Lecture Power Point Chemistry The Molecular Nature of





























- Slides: 72

Lecture Power. Point Chemistry The Molecular Nature of Matter and Change Sixth Edition Martin S. Silberberg 1 -1 Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Chapter 1: Keys to the Study of Chemistry 1. 1 Some Fundamental Definitions 1. 2 Chemical Arts and the Origins of Modern Chemistry 1. 3 The Scientific Approach: Developing a Model 1. 4 Chemical Problem Solving 1. 5 Measurement in Scientific Study 1. 6 Uncertainty in Measurement: Significant Figures 1 -2

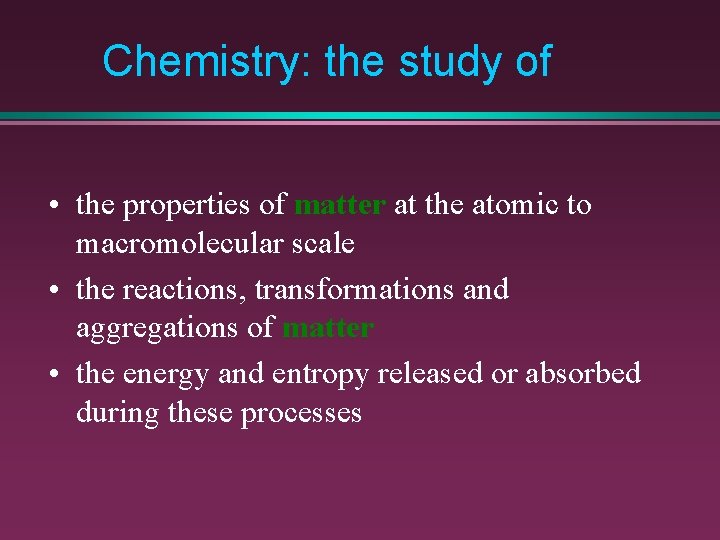
Chemistry: the study of • the properties of matter at the atomic to macromolecular scale • the reactions, transformations and aggregations of matter • the energy and entropy released or absorbed during these processes

Matter: Anything occupying space and having mass.

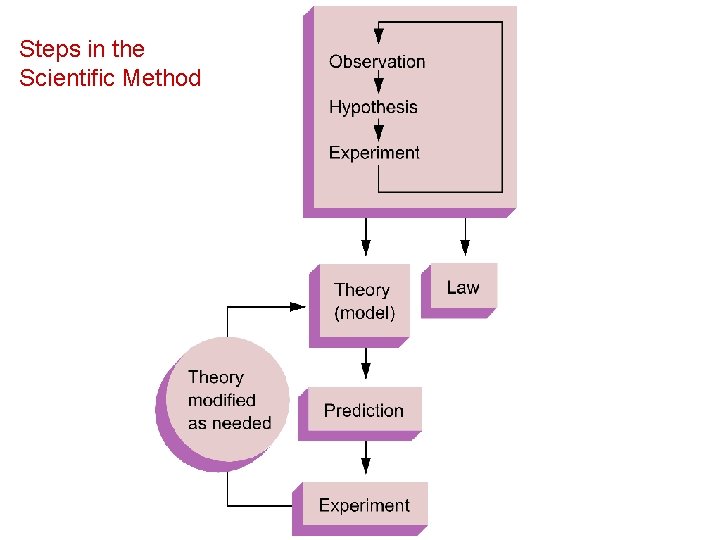
Steps in the Scientific Method

Steps in the Scientific Method 1. Observations quantitative qualitative 2. Formulating hypotheses possible explanation for the observation 3. Performing experiments gathering new information to decide whether the hypothesis is valid

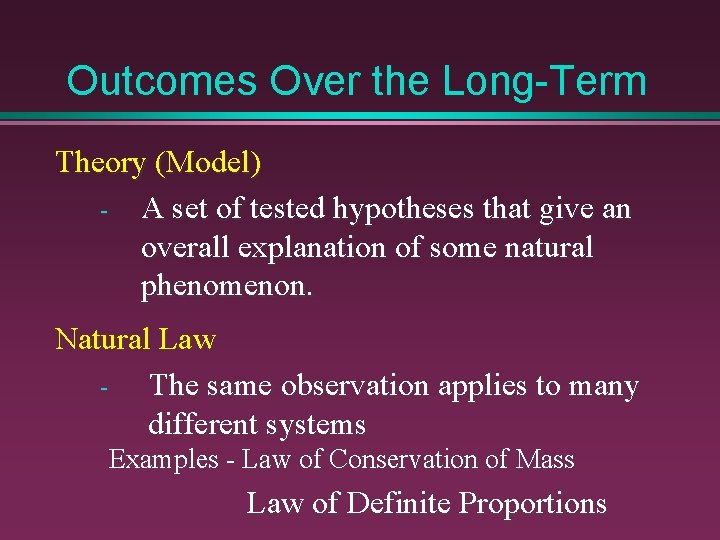
Outcomes Over the Long-Term Theory (Model) - A set of tested hypotheses that give an overall explanation of some natural phenomenon. Natural Law The same observation applies to many different systems Examples - Law of Conservation of Mass Law of Definite Proportions

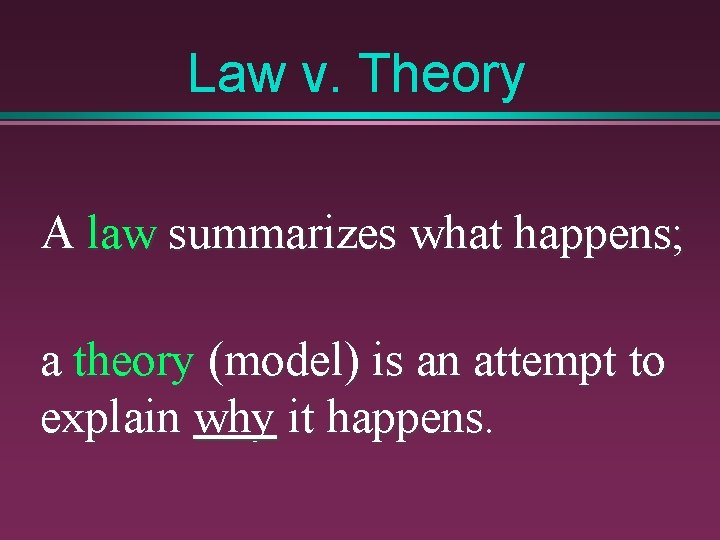
Law v. Theory A law summarizes what happens; a theory (model) is an attempt to explain why it happens.

