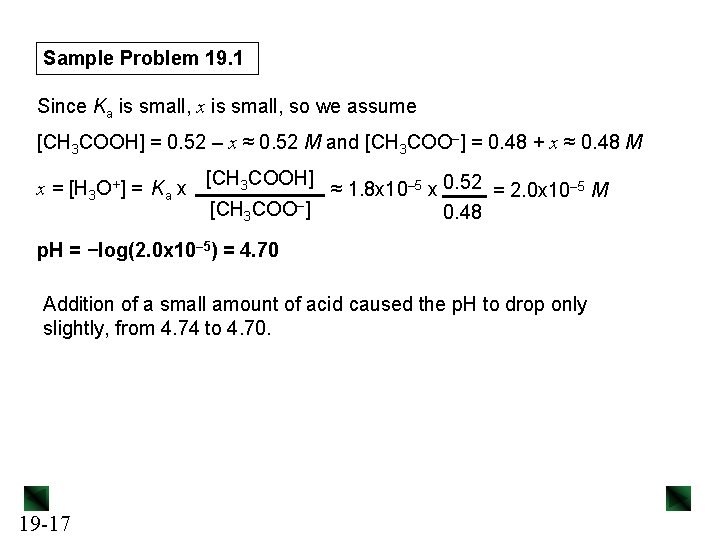
Sample Problem 19. 1 0. 020 mol = 0. 020 M H 3 O+ (c) [H 3 O+]added = 1. 0 L soln Setting up a reaction table for the stoichiometry: Concentration (M) CH 3 COO–(aq) + H 3 O+(aq) → CH 3 COOH(aq) + H 2 O(l) Initial Change Equilibrium 0. 50 − 0. 020 0. 50 +0. 020 - 0. 48 0 0. 52 - Setting up a reaction table for the acid dissociation, using new initial [ ]: Concentration (M) CH 3 COOH(aq) + H 2 O(l) Initial Change Equilibrium 19 -16 0. 52 −x 0. 52 - x - CH 3 COO–(aq) + H 3 O+(aq) 0. 48 +x 0. 48 + x 0 +x x

Sample Problem 19. 1 Since Ka is small, x is small, so we assume [CH 3 COOH] = 0. 52 – x ≈ 0. 52 M and [CH 3 COO–] = 0. 48 + x ≈ 0. 48 M [CH 3 COOH] x = [H 3 O+] = Ka x ≈ 1. 8 x 10– 5 x 0. 52 = 2. 0 x 10– 5 M [CH 3 COO–] 0. 48 p. H = −log(2. 0 x 10– 5) = 4. 70 Addition of a small amount of acid caused the p. H to drop only slightly, from 4. 74 to 4. 70. 19 -17

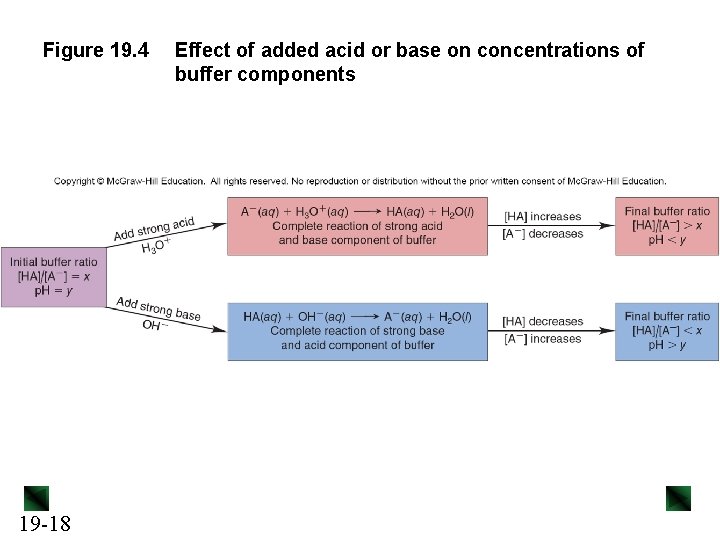
Figure 19. 4 19 -18 Effect of added acid or base on concentrations of buffer components
![The HendersonHasselbalch Equation HAaq H 2 Ol H 3 OA Ka HA The Henderson-Hasselbalch Equation HA(aq) + H 2 O(l) [H 3 O+][A–] Ka = [HA]](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-19.jpg)
The Henderson-Hasselbalch Equation HA(aq) + H 2 O(l) [H 3 O+][A–] Ka = [HA] -log[H 3 O+] A-(aq) + H 3 O+(aq) [H 3 O+] = Ka x [HA] = -log. Ka – log [A–] [base] p. H = p. Ka + log [acid] 19 -19 [HA] [A–]

Buffer Capacity The buffer capacity is a measure of the “strength” of the buffer, its ability to maintain the p. H following addition of strong acid or base. The greater the concentrations of the buffer components, the greater its capacity to resist p. H changes. The closer the component concentrations are to each other, the greater the buffer capacity. 19 -20

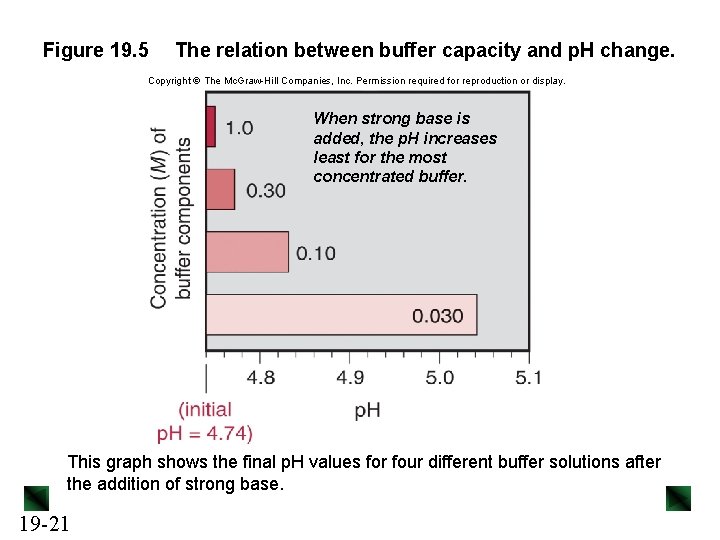
Figure 19. 5 The relation between buffer capacity and p. H change. Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. When strong base is added, the p. H increases least for the most concentrated buffer. This graph shows the final p. H values for four different buffer solutions after the addition of strong base. 19 -21

Buffer Range The buffer range is the p. H range over which the buffer is effective. Buffer range is related to the ratio of buffer component concentrations. [HA] The closer – is to 1, the more effective the buffer. [A ] If the concentration of one component is more than 10 times the concentration of the other, buffering action is poor. Since log 10 = 1, buffers have a usable range within ± 1 p. H unit of the p. Ka of the acid component. 19 -22

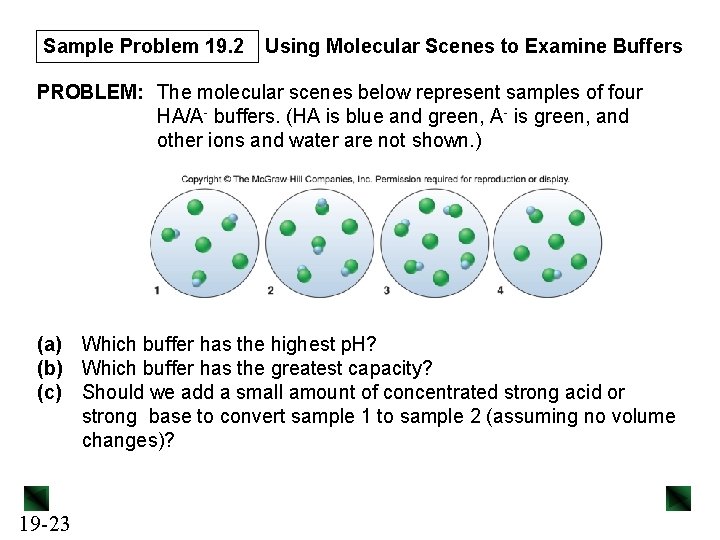
Sample Problem 19. 2 Using Molecular Scenes to Examine Buffers PROBLEM: The molecular scenes below represent samples of four HA/A- buffers. (HA is blue and green, A- is green, and other ions and water are not shown. ) (a) Which buffer has the highest p. H? (b) Which buffer has the greatest capacity? (c) Should we add a small amount of concentrated strong acid or strong base to convert sample 1 to sample 2 (assuming no volume changes)? 19 -23

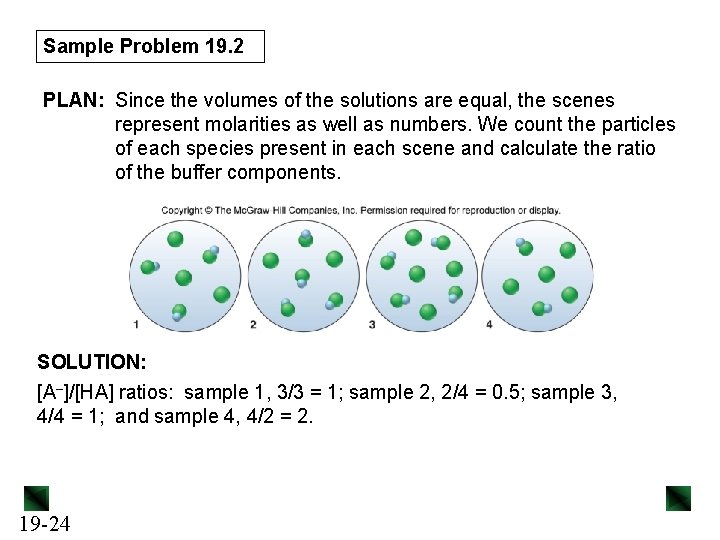
Sample Problem 19. 2 PLAN: Since the volumes of the solutions are equal, the scenes represent molarities as well as numbers. We count the particles of each species present in each scene and calculate the ratio of the buffer components. SOLUTION: [A–]/[HA] ratios: sample 1, 3/3 = 1; sample 2, 2/4 = 0. 5; sample 3, 4/4 = 1; and sample 4, 4/2 = 2. 19 -24

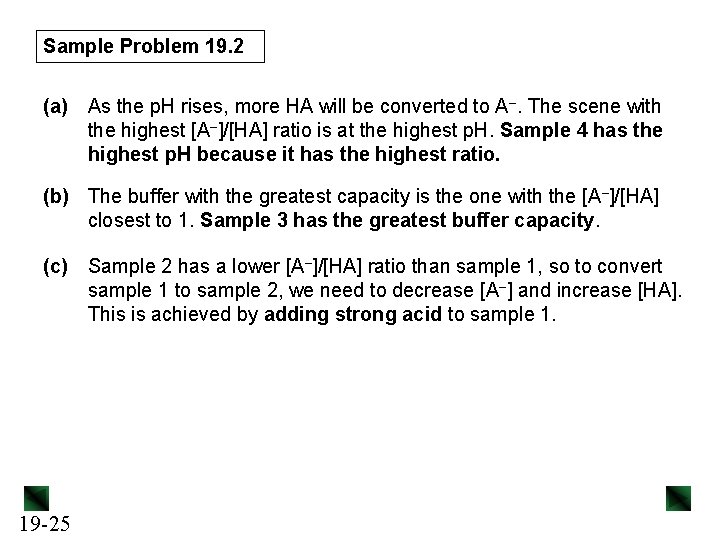
Sample Problem 19. 2 (a) As the p. H rises, more HA will be converted to A–. The scene with the highest [A–]/[HA] ratio is at the highest p. H. Sample 4 has the highest p. H because it has the highest ratio. (b) The buffer with the greatest capacity is the one with the [A–]/[HA] closest to 1. Sample 3 has the greatest buffer capacity. (c) 19 -25 Sample 2 has a lower [A–]/[HA] ratio than sample 1, so to convert sample 1 to sample 2, we need to decrease [A–] and increase [HA]. This is achieved by adding strong acid to sample 1.

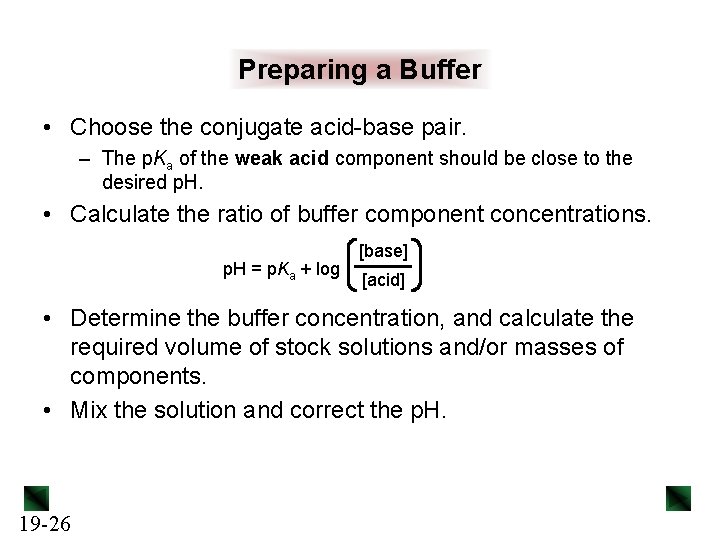
Preparing a Buffer • Choose the conjugate acid-base pair. – The p. Ka of the weak acid component should be close to the desired p. H. • Calculate the ratio of buffer component concentrations. p. H = p. Ka + log [base] [acid] • Determine the buffer concentration, and calculate the required volume of stock solutions and/or masses of components. • Mix the solution and correct the p. H. 19 -26