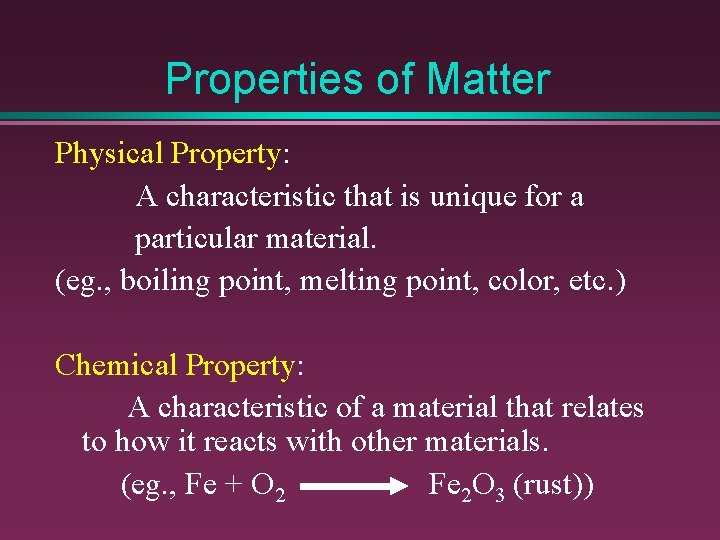
Properties of Matter Physical Property: A characteristic that is unique for a particular material. (eg. , boiling point, melting point, color, etc. ) Chemical Property: A characteristic of a material that relates to how it reacts with other materials. (eg. , Fe + O 2 Fe 2 O 3 (rust))

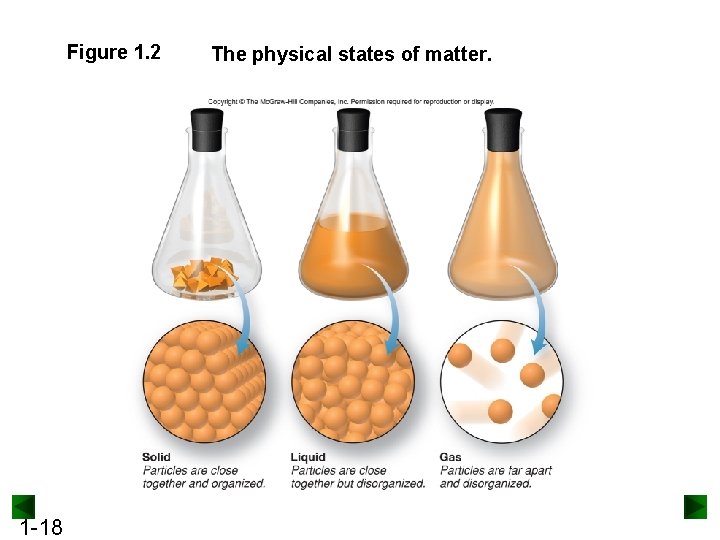
Classification of Matter Physical States of Matter: Solid: rigid - fixed volume and shape Liquid: definite volume but indefinite shape (assumes the shape of its container) Gas: no fixed volume or shape - assumes the shape of its container

Changes of Matter Physical Change: A change of state (from gas to liq. to solid) Dissolving a solid in a liquid (eg. Na. Cl in H 2 O), etc. Chemical Change: A change from one or more forms of matter in to one or more different forms of matter. a. A + b. B c. C + d. D

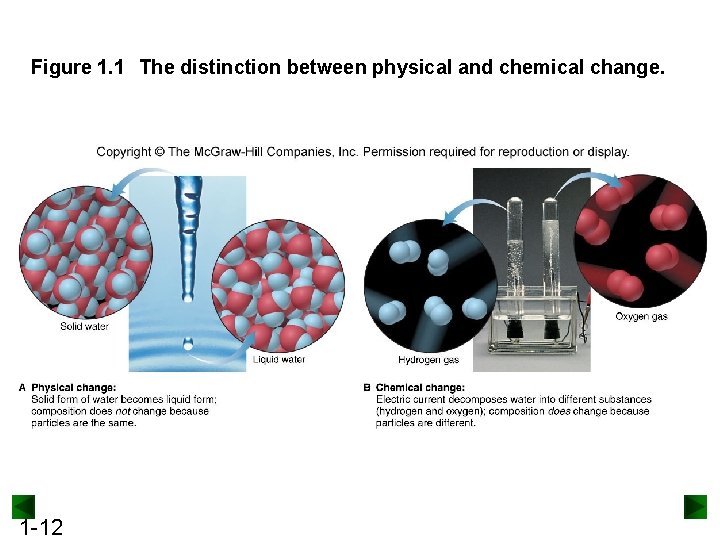
Figure 1. 1 The distinction between physical and chemical change. 1 -12

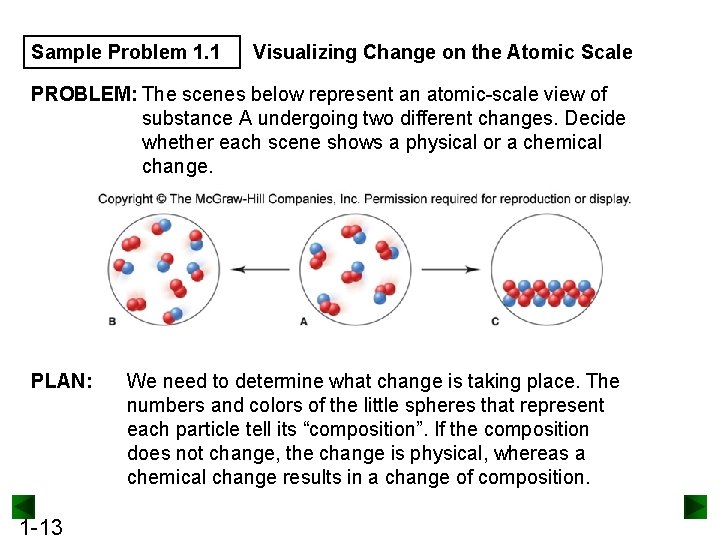
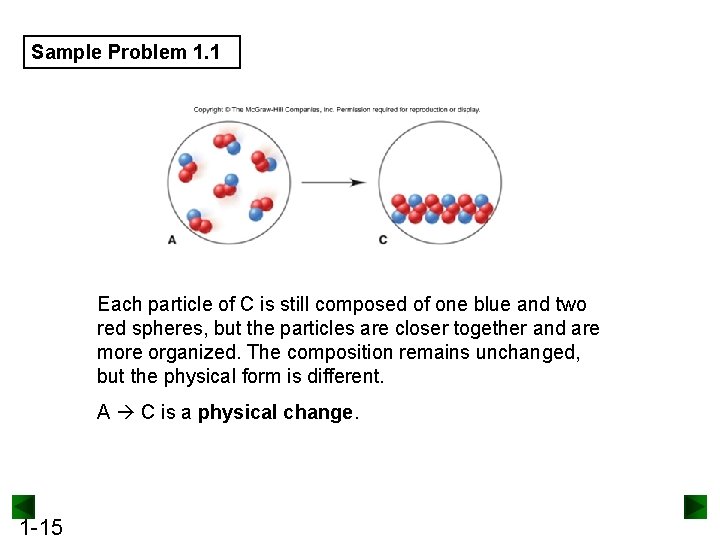
Sample Problem 1. 1 Visualizing Change on the Atomic Scale PROBLEM: The scenes below represent an atomic-scale view of substance A undergoing two different changes. Decide whether each scene shows a physical or a chemical change. PLAN: 1 -13 We need to determine what change is taking place. The numbers and colors of the little spheres that represent each particle tell its “composition”. If the composition does not change, the change is physical, whereas a chemical change results in a change of composition.

Sample Problem 1. 1 SOLUTION: Each particle of substance A is composed of one blue and two red spheres. Sample B is composed of two different types of particles – some have two red spheres while some have one red and one blue. As A changes to B, the chemical composition has changed. A B is a chemical change. 1 -14

Sample Problem 1. 1 Each particle of C is still composed of one blue and two red spheres, but the particles are closer together and are more organized. The composition remains unchanged, but the physical form is different. A C is a physical change. 1 -15

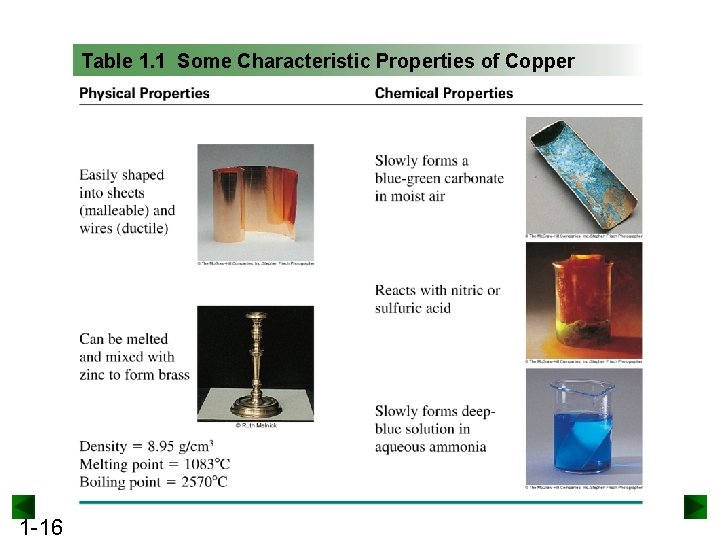
Table 1. 1 Some Characteristic Properties of Copper 1 -16

The States of Matter A solid has a fixed shape and volume. Solids may be hard or soft, rigid or flexible. A liquid has a varying shape that conforms to the shape of the container, but a fixed volume. A liquid has an upper surface. A gas has no fixed shape or volume and therefore does not have a surface. 1 -17

Figure 1. 2 1 -18 The physical states of matter.