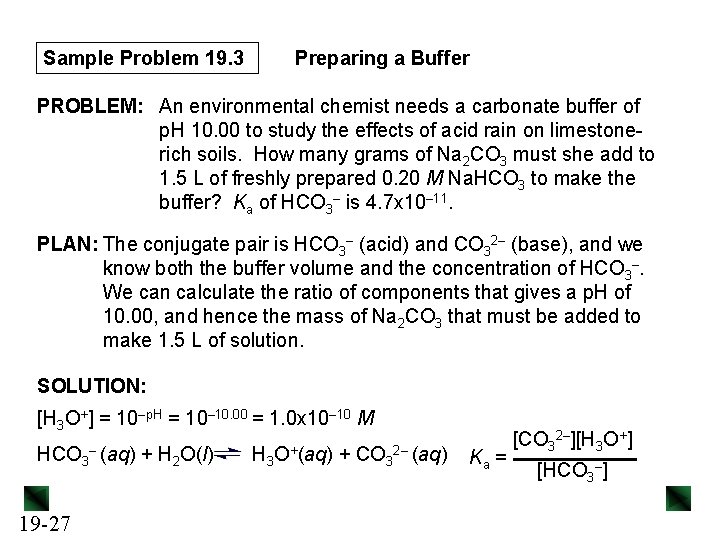
Sample Problem 19. 3 Preparing a Buffer PROBLEM: An environmental chemist needs a carbonate buffer of p. H 10. 00 to study the effects of acid rain on limestonerich soils. How many grams of Na 2 CO 3 must she add to 1. 5 L of freshly prepared 0. 20 M Na. HCO 3 to make the buffer? Ka of HCO 3– is 4. 7 x 10– 11. PLAN: The conjugate pair is HCO 3– (acid) and CO 32– (base), and we know both the buffer volume and the concentration of HCO 3–. We can calculate the ratio of components that gives a p. H of 10. 00, and hence the mass of Na 2 CO 3 that must be added to make 1. 5 L of solution. SOLUTION: [H 3 O+] = 10–p. H = 10– 10. 00 = 1. 0 x 10– 10 M HCO 3 19 -27 – (aq) + H 2 O(l) H 3 O+(aq) + CO 3 2– (aq) Ka = [CO 32–][H 3 O+] [HCO 3–]
![Sample Problem 19 3 CO 3 2 KaHCO 3 H 3 O Sample Problem 19. 3 [CO 3 2–] = Ka[HCO 3–] [H 3 O+ ]](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-28.jpg)
Sample Problem 19. 3 [CO 3 2–] = Ka[HCO 3–] [H 3 O+ ] Preparing a Buffer = (4. 7 x 10– 11)(0. 20) 1. 0 x 10– 10 = 0. 094 M 2– Amount (mol) of CO 32– needed = 1. 5 L soln x 0. 094 mol CO 3 1 L soln = 0. 14 mol CO 32– 0. 14 mol Na 2 CO 3 x 105. 99 g Na 2 CO 3 1 mol Na 2 CO 3 = 15 g Na 2 CO 3 The chemist should dissolve 15 g Na 2 CO 3 in about 1. 3 L of 0. 20 M Na. HCO 3 and add more 0. 20 M Na. HCO 3 to make 1. 5 L. Using a p. H meter, she can then adjust the p. H to 10. 00 by dropwise addition of concentrated strong acid or base. 19 -28

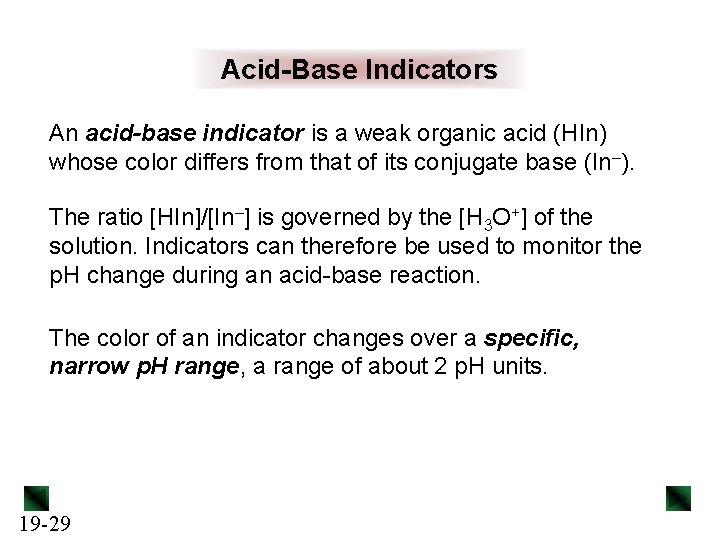
Acid-Base Indicators An acid-base indicator is a weak organic acid (HIn) whose color differs from that of its conjugate base (In–). The ratio [HIn]/[In–] is governed by the [H 3 O+] of the solution. Indicators can therefore be used to monitor the p. H change during an acid-base reaction. The color of an indicator changes over a specific, narrow p. H range, a range of about 2 p. H units. 19 -29

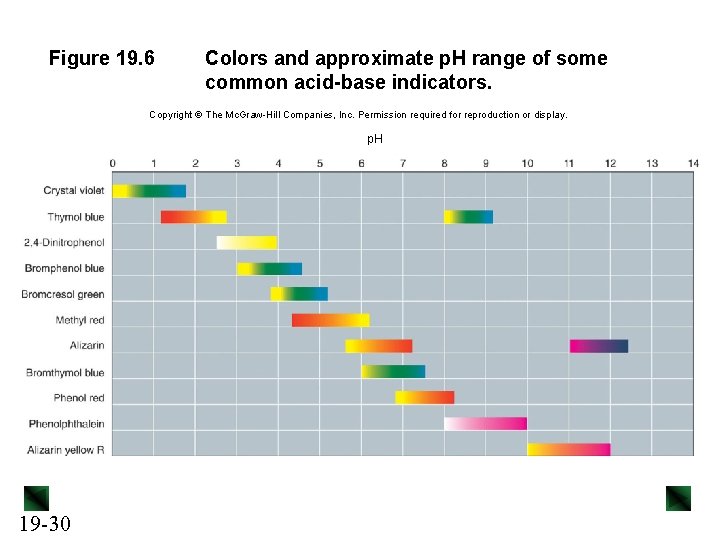
Figure 19. 6 Colors and approximate p. H range of some common acid-base indicators. Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. p. H 19 -30

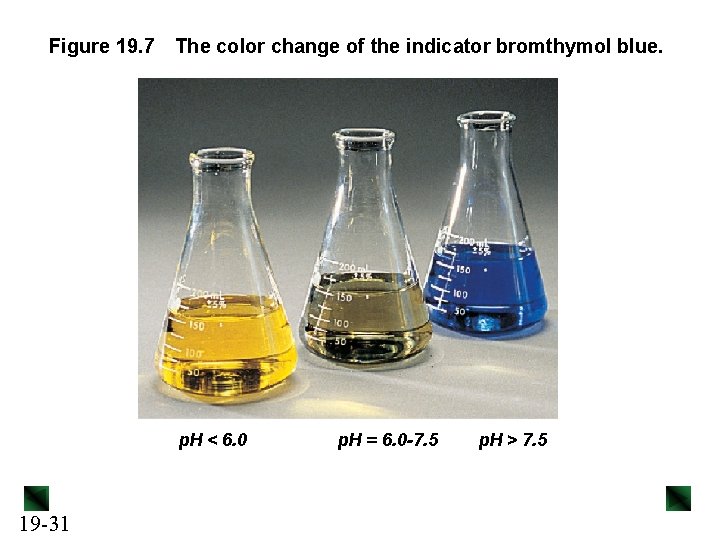
Figure 19. 7 The color change of the indicator bromthymol blue. p. H < 6. 0 19 -31 p. H = 6. 0 -7. 5 p. H > 7. 5

Acid-Base Titrations In an acid-base titration, the concentration of an acid (or a base) is determined by neutralizing the acid (or base) with a solution of base (or acid) of known concentration. The equivalence point of the reaction occurs when the number of moles of OH– added equals the number of moles of H 3 O+ originally present, or vice versa. The end point occurs when the indicator changes color. - The indicator should be selected so that its color change occurs at a p. H close to that of the equivalence point. 19 -32

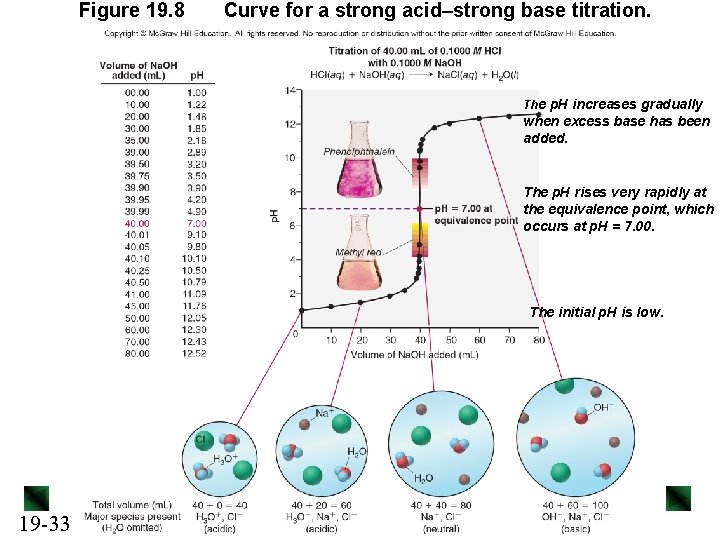
Figure 19. 8 Curve for a strong acid–strong base titration. The p. H increases gradually when excess base has been added. The p. H rises very rapidly at the equivalence point, which occurs at p. H = 7. 00. The initial p. H is low. 19 -33

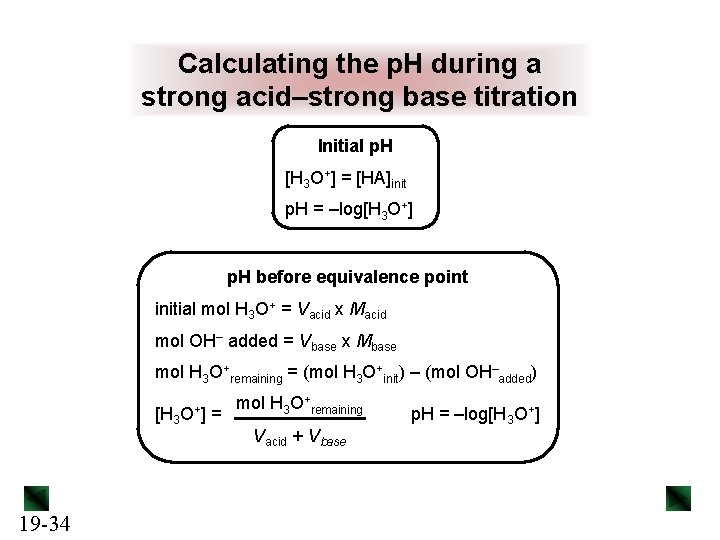
Calculating the p. H during a strong acid–strong base titration Initial p. H [H 3 O+] = [HA]init p. H = –log[H 3 O+] p. H before equivalence point initial mol H 3 O+ = Vacid x Macid mol OH– added = Vbase x Mbase mol H 3 O+remaining = (mol H 3 O+init) – (mol OH–added) [H 3 19 -34 O+ ] = mol H 3 O+remaining Vacid + Vbase p. H = –log[H 3 O+]

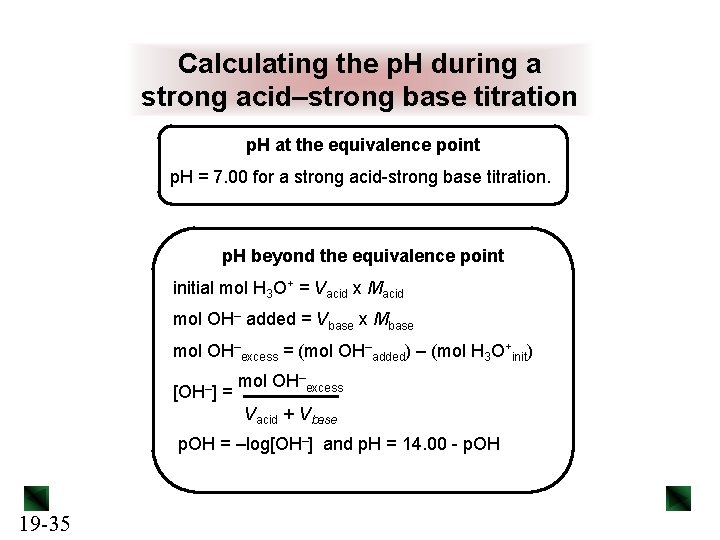
Calculating the p. H during a strong acid–strong base titration p. H at the equivalence point p. H = 7. 00 for a strong acid-strong base titration. p. H beyond the equivalence point initial mol H 3 O+ = Vacid x Macid mol OH– added = Vbase x Mbase mol OH–excess = (mol OH–added) – (mol H 3 O+init) [OH–] = mol OH–excess Vacid + Vbase p. OH = –log[OH–] and p. H = 14. 00 - p. OH 19 -35

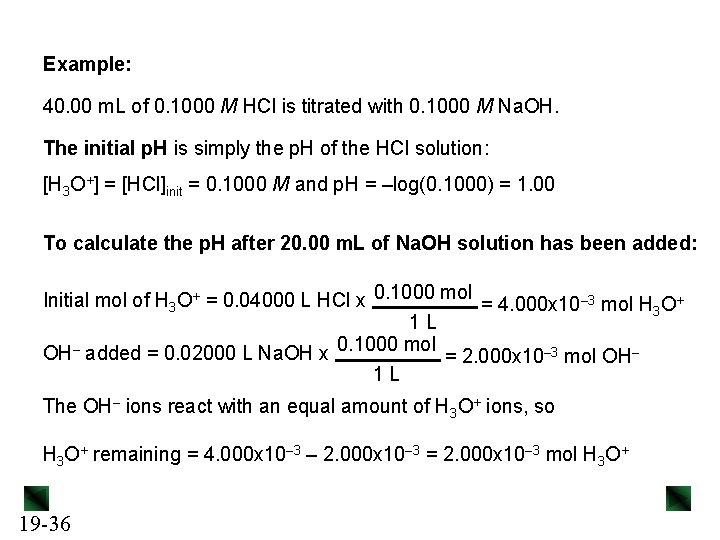
Example: 40. 00 m. L of 0. 1000 M HCl is titrated with 0. 1000 M Na. OH. The initial p. H is simply the p. H of the HCl solution: [H 3 O+] = [HCl]init = 0. 1000 M and p. H = –log(0. 1000) = 1. 00 To calculate the p. H after 20. 00 m. L of Na. OH solution has been added: Initial mol of H 3 O+ = 0. 04000 L HCl x 0. 1000 mol = 4. 000 x 10– 3 mol H 3 O+ 1 L OH– added = 0. 02000 L Na. OH x 0. 1000 mol = 2. 000 x 10– 3 mol OH– 1 L The OH– ions react with an equal amount of H 3 O+ ions, so H 3 O+ remaining = 4. 000 x 10– 3 – 2. 000 x 10– 3 = 2. 000 x 10– 3 mol H 3 O+ 19 -36
![H 3 O 2 000 x 10 3 mol 0 03333 [H 3 O+ ] 2. 000 x 10– 3 mol = = 0. 03333](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-37.jpg)
[H 3 O+ ] 2. 000 x 10– 3 mol = = 0. 03333 M 0. 04000 L + 0. 02000 L p. H = –log(0. 03333) = 1. 48 The equivalence point occurs when mol of OH– added = initial mol of HCl, so when 40. 00 m. L of Na. OH has been added. To calculate the p. H after 50. 00 m. L of Na. OH solution has been added: OH– added = 0. 05000 L Na. OH x 0. 1000 mol = 5. 000 x 10– 3 mol OH– 1 L OH– in excess = 5. 000 x 10– 3 – 4. 000 x 10– 3 = 1. 000 x 10– 3 mol OH– [OH–] = 1. 000 x 10– 3 mol = 0. 01111 M 0. 04000 L + 0. 05000 L p. OH = –log(0. 01111) = 1. 95 19 -37 p. H = 14. 00 – 1. 95 = 12. 05