Temperature and Change of State • A change of state is a physical change. – Physical form changes, composition does not. • Changes in physical state are reversible – by changing the temperature. • A chemical change cannot simply be reversed by a change in temperature. 1 -19

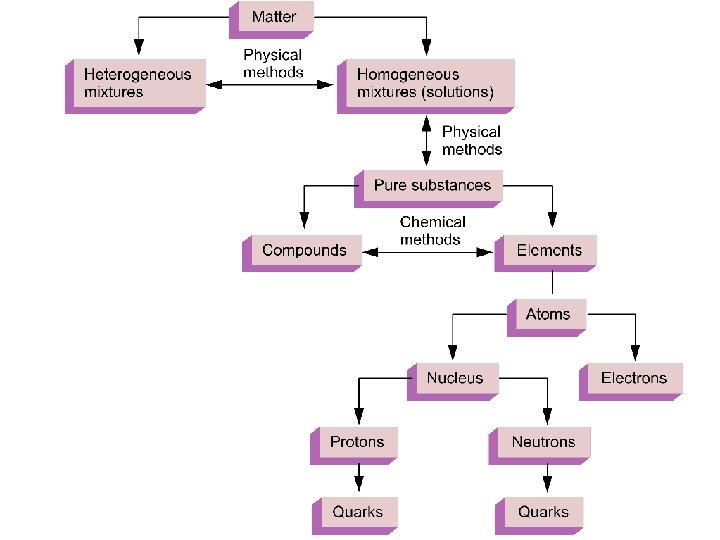
Pure Substances Have a unique set of properties All Particles are the same Cannot be separated into different species by physical means

Types of Matter Compound: A substance with a constant composition that can be broken down into elements by chemical processes. Element: A substance that cannot be decomposed into simpler substances by chemical means.

Mixtures Homogeneous Properties are the same throughout the sample (eg. H 2 O/Na. Cl, vinegar, etc. ). Heterogeneous Properties vary from one part of the sample to another (eg. blood, lemonade, italian salad dressing).


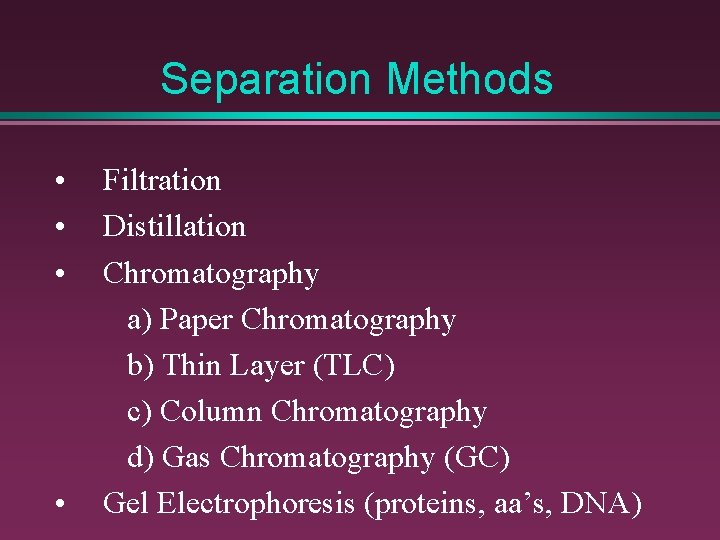
Separation Methods • • Filtration Distillation Chromatography a) Paper Chromatography b) Thin Layer (TLC) c) Column Chromatography d) Gas Chromatography (GC) Gel Electrophoresis (proteins, aa’s, DNA)

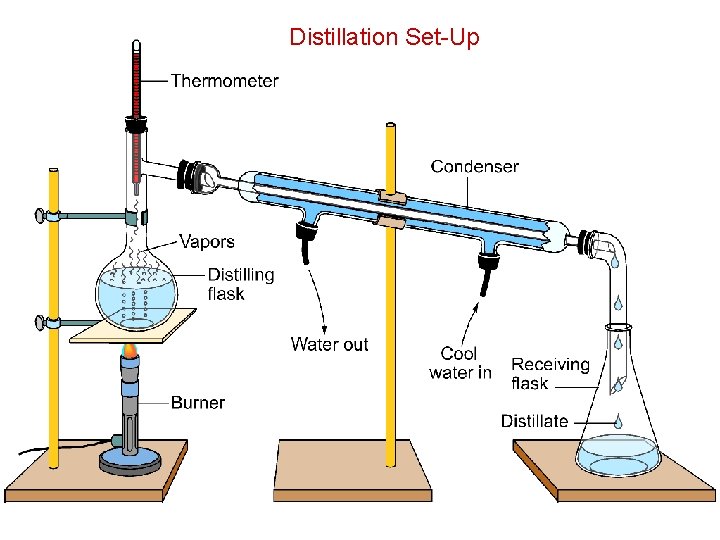
Distillation Set-Up

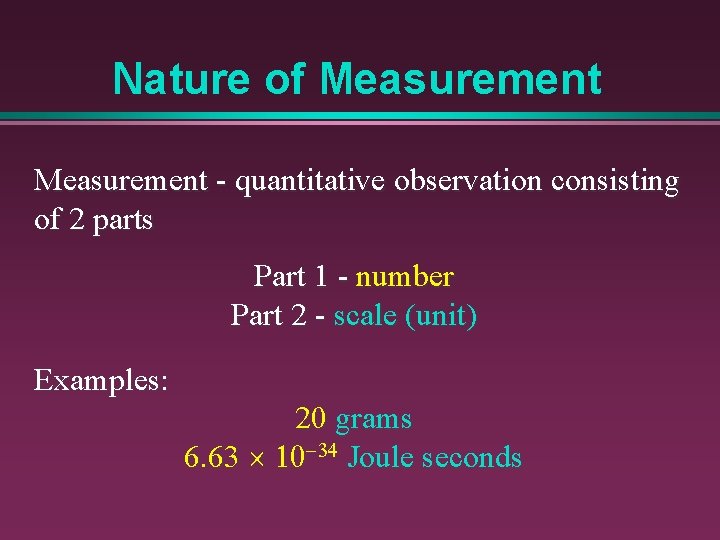
Nature of Measurement - quantitative observation consisting of 2 parts Part 1 - number Part 2 - scale (unit) Examples: 20 grams 6. 63 ´ 10 -34 Joule seconds

International System (le Système International) Based on metric system and units derived from metric system. Review scientific notation

Scientific Notation Express the following in scientific notation: 238, 000 1, 500, 000 0. 00043 0. 089 2. 38 x 105 1. 5 x 106 4. 3 x 10 -4 8. 9 x 10 -2

The Fundamental SI Units See Table 1. 1 for SI Units Note: SI Prefixes may also be used in Scientific Notation

Prefixes in the Metric System Table 1. 2 Prefix Symbol mega kilo deci milli micro nano M k d m m n Meaning 10 X 1, 000 1000 0. 1 0. 000001 0. 00001 106 103 10 -1 10 -3 10 -6 10 -9

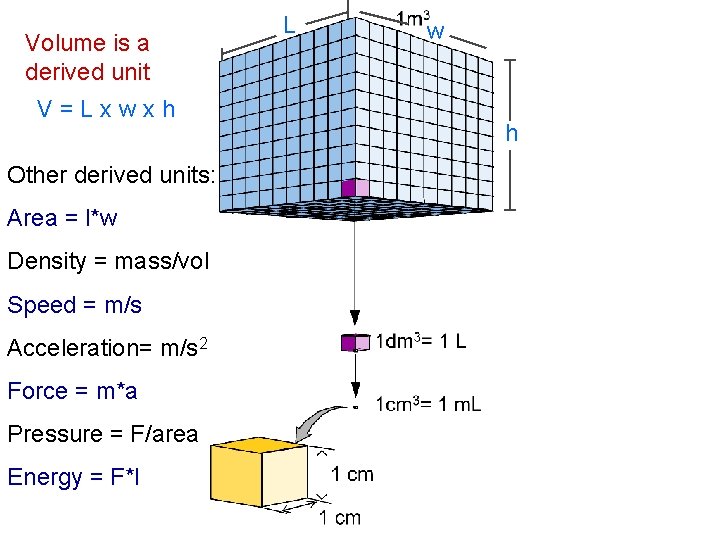
Volume is a derived unit V=Lxwxh Other derived units: Area = l*w Density = mass/vol Speed = m/s Acceleration= m/s 2 Force = m*a Pressure = F/area Energy = F*l L w h

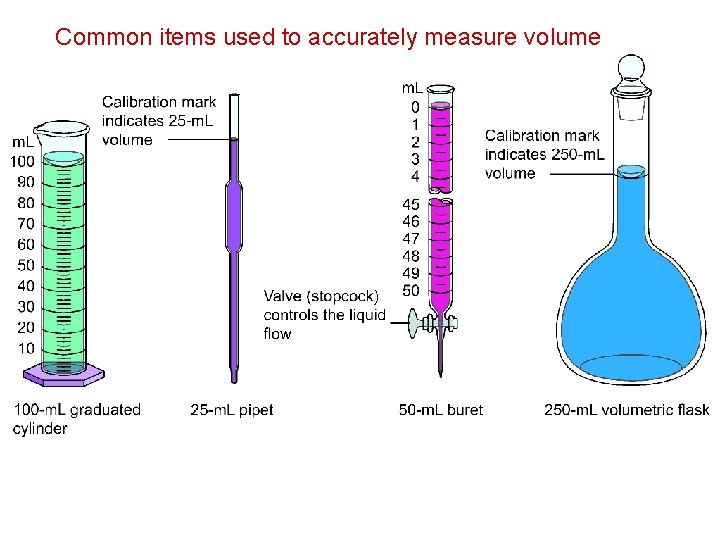
Common items used to accurately measure volume

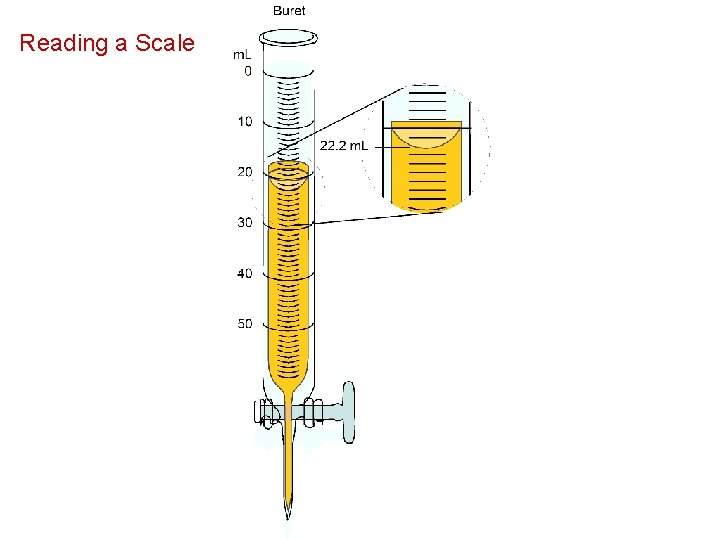
Reading a Scale

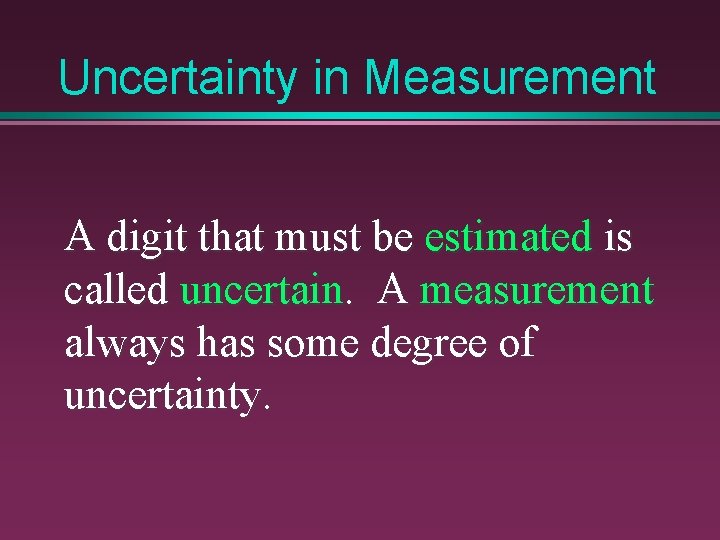
Uncertainty in Measurement A digit that must be estimated is called uncertain. A measurement always has some degree of uncertainty.