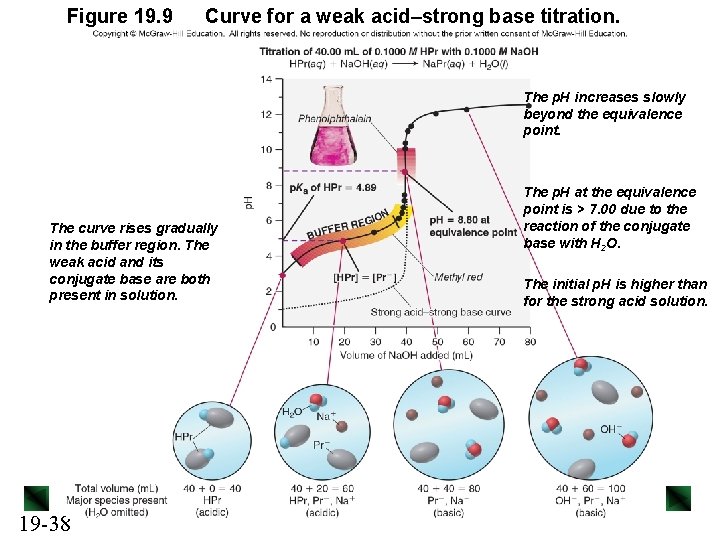
Figure 19. 9 Curve for a weak acid–strong base titration. The p. H increases slowly beyond the equivalence point. The curve rises gradually in the buffer region. The weak acid and its conjugate base are both present in solution. 19 -38 The p. H at the equivalence point is > 7. 00 due to the reaction of the conjugate base with H 2 O. The initial p. H is higher than for the strong acid solution.

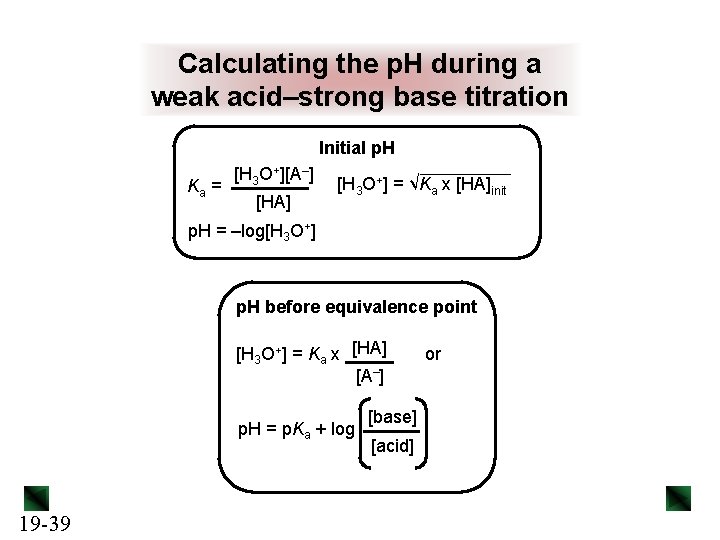
Calculating the p. H during a weak acid–strong base titration Initial p. H Ka = [H 3 O+][A–] [HA] [H 3 O+] = √Ka x [HA]init p. H = –log[H 3 O+] p. H before equivalence point [H 3 O+] = Ka x [HA] [A–] p. H = p. Ka + log 19 -39 [base] [acid] or

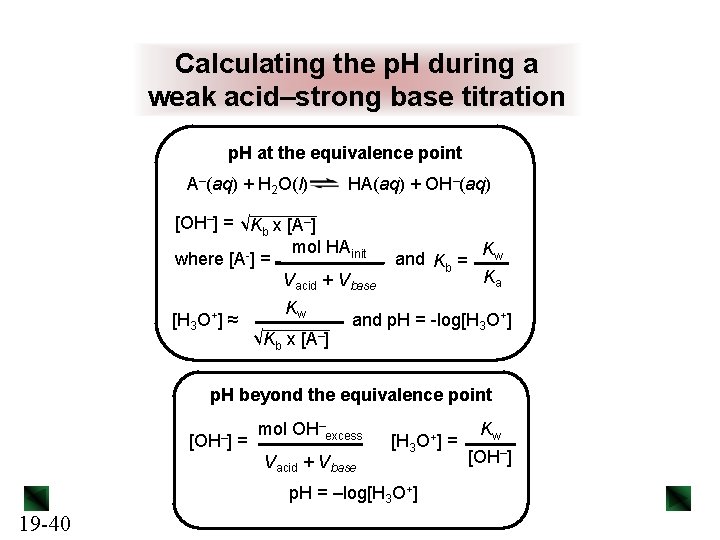
Calculating the p. H during a weak acid–strong base titration p. H at the equivalence point A–(aq) + H 2 O(l) HA(aq) + OH–(aq) [OH–] = √Kb x [A–] mol HAinit where [A-] = Vacid + Vbase [H 3 O+] ≈ Kw √Kb x [A–] and Kb = Kw Ka and p. H = -log[H 3 O+] p. H beyond the equivalence point [OH–] = mol OH–excess Vacid + Vbase [H 3 O+] = p. H = –log[H 3 O+] 19 -40 Kw [OH–]

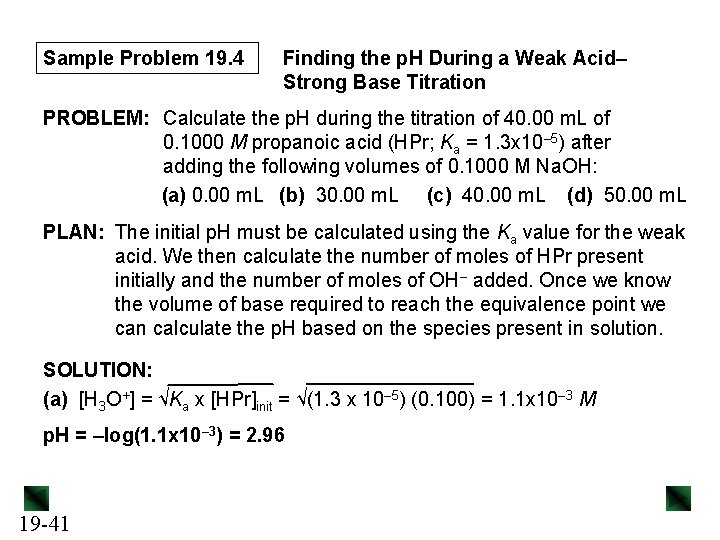
Sample Problem 19. 4 Finding the p. H During a Weak Acid– Strong Base Titration PROBLEM: Calculate the p. H during the titration of 40. 00 m. L of 0. 1000 M propanoic acid (HPr; Ka = 1. 3 x 10– 5) after adding the following volumes of 0. 1000 M Na. OH: (a) 0. 00 m. L (b) 30. 00 m. L (c) 40. 00 m. L (d) 50. 00 m. L PLAN: The initial p. H must be calculated using the Ka value for the weak acid. We then calculate the number of moles of HPr present initially and the number of moles of OH– added. Once we know the volume of base required to reach the equivalence point we can calculate the p. H based on the species present in solution. SOLUTION: (a) [H 3 O+] = √Ka x [HPr]init = √(1. 3 x 10– 5) (0. 100) = 1. 1 x 10– 3 M p. H = –log(1. 1 x 10– 3) = 2. 96 19 -41

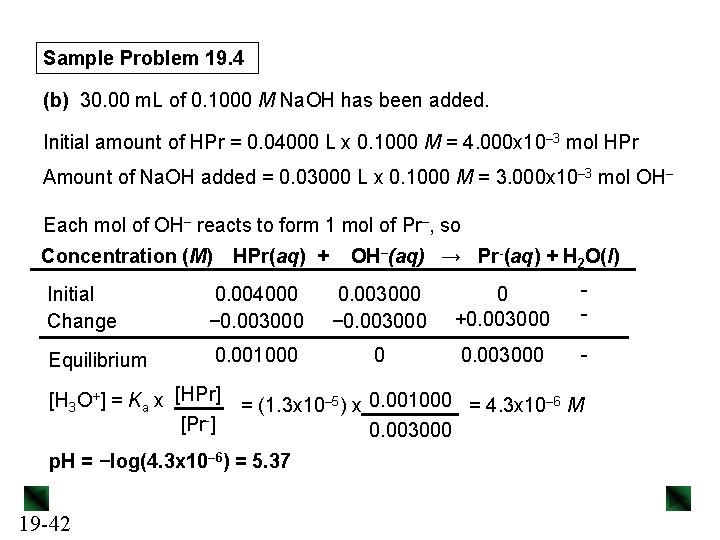
Sample Problem 19. 4 (b) 30. 00 m. L of 0. 1000 M Na. OH has been added. Initial amount of HPr = 0. 04000 L x 0. 1000 M = 4. 000 x 10– 3 mol HPr Amount of Na. OH added = 0. 03000 L x 0. 1000 M = 3. 000 x 10– 3 mol OH– Each mol of OH– reacts to form 1 mol of Pr–, so Concentration (M) HPr(aq) + OH–(aq) → Pr-(aq) + H 2 O(l) Initial Change 0. 004000 − 0. 003000 0 +0. 003000 - Equilibrium 0. 001000 0 0. 003000 - [H 3 O+] = Ka x [HPr] [Pr-] = (1. 3 x 10– 5) x 0. 001000 = 4. 3 x 10– 6 M 0. 003000 p. H = −log(4. 3 x 10– 6) = 5. 37 19 -42

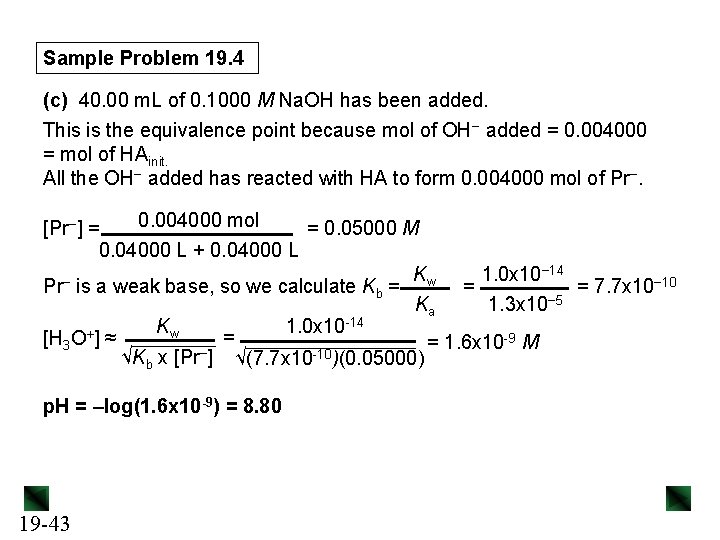
Sample Problem 19. 4 (c) 40. 00 m. L of 0. 1000 M Na. OH has been added. This is the equivalence point because mol of OH− added = 0. 004000 = mol of HAinit. All the OH− added has reacted with HA to form 0. 004000 mol of Pr−. 0. 004000 mol = 0. 05000 M [Pr−] = 0. 04000 L + 0. 04000 L Kw 1. 0 x 10− 14 − = Pr is a weak base, so we calculate Kb = = 7. 7 x 10− 10 Ka 1. 3 x 10− 5 Kw 1. 0 x 10 -14 + -9 M = [H 3 O ] ≈ = 1. 6 x 10 √Kb x [Pr−] √(7. 7 x 10 -10)(0. 05000) p. H = –log(1. 6 x 10 -9) = 8. 80 19 -43

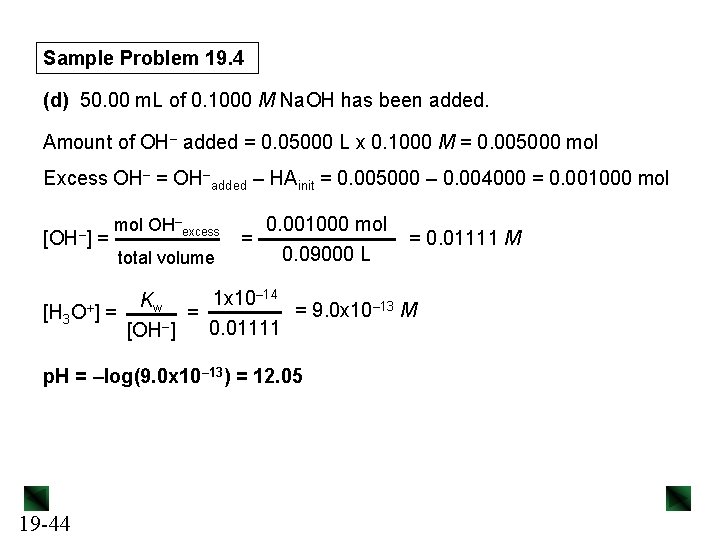
Sample Problem 19. 4 (d) 50. 00 m. L of 0. 1000 M Na. OH has been added. Amount of OH– added = 0. 05000 L x 0. 1000 M = 0. 005000 mol Excess OH– = OH–added – HAinit = 0. 005000 – 0. 004000 = 0. 001000 mol [OH–] = mol OH–excess total volume 0. 001000 mol = 0. 09000 L = 0. 01111 M – 14 1 x 10 K w = 9. 0 x 10– 13 M [H 3 O+] = = 0. 01111 [OH–] p. H = –log(9. 0 x 10– 13) = 12. 05 19 -44

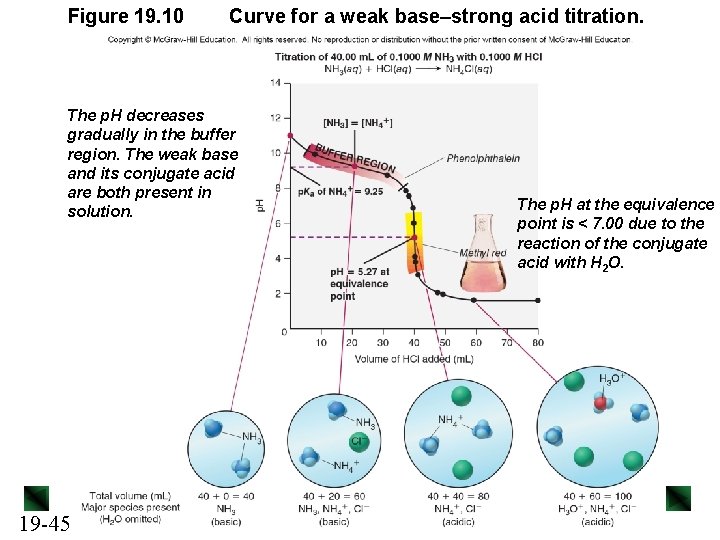
Figure 19. 10 Curve for a weak base–strong acid titration. The p. H decreases gradually in the buffer region. The weak base and its conjugate acid are both present in solution. 19 -45 The p. H at the equivalence point is < 7. 00 due to the reaction of the conjugate acid with H 2 O.

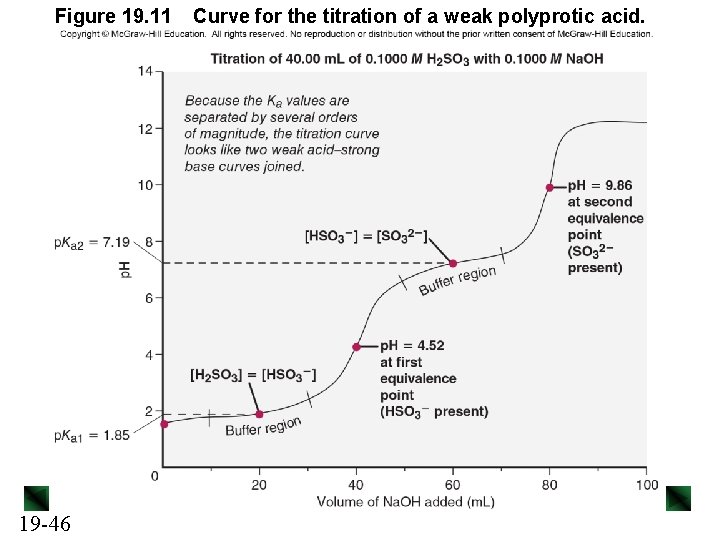
Figure 19. 11 19 -46 Curve for the titration of a weak polyprotic acid.

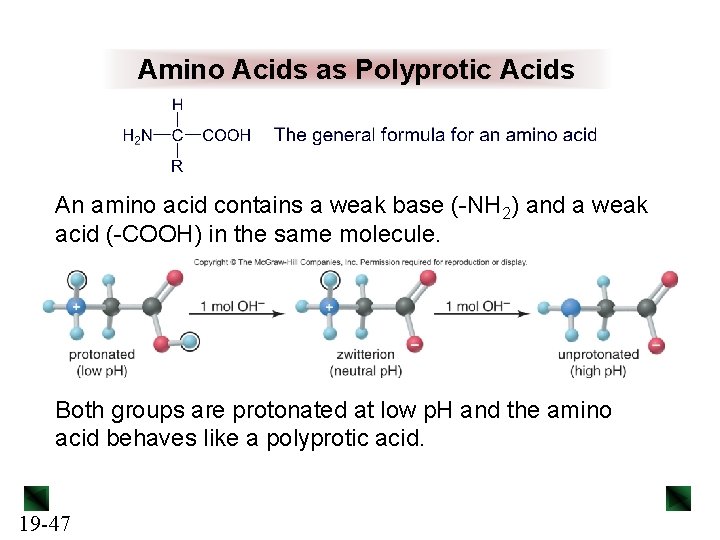
Amino Acids as Polyprotic Acids An amino acid contains a weak base (-NH 2) and a weak acid (-COOH) in the same molecule. Both groups are protonated at low p. H and the amino acid behaves like a polyprotic acid. 19 -47

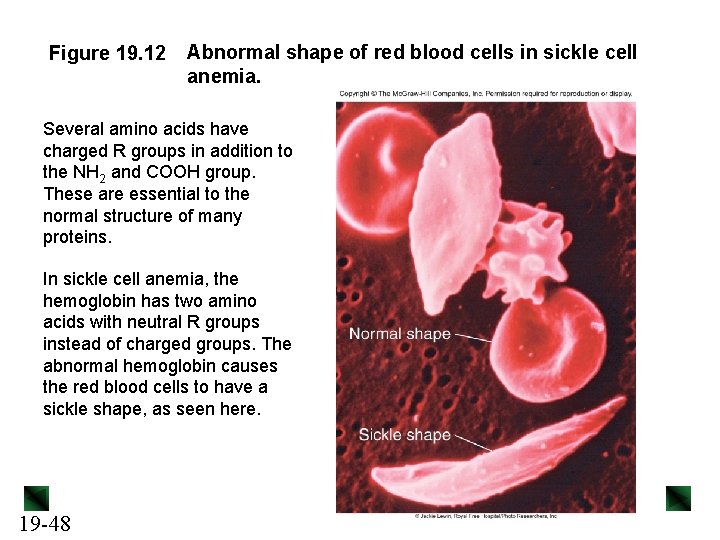
Figure 19. 12 Abnormal shape of red blood cells in sickle cell anemia. Several amino acids have charged R groups in addition to the NH 2 and COOH group. These are essential to the normal structure of many proteins. In sickle cell anemia, the hemoglobin has two amino acids with neutral R groups instead of charged groups. The abnormal hemoglobin causes the red blood cells to have a sickle shape, as seen here. 19 -48

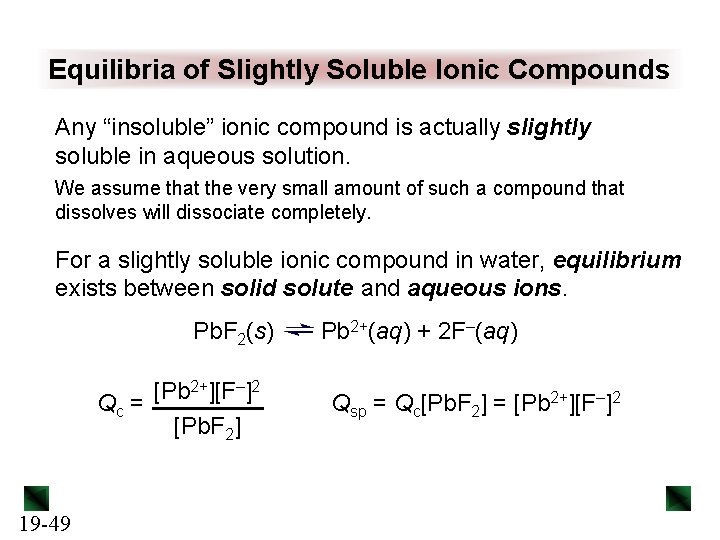
Equilibria of Slightly Soluble Ionic Compounds Any “insoluble” ionic compound is actually slightly soluble in aqueous solution. We assume that the very small amount of such a compound that dissolves will dissociate completely. For a slightly soluble ionic compound in water, equilibrium exists between solid solute and aqueous ions. Pb. F 2(s) [Pb 2+][F–]2 Qc = [Pb. F 2] 19 -49 Pb 2+(aq) + 2 F–(aq) Qsp = Qc[Pb. F 2] = [Pb 2+][F–]2

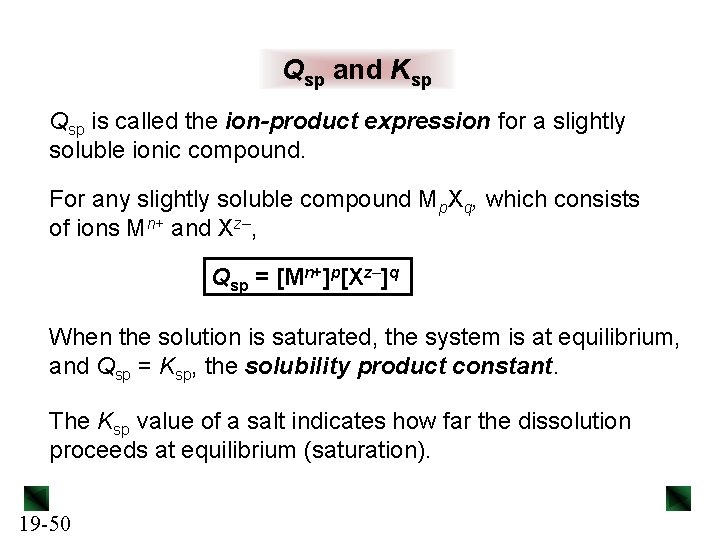
Qsp and Ksp Qsp is called the ion-product expression for a slightly soluble ionic compound. For any slightly soluble compound Mp. Xq, which consists of ions Mn+ and Xz–, Qsp = [Mn+]p[Xz–]q When the solution is saturated, the system is at equilibrium, and Qsp = Ksp, the solubility product constant. The Ksp value of a salt indicates how far the dissolution proceeds at equilibrium (saturation). 19 -50

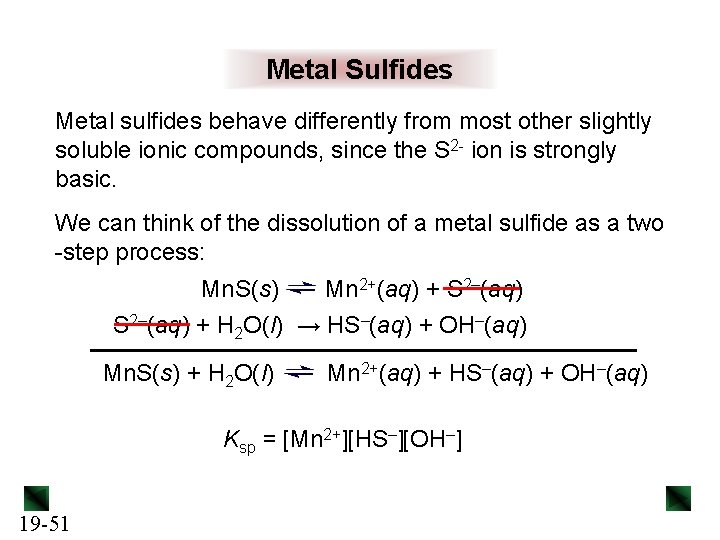
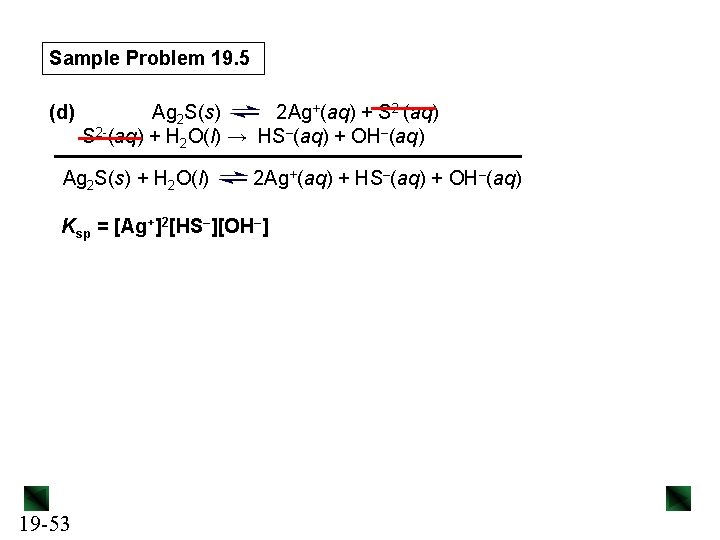
Metal Sulfides Metal sulfides behave differently from most other slightly soluble ionic compounds, since the S 2 - ion is strongly basic. We can think of the dissolution of a metal sulfide as a two -step process: Mn. S(s) Mn 2+(aq) + S 2–(aq) + H 2 O(l) → HS–(aq) + OH–(aq) Mn. S(s) + H 2 O(l) Mn 2+(aq) + HS–(aq) + OH–(aq) Ksp = [Mn 2+][HS–][OH–] 19 -51

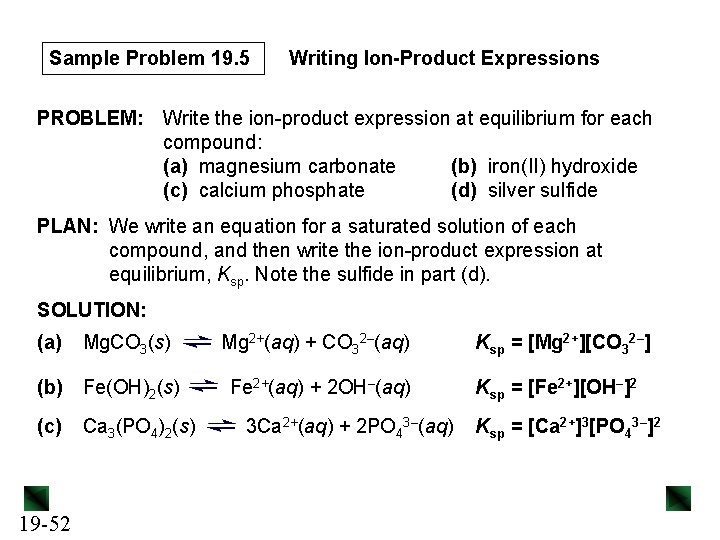
Sample Problem 19. 5 Writing Ion-Product Expressions PROBLEM: Write the ion-product expression at equilibrium for each compound: (a) magnesium carbonate (b) iron(II) hydroxide (c) calcium phosphate (d) silver sulfide PLAN: We write an equation for a saturated solution of each compound, and then write the ion-product expression at equilibrium, Ksp. Note the sulfide in part (d). SOLUTION: (a) Mg. CO 3(s) (b) Fe(OH)2(s) (c) 19 -52 Ca 3(PO 4)2(s) Mg 2+(aq) + CO 32–(aq) Fe 2+(aq) + 2 OH–(aq) 3 Ca 2+(aq) + 2 PO 43–(aq) Ksp = [Mg 2+][CO 32–] Ksp = [Fe 2+][OH–]2 Ksp = [Ca 2+]3[PO 43–]2