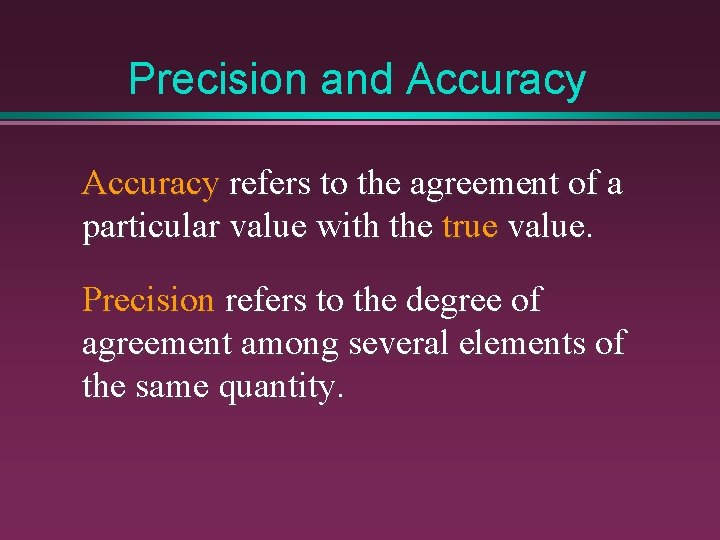
Precision and Accuracy refers to the agreement of a particular value with the true value. Precision refers to the degree of agreement among several elements of the same quantity.

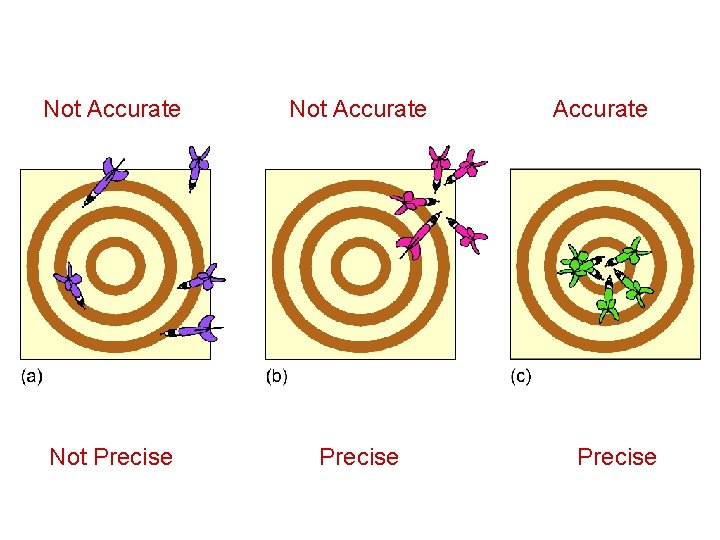
Not Accurate Not Precise Accurate Precise

Types of Error Random Error (Indeterminate Error) measurement has an equal probability of being high or low. Systematic Error (Determinate Error) Occurs in the same direction each time (high or low), often resulting from poor technique, or uncalibrated instruments.

Measurements Person 1 2 3 4 5 Measurement 2. 85 cm 2. 84 cm 2. 86 cm 2. 85 cm 2. 86 cm Are these measurements an example of random error or systematic error? Random error

Significant Figures Fig. 2. 6 The digital readout on an electronic calculator usually shows more digits than are needed.

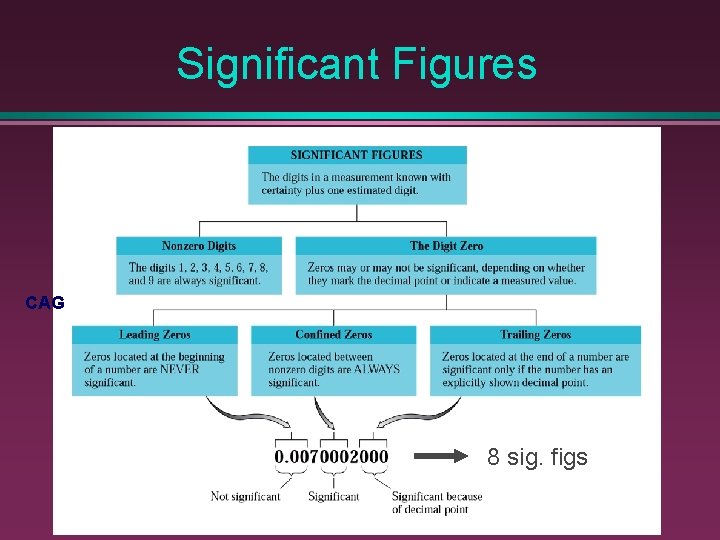
Rules for Counting Significant Figures - Overview 1. Nonzero integers 2. Zeros leading zeros captive zeros trailing zeros 3. Exact numbers

Rules for Counting Significant Figures - Details Nonzero integers always count as significant figures. 3456 has 4 sig figs.

Rules for Counting Significant Figures - Details Zeros - Leading zeros do not count as significant figures. 0. 0486 has 3 sig figs.

Rules for Counting Significant Figures - Details Zeros - Captive zeros always count as significant figures. 16. 07 has 4 sig figs.