Sample Problem 19. 5 (d) Ag 2 S(s) 2 Ag+(aq) + S 2 -(aq) + H 2 O(l) → HS–(aq) + OH–(aq) Ag 2 S(s) + H 2 O(l) 2 Ag+(aq) + HS–(aq) + OH–(aq) Ksp = [Ag+]2[HS–][OH–] 19 -53

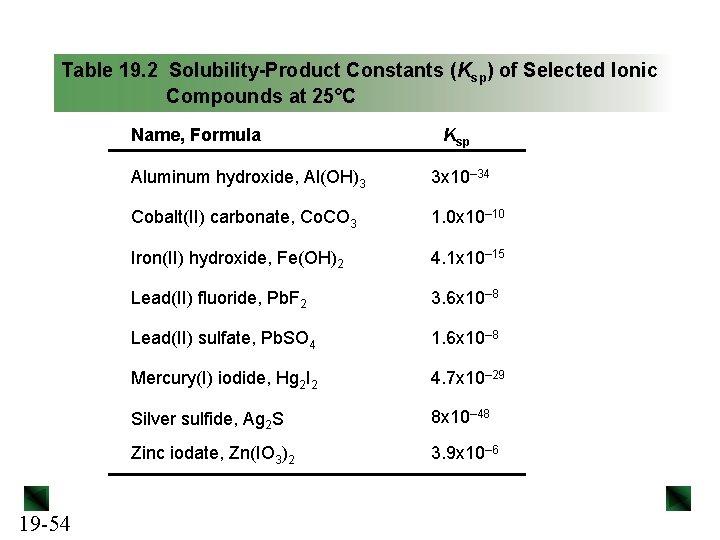
Table 19. 2 Solubility-Product Constants (Ksp) of Selected Ionic Compounds at 25°C Name, Formula 19 -54 Ksp Aluminum hydroxide, Al(OH)3 3 x 10– 34 Cobalt(II) carbonate, Co. CO 3 1. 0 x 10– 10 Iron(II) hydroxide, Fe(OH)2 4. 1 x 10– 15 Lead(II) fluoride, Pb. F 2 3. 6 x 10– 8 Lead(II) sulfate, Pb. SO 4 1. 6 x 10– 8 Mercury(I) iodide, Hg 2 I 2 4. 7 x 10– 29 Silver sulfide, Ag 2 S 8 x 10– 48 Zinc iodate, Zn(IO 3)2 3. 9 x 10– 6

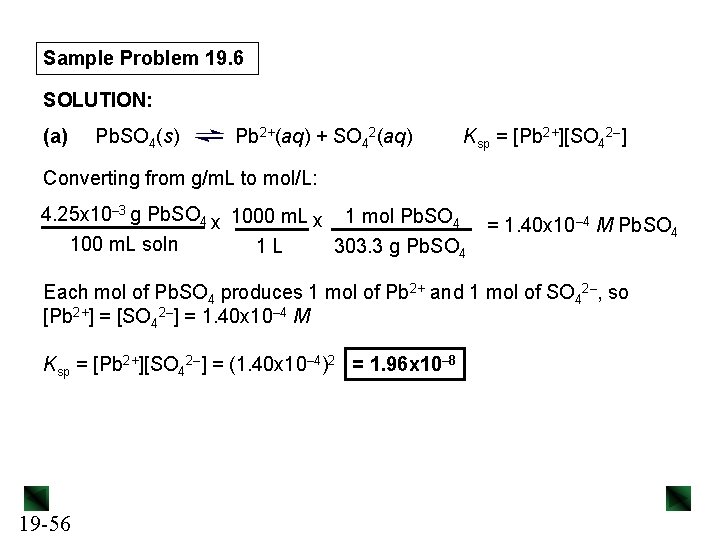
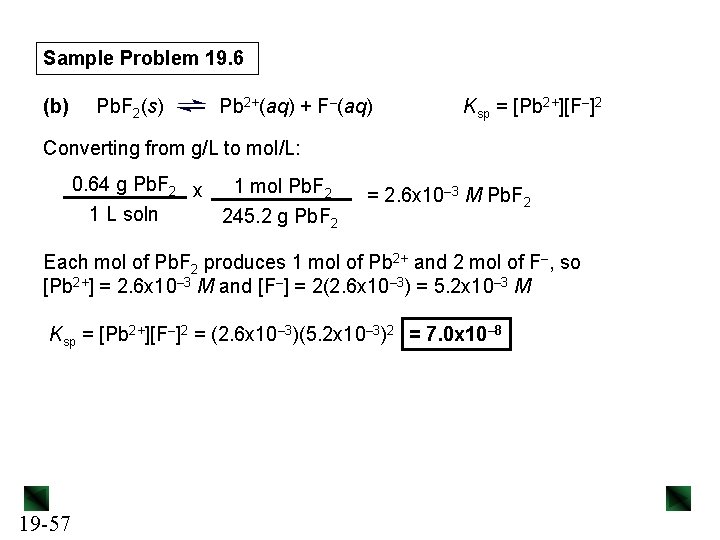
Sample Problem 19. 6 Determining Ksp from Solubility PROBLEM: (a) Lead(II) sulfate (Pb. SO 4) is a key component in leadacid car batteries. Its solubility in water at 25°C is 4. 25 x 10– 3 g/100 m. L solution. What is the Ksp of Pb. SO 4? (b) When lead(II) fluoride (Pb. F 2) is shaken with pure water at 25°C, the solubility is found to be 0. 64 g/L. Calculate the Ksp of Pb. F 2. PLAN: We write the dissolution equation and the ion-product expression for each compound. This tells us the number of moles of each ion formed. We use the molar mass to convert the solubility of the compound to molar solubility (molarity), then use it to find the molarity of each ion, which we can substitute into the Ksp expression. 19 -55

Sample Problem 19. 6 SOLUTION: (a) Pb. SO 4(s) Pb 2+(aq) + SO 42(aq) Ksp = [Pb 2+][SO 42–] Converting from g/m. L to mol/L: 4. 25 x 10– 3 g Pb. SO 4 x 1000 m. L x 1 mol Pb. SO 4 100 m. L soln 1 L 303. 3 g Pb. SO 4 = 1. 40 x 10– 4 M Pb. SO 4 Each mol of Pb. SO 4 produces 1 mol of Pb 2+ and 1 mol of SO 42–, so [Pb 2+] = [SO 42–] = 1. 40 x 10– 4 M Ksp = [Pb 2+][SO 42–] = (1. 40 x 10– 4)2 = 1. 96 x 10– 8 19 -56

Sample Problem 19. 6 (b) Pb. F 2(s) Pb 2+(aq) + F–(aq) Ksp = [Pb 2+][F–]2 Converting from g/L to mol/L: 0. 64 g Pb. F 2 x 1 mol Pb. F 2 1 L soln 245. 2 g Pb. F 2 = 2. 6 x 10– 3 M Pb. F 2 Each mol of Pb. F 2 produces 1 mol of Pb 2+ and 2 mol of F–, so [Pb 2+] = 2. 6 x 10– 3 M and [F–] = 2(2. 6 x 10– 3) = 5. 2 x 10– 3 M Ksp = [Pb 2+][F–]2 = (2. 6 x 10– 3)(5. 2 x 10– 3)2 = 7. 0 x 10– 8 19 -57

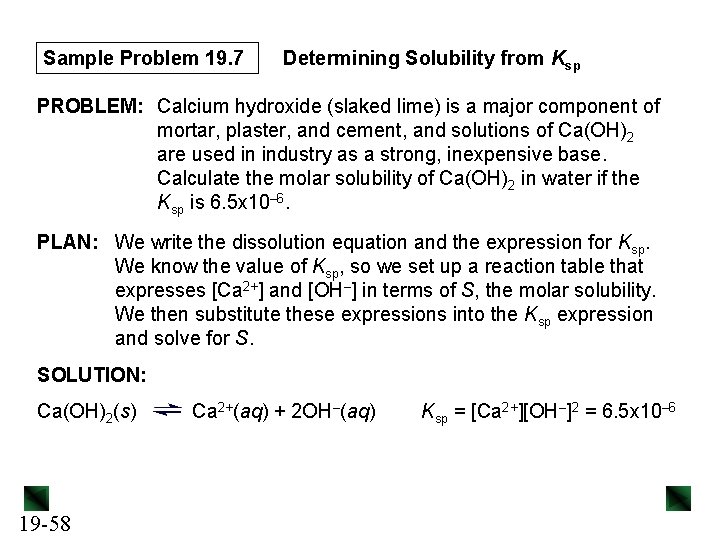
Sample Problem 19. 7 Determining Solubility from Ksp PROBLEM: Calcium hydroxide (slaked lime) is a major component of mortar, plaster, and cement, and solutions of Ca(OH)2 are used in industry as a strong, inexpensive base. Calculate the molar solubility of Ca(OH)2 in water if the Ksp is 6. 5 x 10– 6. PLAN: We write the dissolution equation and the expression for Ksp. We know the value of Ksp, so we set up a reaction table that expresses [Ca 2+] and [OH–] in terms of S, the molar solubility. We then substitute these expressions into the Ksp expression and solve for S. SOLUTION: Ca(OH)2(s) 19 -58 Ca 2+(aq) + 2 OH–(aq) Ksp = [Ca 2+][OH–]2 = 6. 5 x 10– 6

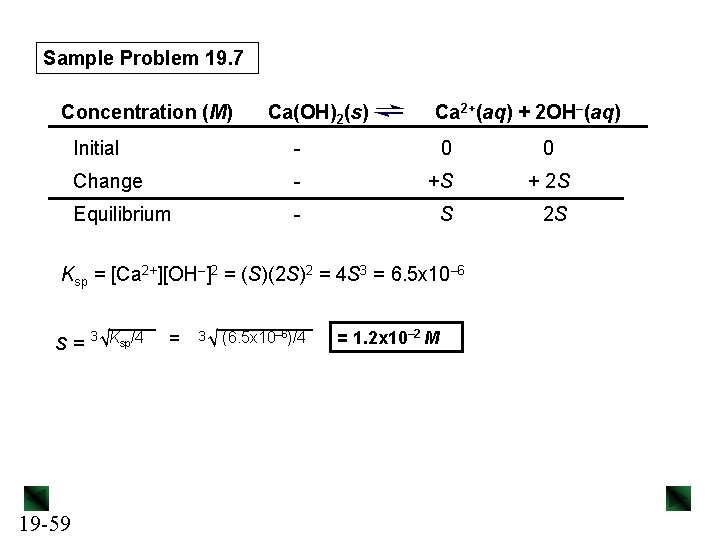
Sample Problem 19. 7 Concentration (M) Ca(OH)2(s) Ca 2+(aq) + 2 OH–(aq) Initial - 0 Change - +S + 2 S Equilibrium - S 2 S Ksp = [Ca 2+][OH–]2 = (S)(2 S)2 = 4 S 3 = 6. 5 x 10– 6 S = 3√Ksp/4 19 -59 = 3√ (6. 5 x 10– 6)/4 = 1. 2 x 10– 2 M 0

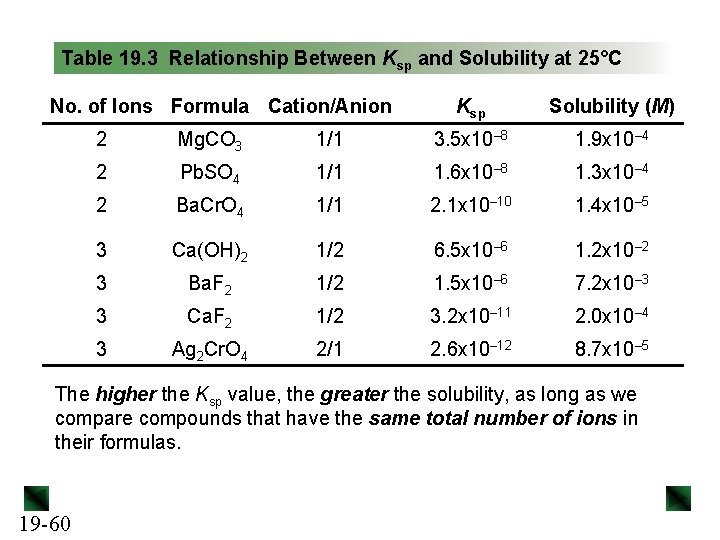
Table 19. 3 Relationship Between Ksp and Solubility at 25°C No. of Ions Formula Cation/Anion Ksp Solubility (M) 2 Mg. CO 3 1/1 3. 5 x 10– 8 1. 9 x 10– 4 2 Pb. SO 4 1/1 1. 6 x 10– 8 1. 3 x 10– 4 2 Ba. Cr. O 4 1/1 2. 1 x 10– 10 1. 4 x 10– 5 3 Ca(OH)2 1/2 6. 5 x 10– 6 1. 2 x 10– 2 3 Ba. F 2 1/2 1. 5 x 10– 6 7. 2 x 10– 3 3 Ca. F 2 1/2 3. 2 x 10– 11 2. 0 x 10– 4 3 Ag 2 Cr. O 4 2/1 2. 6 x 10– 12 8. 7 x 10– 5 The higher the Ksp value, the greater the solubility, as long as we compare compounds that have the same total number of ions in their formulas. 19 -60

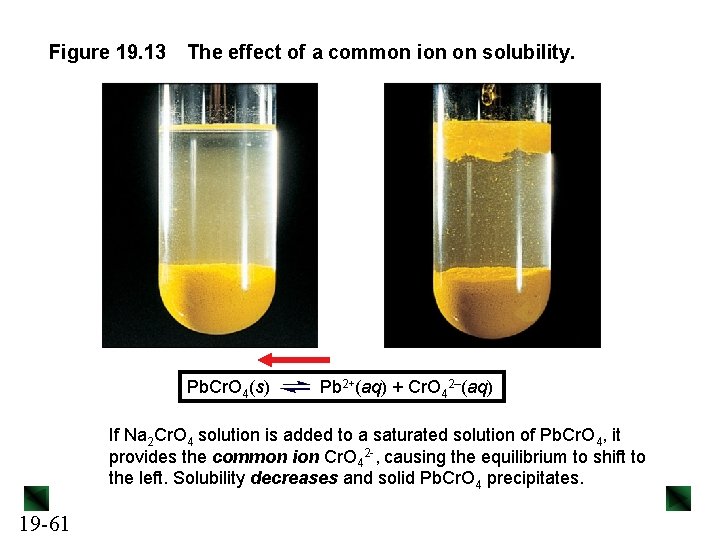
Figure 19. 13 The effect of a common ion on solubility. Pb. Cr. O 4(s) Pb 2+(aq) + Cr. O 42–(aq) If Na 2 Cr. O 4 solution is added to a saturated solution of Pb. Cr. O 4, it provides the common ion Cr. O 42 -, causing the equilibrium to shift to the left. Solubility decreases and solid Pb. Cr. O 4 precipitates. 19 -61