Rules for Counting Significant Figures - Details Zeros - Trailing zeros are significant only if the number contains a decimal point. 9. 300 has 4 sig figs.

Significant Figures CAG 8 sig. figs

Rules for Counting Significant Figures - Details Exact numbers have an infinite number of significant figures. 1 inch = 2. 54 cm, exactly

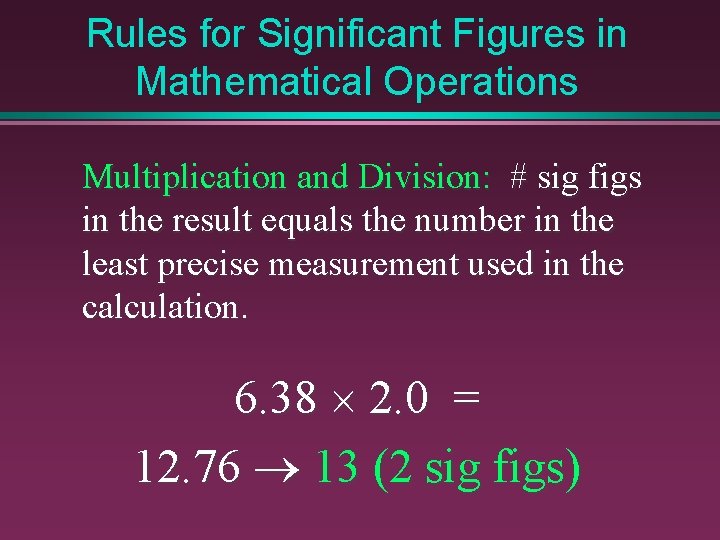
Rules for Significant Figures in Mathematical Operations Multiplication and Division: # sig figs in the result equals the number in the least precise measurement used in the calculation. 6. 38 ´ 2. 0 = 12. 76 ® 13 (2 sig figs)

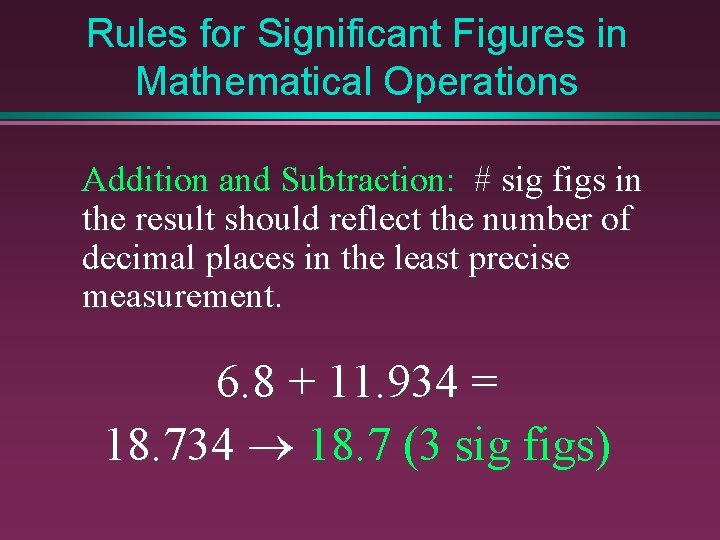
Rules for Significant Figures in Mathematical Operations Addition and Subtraction: # sig figs in the result should reflect the number of decimal places in the least precise measurement. 6. 8 + 11. 934 = 18. 734 ® 18. 7 (3 sig figs)

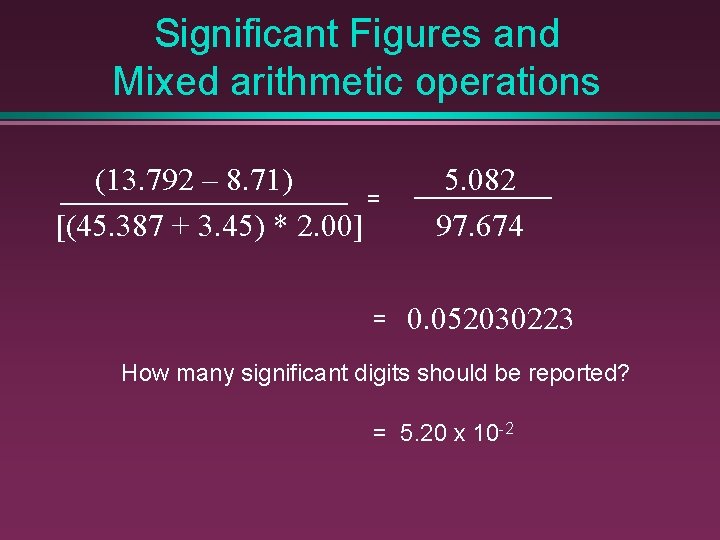
Significant Figures and Mixed arithmetic operations (13. 792 – 8. 71) = [(45. 387 + 3. 45) * 2. 00] = 5. 082 97. 674 0. 052030223 How many significant digits should be reported? = 5. 20 x 10 -2

Rounding off Numbers 2 Rules for rounding off numbers: 1. If the last digit is 4 or less, it is dropped eg. 3. 72456 3. 72 for 3 sig figs. 2. If the last digit is 5 or greater, it is dropped, and the preceeding number is increased by 1 eg. , 5. 00673 5. 01 for 3 sig figs. NOTE: For a series of calculations, round off at the end.

Density is the mass of substance per unit volume of the substance:

Density cont’d Fig. 2. 8 Both of these items have a mass of 23 grams, but they have very different volumes; therefore, their densities are different as well.

Measurements in Chemistry cont’d Fig. 2. 9 The penny is less dense than the mercury it floats on.