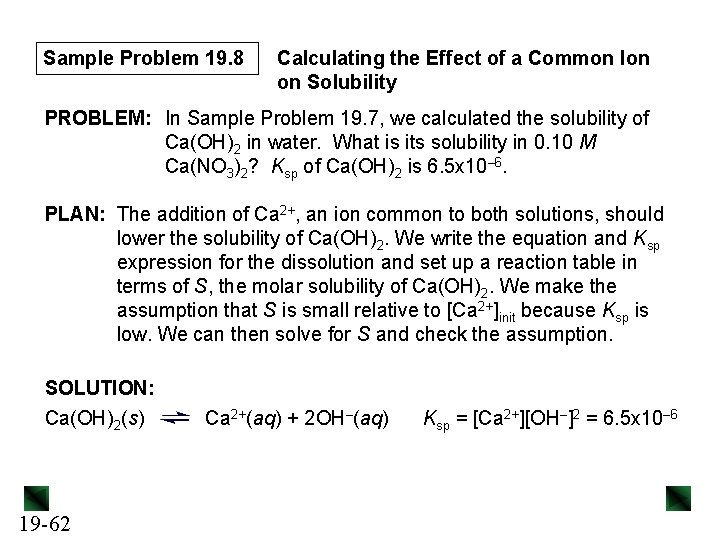
Sample Problem 19. 8 Calculating the Effect of a Common Ion on Solubility PROBLEM: In Sample Problem 19. 7, we calculated the solubility of Ca(OH)2 in water. What is its solubility in 0. 10 M Ca(NO 3)2? Ksp of Ca(OH)2 is 6. 5 x 10– 6. PLAN: The addition of Ca 2+, an ion common to both solutions, should lower the solubility of Ca(OH)2. We write the equation and Ksp expression for the dissolution and set up a reaction table in terms of S, the molar solubility of Ca(OH)2. We make the assumption that S is small relative to [Ca 2+]init because Ksp is low. We can then solve for S and check the assumption. SOLUTION: Ca(OH)2(s) 19 -62 Ca 2+(aq) + 2 OH–(aq) Ksp = [Ca 2+][OH–]2 = 6. 5 x 10– 6
![Sample Problem 19 8 Ca 2init 0 10 M because CaNO 32 is Sample Problem 19. 8 [Ca 2+]init = 0. 10 M because Ca(NO 3)2 is](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-63.jpg)
Sample Problem 19. 8 [Ca 2+]init = 0. 10 M because Ca(NO 3)2 is a soluble salt, and dissociates completely in solution. Concentration (M) Ca(OH)2(s) Ca 2+(aq) + 2 OH–(aq) Initial - 0. 10 0 Change - +S + 2 S Equilibrium - 0. 10 + S 2 S Ksp = [Ca 2+][OH–]2 = 6. 5 x 10– 6 ≈ (0. 10)(2 S)2 = (0. 10)(4 S 2) 4 S 2 ≈ 6. 5 x 10– 6 0. 10 so S ≈ √(6. 5 x 10 -5)/4 = 4. 0 x 10– 3 M -3 Checking the assumption: 4. 0 x 10 M x 100 = 4. 0% < 5% 0. 10 M 19 -63

Effect of p. H on Solubility Changes in p. H affects the solubility of many slightly soluble ionic compounds. The addition of H 3 O+ will increase the solubility of a salt that contains the anion of a weak acid. Ca. CO 3(s) Ca 2+(aq) + CO 32–(aq) + H 3 O+(aq) → HCO 3–(aq) + H 2 O(l) HCO 3–(aq) + H 3 O+(aq) → [H 2 CO 3(aq)] + H 2 O(l) → CO 2(g) + 2 H 2 O(l) The net effect of adding H 3 O+ to Ca. CO 3 is the removal of CO 32– ions, which causes an equilibrium shift to the right. More Ca. CO 3 will dissolve. 19 -64

Figure 19. 14 Test for the presence of a carbonate. When a carbonate mineral is treated with HCl, bubbles of CO 2 form. 19 -65

Sample Problem 19. 9 Predicting the Effect on Solubility of Adding Strong Acid PROBLEM: Write balanced equations to explain whether addition of H 3 O+ from a strong acid affects the solubility of each ionic compound: (a) lead(II) bromide (b) copper(II) hydroxide (c) iron(II) sulfide PLAN: We write the balanced dissolution equation for each compound and note the anion. The anion of a weak acid reacts with H 3 O+, causing an increase in solubility. SOLUTION: (a) Pb. Br 2(s) Pb 2+(aq) + 2 Br–(aq) Br– is the anion of HBr, a strong acid, so it does not react with H 3 O+. The addition of strong acid has no effect on its solubility. 19 -66

Sample Problem 19. 9 (b) Cu(OH)2(s) Cu 2+(aq) + 2 OH–(aq) OH– is the anion of H 2 O, a very weak acid, and is in fact a strong base. It will react with H 3 O+: OH–(aq) + H 3 O+(aq) → 2 H 2 O(l) The addition of strong acid will cause an increase in solubility. (c) Fe. S(s) Fe 2+(aq) + S 2–(aq) S 2– is the anion of HS–, a weak acid, and is a strong base. It will react completely with water to form HS– and OH–. Both these ions will react with added H 3 O+: HS–(aq) + H 3 O+(aq) → H 2 S(aq) + H 2 O(l) OH–(aq) + H 3 O+(aq) → 2 H 2 O(l) The addition of strong acid will cause an increase in solubility. 19 -67

Figure 19. 15 Limestone cave in Nerja, Málaga, Spain. Limestone is mostly Ca. CO 3 (Ksp = 3. 3 x 10– 9). Ground water rich in CO 2 trickles over Ca. CO 3, causing it to dissolve. This gradually carves out a cave. Water containing HCO 3 - and Ca 2+ ions drips from the cave ceiling. The air has a lower PCO than the soil, causing CO 2 2 to come out of solution. A shift in equilibrium results in the precipitation of Ca. CO 3 to form stalagmites and stalactites. CO 2(g) CO 2(aq) + 2 H 2 O(l) H 3 O+(aq) + HCO 3–(aq) Ca. CO 3(s) + CO 2(aq) + H 2 O(l) 19 -68 Ca 2+(aq) + 2 HCO 3– (aq)

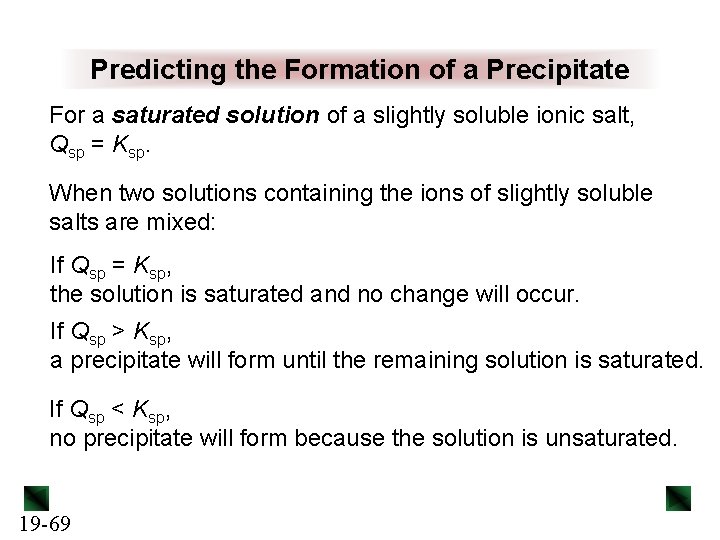
Predicting the Formation of a Precipitate For a saturated solution of a slightly soluble ionic salt, Qsp = Ksp. When two solutions containing the ions of slightly soluble salts are mixed: If Qsp = Ksp, the solution is saturated and no change will occur. If Qsp > Ksp, a precipitate will form until the remaining solution is saturated. If Qsp < Ksp, no precipitate will form because the solution is unsaturated. 19 -69

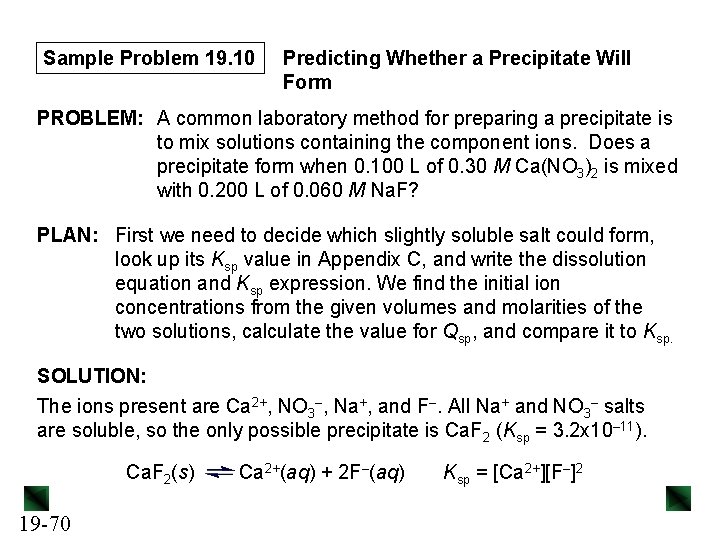
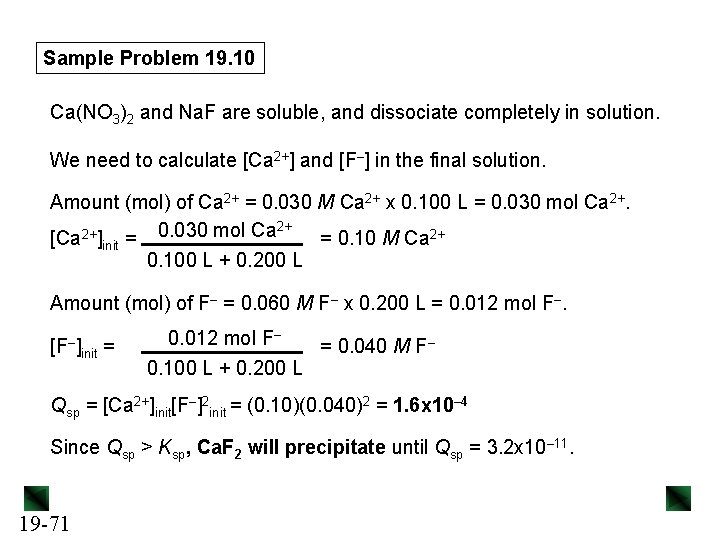
Sample Problem 19. 10 Predicting Whether a Precipitate Will Form PROBLEM: A common laboratory method for preparing a precipitate is to mix solutions containing the component ions. Does a precipitate form when 0. 100 L of 0. 30 M Ca(NO 3)2 is mixed with 0. 200 L of 0. 060 M Na. F? PLAN: First we need to decide which slightly soluble salt could form, look up its Ksp value in Appendix C, and write the dissolution equation and Ksp expression. We find the initial ion concentrations from the given volumes and molarities of the two solutions, calculate the value for Qsp, and compare it to Ksp. SOLUTION: The ions present are Ca 2+, NO 3–, Na+, and F–. All Na+ and NO 3– salts are soluble, so the only possible precipitate is Ca. F 2 (Ksp = 3. 2 x 10– 11). Ca. F 2(s) 19 -70 Ca 2+(aq) + 2 F–(aq) Ksp = [Ca 2+][F–]2

Sample Problem 19. 10 Ca(NO 3)2 and Na. F are soluble, and dissociate completely in solution. We need to calculate [Ca 2+] and [F–] in the final solution. Amount (mol) of Ca 2+ = 0. 030 M Ca 2+ x 0. 100 L = 0. 030 mol Ca 2+. 2+ 0. 030 mol Ca 2+ [Ca ]init = = 0. 10 M Ca 2+ 0. 100 L + 0. 200 L Amount (mol) of F– = 0. 060 M F– x 0. 200 L = 0. 012 mol F–. [F–] init = 0. 012 mol F– = 0. 040 M F– 0. 100 L + 0. 200 L Qsp = [Ca 2+]init[F–]2 init = (0. 10)(0. 040)2 = 1. 6 x 10– 4 Since Qsp > Ksp, Ca. F 2 will precipitate until Qsp = 3. 2 x 10– 11. 19 -71

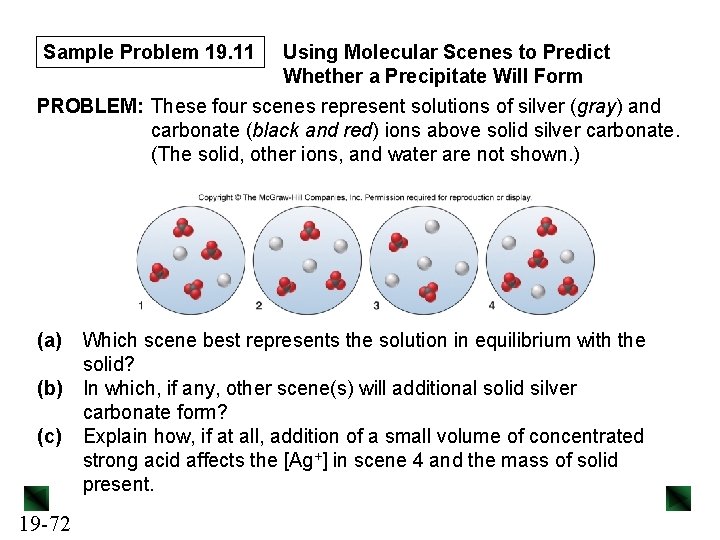
Sample Problem 19. 11 Using Molecular Scenes to Predict Whether a Precipitate Will Form PROBLEM: These four scenes represent solutions of silver (gray) and carbonate (black and red) ions above solid silver carbonate. (The solid, other ions, and water are not shown. ) (a) Which scene best represents the solution in equilibrium with the solid? (b) In which, if any, other scene(s) will additional solid silver carbonate form? (c) Explain how, if at all, addition of a small volume of concentrated strong acid affects the [Ag+] in scene 4 and the mass of solid present. 19 -72

Sample Problem 19. 11 PLAN: We need to determine the ratio of the different types of ion in each solution. A saturated solution of Ag 2 CO 3 should have 2 Ag+ ions for every 1 CO 32– ion. For (b) we need to compare Qsp to Ksp. For (c) we recall that CO 32– reacts with H 3 O+. SOLUTION: First we determine the Ag+/CO 32– ratios for each scene. Scene 1: 2/4 or 1/2 Scene 2: 3/3 or 1/1 Scene 3: 4/2 or 2/1 Scene 4: 3/4 (a) Scene 3 is the only one that has an Ag+/CO 32 - ratio of 2/1, so this scene represents the solution in equilibrium with the solid. 19 -73

Sample Problem 19. 11 (b) We use the ion count for each solution to determine Qsp for each one. Since Scene 3 is at equilibrium, its Qsp value = Ksp. Scene 1: Qsp = (2)2(4) = 16 Scene 2: Qsp = (3)2(3) = 27 Scene 3: Qsp = (4)2(2) = 32 Scene 4: Qsp = (3)2(4) = 36 Scene 4 is the only one that has Qsp > Ksp, so a precipitate forms in this solution. (c) Ag 2 CO 3(s) 2 Ag+(aq) + CO 32–(aq) + 2 H 3 O+(aq) → [H 2 CO 3(aq)] + 2 H 2 O(l) → 3 H 2 O(l) + CO 2(g) The CO 2 leaves as a gas, so adding H 3 O+ decreases the [CO 32–] in solution, causing more Ag 2 CO 3 to dissolve. [Ag+] increases and the mass of Ag 2 CO 3 decreases. 19 -74

Selective Precipitation Selective precipitation is used to separate a solution containing a mixture of ions. A precipitating ion is added to the solution until the Qsp of the more soluble compound is almost equal to its Ksp. The less soluble compound will precipitate in as large a quantity as possible, leaving behind the ion of the more soluble compound. 19 -75

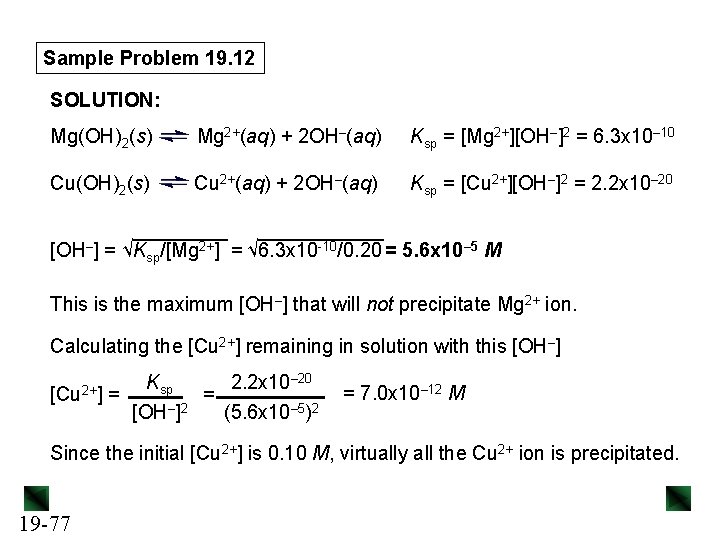
Sample Problem 19. 12 Separating Ions by Selective Precipitation PROBLEM: A solution consists of 0. 20 M Mg. Cl 2 and 0. 10 M Cu. Cl 2. Calculate the [OH–] that would separate the metal ions as their hydroxides. Ksp of Mg(OH)2= is 6. 3 x 10– 10; Ksp of Cu(OH)2 is 2. 2 x 10– 20. PLAN: Both compounds have 1/2 ratios of cation/anion, so we can compare their solubilities by comparing their Ksp values. Mg(OH)2 is 1010 times more soluble than Cu(OH)2, so Cu(OH)2 will precipitate first. We write the dissolution equations and Ksp expressions. Using the given cation concentrations, we solve for the [OH–] that gives a saturated solution of Mg(OH)2. Then we calculate the [Cu 2+] remaining to see if the separation was successful. 19 -76

Sample Problem 19. 12 SOLUTION: Mg(OH)2(s) Mg 2+(aq) + 2 OH–(aq) Ksp = [Mg 2+][OH–]2 = 6. 3 x 10– 10 Cu(OH)2(s) Cu 2+(aq) + 2 OH–(aq) Ksp = [Cu 2+][OH–]2 = 2. 2 x 10– 20 [OH–] = √Ksp/[Mg 2+] = √ 6. 3 x 10 -10/0. 20 = 5. 6 x 10– 5 M This is the maximum [OH–] that will not precipitate Mg 2+ ion. Calculating the [Cu 2+] remaining in solution with this [OH–] [Cu 2+] Ksp 2. 2 x 10– 20 = = – 2 [OH ] (5. 6 x 10– 5)2 = 7. 0 x 10– 12 M Since the initial [Cu 2+] is 0. 10 M, virtually all the Cu 2+ ion is precipitated. 19 -77

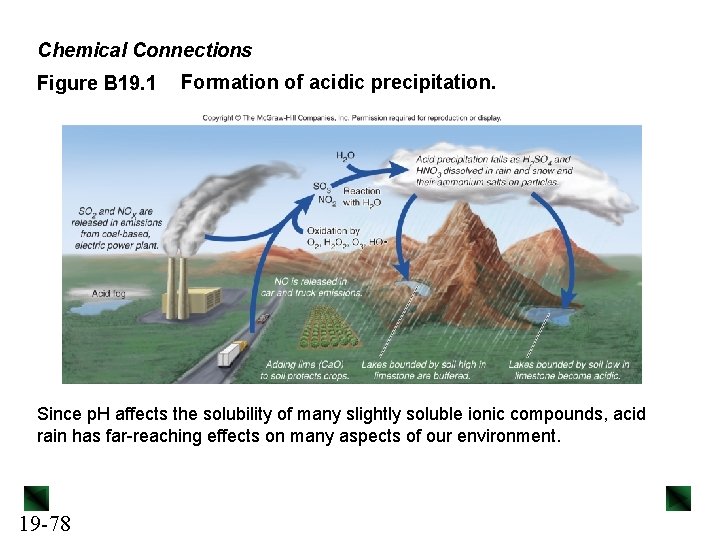
Chemical Connections Figure B 19. 1 Formation of acidic precipitation. Since p. H affects the solubility of many slightly soluble ionic compounds, acid rain has far-reaching effects on many aspects of our environment. 19 -78

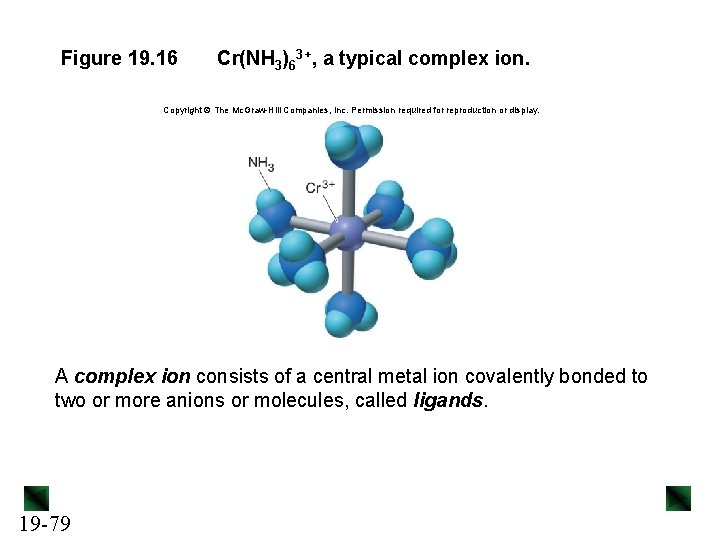
Figure 19. 16 Cr(NH 3)63+, a typical complex ion. Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. A complex ion consists of a central metal ion covalently bonded to two or more anions or molecules, called ligands. 19 -79

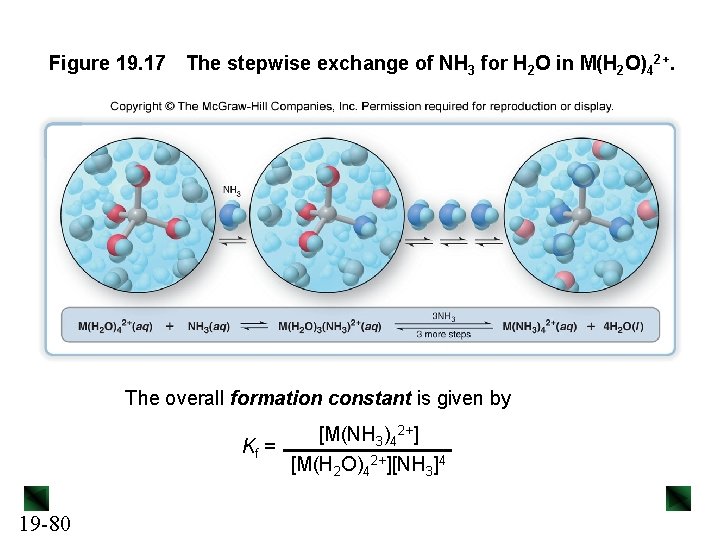
Figure 19. 17 The stepwise exchange of NH 3 for H 2 O in M(H 2 O)42+. The overall formation constant is given by [M(NH 3)42+] Kf = [M(H 2 O)42+][NH 3]4 19 -80

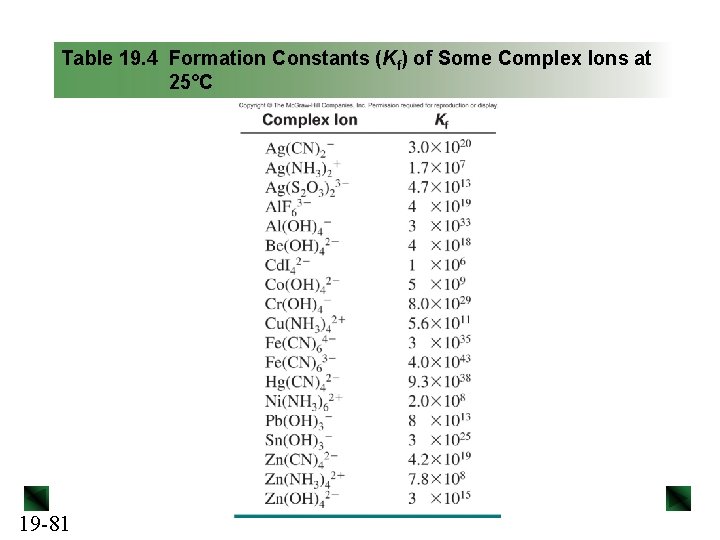
Table 19. 4 Formation Constants (Kf) of Some Complex Ions at 25°C 19 -81

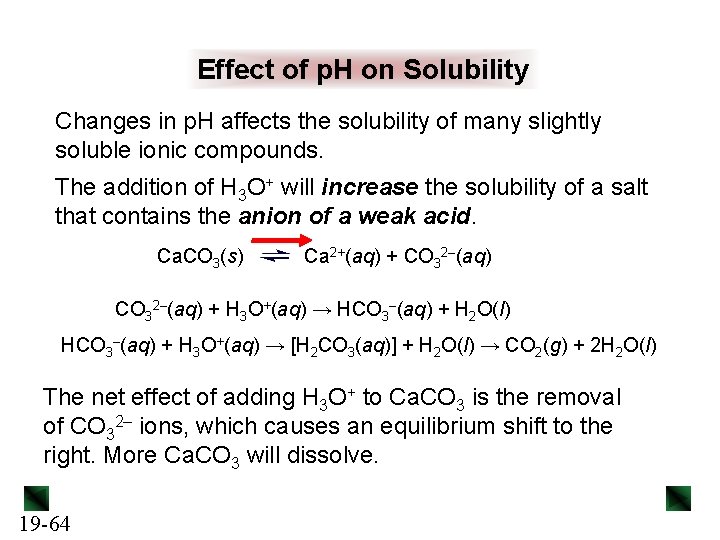
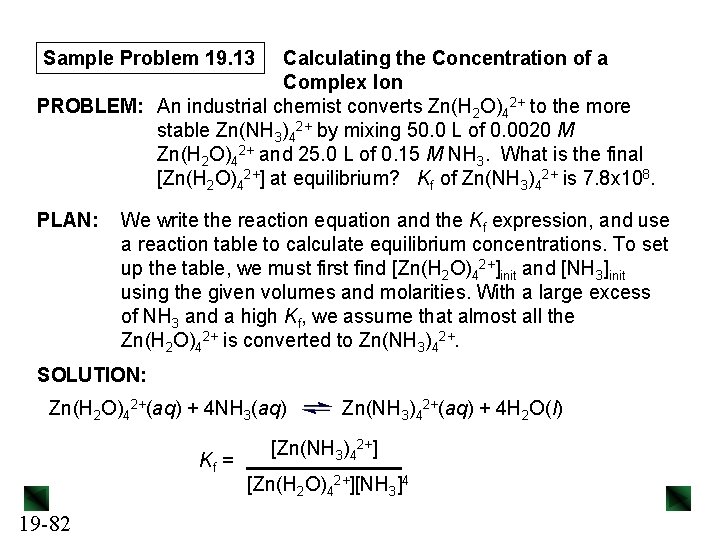
Sample Problem 19. 13 Calculating the Concentration of a Complex Ion PROBLEM: An industrial chemist converts Zn(H 2 O)42+ to the more stable Zn(NH 3)42+ by mixing 50. 0 L of 0. 0020 M Zn(H 2 O)42+ and 25. 0 L of 0. 15 M NH 3. What is the final [Zn(H 2 O)42+] at equilibrium? Kf of Zn(NH 3)42+ is 7. 8 x 108. PLAN: We write the reaction equation and the Kf expression, and use a reaction table to calculate equilibrium concentrations. To set up the table, we must first find [Zn(H 2 O)42+]init and [NH 3]init using the given volumes and molarities. With a large excess of NH 3 and a high Kf, we assume that almost all the Zn(H 2 O)42+ is converted to Zn(NH 3)42+. SOLUTION: Zn(H 2 O)42+(aq) + 4 NH 3(aq) Kf = 19 -82 Zn(NH 3)42+(aq) + 4 H 2 O(l) [Zn(NH 3)42+] [Zn(H 2 O)42+][NH 3]4
![Sample Problem 19 13 ZnH 2 O42initial 50 0 L x 0 0020 Sample Problem 19. 13 [Zn(H 2 O)42+]initial = 50. 0 L x 0. 0020](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-83.jpg)
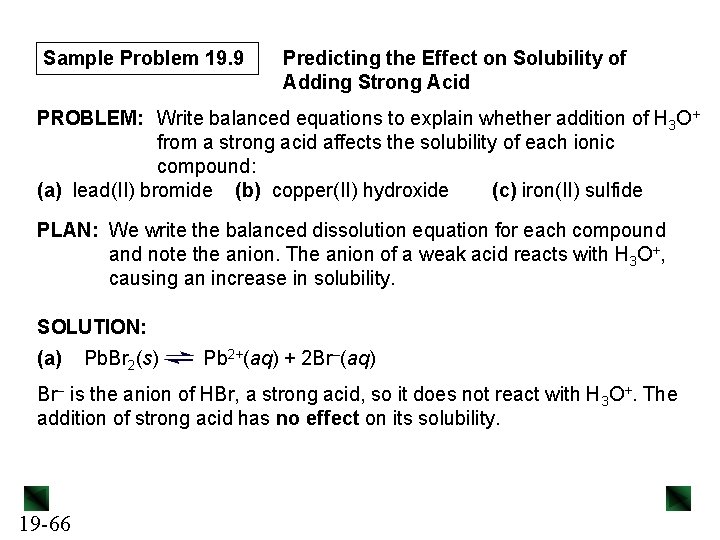
Sample Problem 19. 13 [Zn(H 2 O)42+]initial = 50. 0 L x 0. 0020 M = 1. 3 x 10– 3 M 50. 0 L + 25. 0 L x 0. 15 M = 5. 0 x 10– 2 M 50. 0 L + 25. 0 L 4 mol of NH 3 is needed per mol of Zn(H 2 O 4)2+, so [NH 3]reacted ≈ 4(1. 3 x 10– 3 M) = 5. 2 x 10– 3 M and [Zn(NH 3)42+] ≈ 1. 3 x 10– 3 M [NH 3]initial = Concentration (M) Initial Change Equilibrium 19 -83 Zn(H 2 O)42+(aq) + 4 NH 3(aq) 1. 3 x 10– 3 ~(− 1. 3 x 10– 3) x Zn(NH 3)42+(aq) + 4 H 2 O(l) 5. 0 x 10– 2 0 - ~(− 5. 2 x 10– 3) ~(+1. 3 x 10– 3) - 4. 5 x 10– 2 1. 3 x 10– 3 -
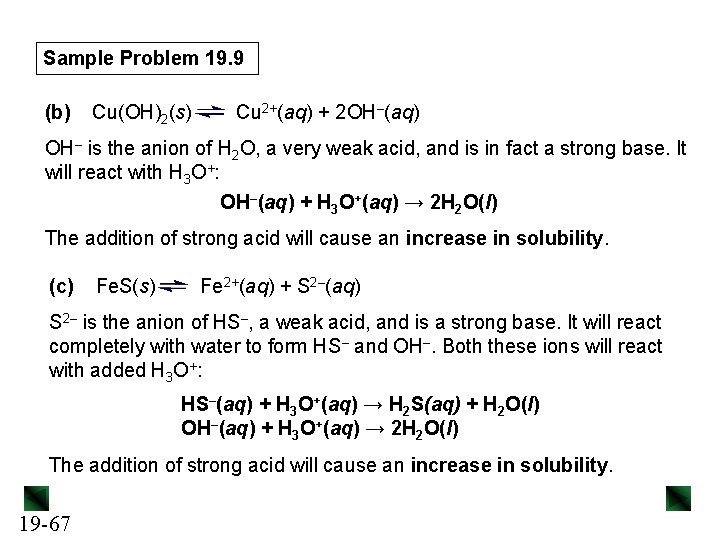
![Sample Problem 19 13 Kf ZnNH 342 ZnH 2 O42NH 34 7 Sample Problem 19. 13 Kf = [Zn(NH 3)42+] [Zn(H 2 O)42+][NH 3]4 = 7.](https://slidetodoc.com/presentation_image/02f29cbb4630847cefb4d3687eb0c56f/image-84.jpg)
Sample Problem 19. 13 Kf = [Zn(NH 3)42+] [Zn(H 2 O)42+][NH 3]4 = 7. 8 x 108 x = [Zn(H 2 O)42+] ≈ 4. 1 x 10– 7 M 19 -84 ≈ (1. 3 x 10– 3) x(4. 5 x 10– 2)4

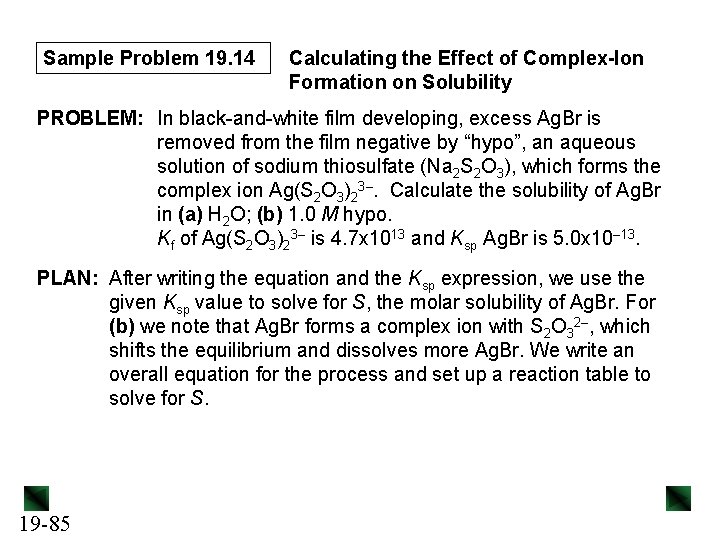
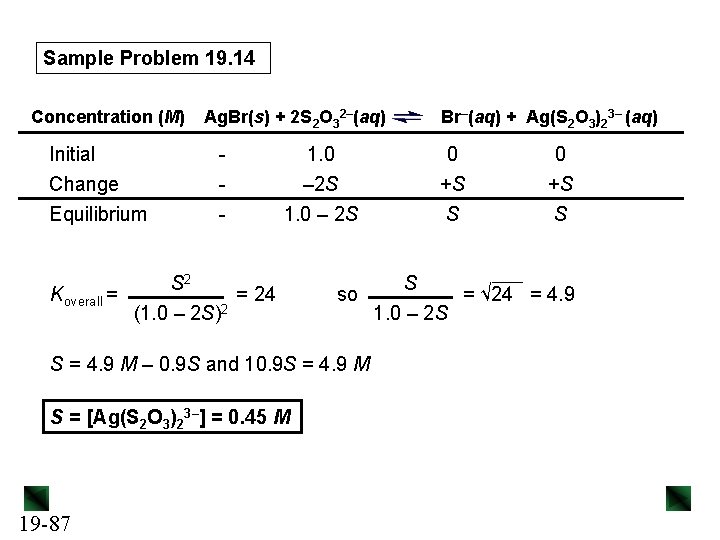
Sample Problem 19. 14 Calculating the Effect of Complex-Ion Formation on Solubility PROBLEM: In black-and-white film developing, excess Ag. Br is removed from the film negative by “hypo”, an aqueous solution of sodium thiosulfate (Na 2 S 2 O 3), which forms the complex ion Ag(S 2 O 3)23–. Calculate the solubility of Ag. Br in (a) H 2 O; (b) 1. 0 M hypo. Kf of Ag(S 2 O 3)23– is 4. 7 x 1013 and Ksp Ag. Br is 5. 0 x 10– 13. PLAN: After writing the equation and the Ksp expression, we use the given Ksp value to solve for S, the molar solubility of Ag. Br. For (b) we note that Ag. Br forms a complex ion with S 2 O 32–, which shifts the equilibrium and dissolves more Ag. Br. We write an overall equation for the process and set up a reaction table to solve for S. 19 -85

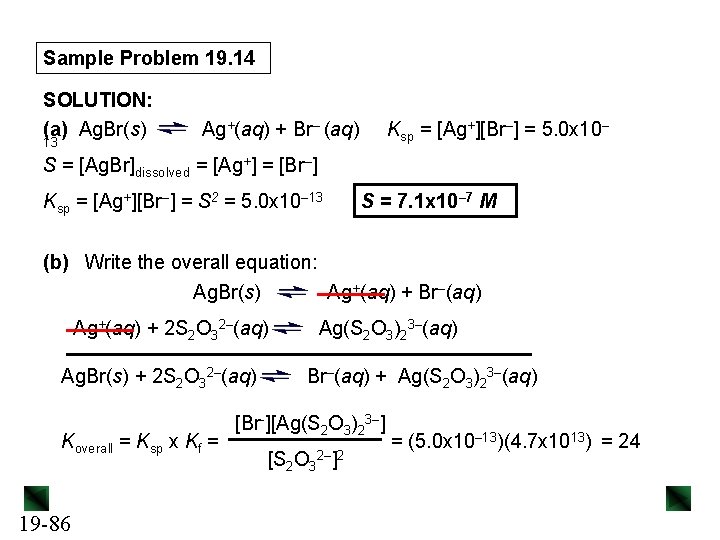
Sample Problem 19. 14 SOLUTION: (a) Ag. Br(s) 13 Ag+(aq) + Br– (aq) Ksp = [Ag+][Br–] = 5. 0 x 10– S = [Ag. Br]dissolved = [Ag+] = [Br–] Ksp = [Ag+][Br–] = S 2 = 5. 0 x 10– 13 S = 7. 1 x 10– 7 M (b) Write the overall equation: Ag. Br(s) Ag+(aq) + Br–(aq) Ag+(aq) + 2 S 2 O 32–(aq) Ag. Br(s) + 2 S 2 O 32–(aq) Koverall = Ksp x Kf = 19 -86 Ag(S 2 O 3)23–(aq) Br–(aq) + Ag(S 2 O 3)23–(aq) [Br-][Ag(S 2 O 3)23–] [S 2 O 32–]2 = (5. 0 x 10– 13)(4. 7 x 1013) = 24

Sample Problem 19. 14 Ag. Br(s) + 2 S 2 O 32–(aq) Concentration (M) Initial Change Equilibrium Koverall = S 2 (1. 0 – 2 S)2 1. 0 – 2 S = 24 so S = 4. 9 M – 0. 9 S and 10. 9 S = 4. 9 M S = [Ag(S 2 O 3)23–] = 0. 45 M 19 -87 Br–(aq) + Ag(S 2 O 3)23– (aq) 0 +S S S 1. 0 – 2 S 0 +S S = √ 24 = 4. 9

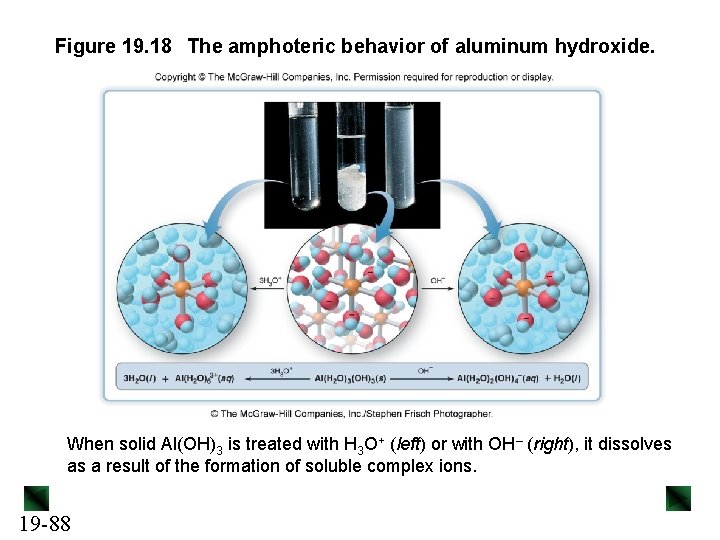
Figure 19. 18 The amphoteric behavior of aluminum hydroxide. When solid Al(OH)3 is treated with H 3 O+ (left) or with OH– (right), it dissolves as a result of the formation of soluble complex ions. 19 -88
 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Molecular biology lectures
Molecular biology lectures Enteroendocrine cell
Enteroendocrine cell Molecular biology of the cell
Molecular biology of the cell Physical state of covalent compounds
Physical state of covalent compounds Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Draw power triangle
Draw power triangle Power bi power point
Power bi power point Point point power
Point point power Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Ap chemistry molecular geometry
Ap chemistry molecular geometry Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Zline 667-36
Zline 667-36 Power semiconductor devices lecture notes
Power semiconductor devices lecture notes Switch mode power supply lecture notes
Switch mode power supply lecture notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Things fall apart customs
Things fall apart customs Nature and nature's law lay hid in night meaning
Nature and nature's law lay hid in night meaning Nature nature controversy
Nature nature controversy Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thang điểm glasgow
Thang điểm glasgow Hát lên người ơi
Hát lên người ơi Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Ví dụ giọng cùng tên
Ví dụ giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Số nguyên tố là số gì
Số nguyên tố là số gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Kamikaze power of nature
Kamikaze power of nature Nature and power of prejudice in social psychology
Nature and power of prejudice in social psychology Ozymandias key quotes gcse
Ozymandias key quotes gcse Storm on the island power of nature
Storm on the island power of nature The nature of power politics and government
The nature of power politics and government Stationary point of a function
Stationary point of a function Hcl point group
Hcl point group Iron pentacarbonyl point group
Iron pentacarbonyl point group Solar power satellites and microwave power transmission
Solar power satellites and microwave power transmission Actual power
Actual power Flex power power supply
Flex power power supply Define dispersive power of a grating.
Define dispersive power of a grating. Power of a power property
Power of a power property Chain rule範例
Chain rule範例 Power angle curve in power system stability
Power angle curve in power system stability Power absorbed or supplied
Power absorbed or supplied Evangelio del domingo en power point
Evangelio del domingo en power point Aplausos sonido
Aplausos sonido La boutique del power point
La boutique del power point Power point tennis
Power point tennis