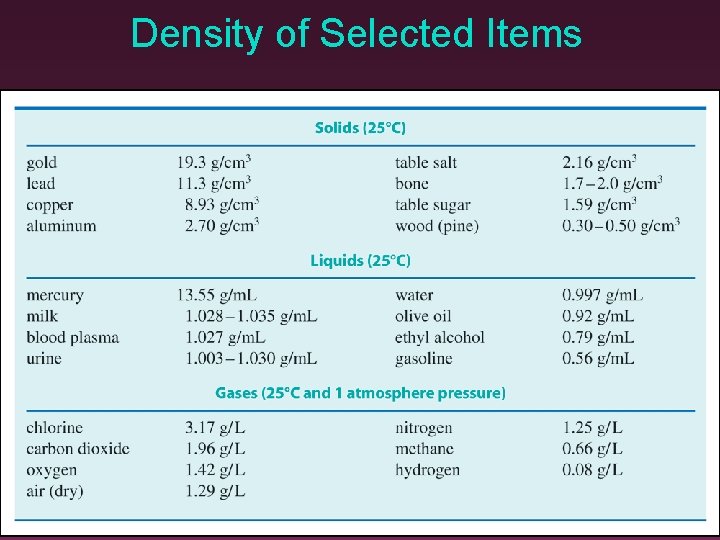
Density of Selected Items

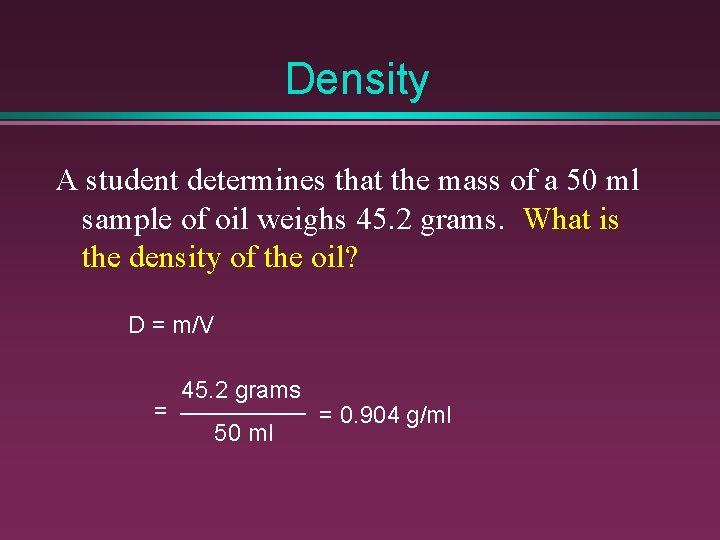
Density A student determines that the mass of a 50 ml sample of oil weighs 45. 2 grams. What is the density of the oil? D = m/V = 45. 2 grams 50 ml = 0. 904 g/ml

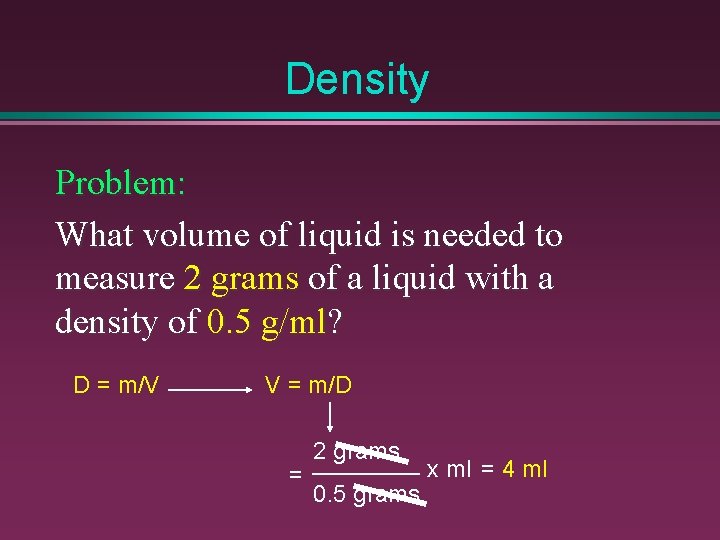
Density Problem: What volume of liquid is needed to measure 2 grams of a liquid with a density of 0. 5 g/ml? D = m/V V = m/D = 2 grams 0. 5 grams x ml = 4 ml

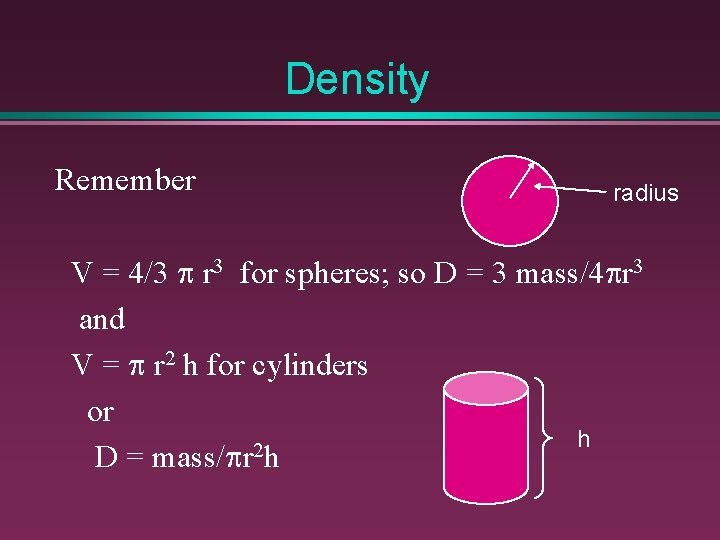
Density Remember radius V = 4/3 p r 3 for spheres; so D = 3 mass/4 pr 3 and V = p r 2 h for cylinders or h 2 D = mass/pr h

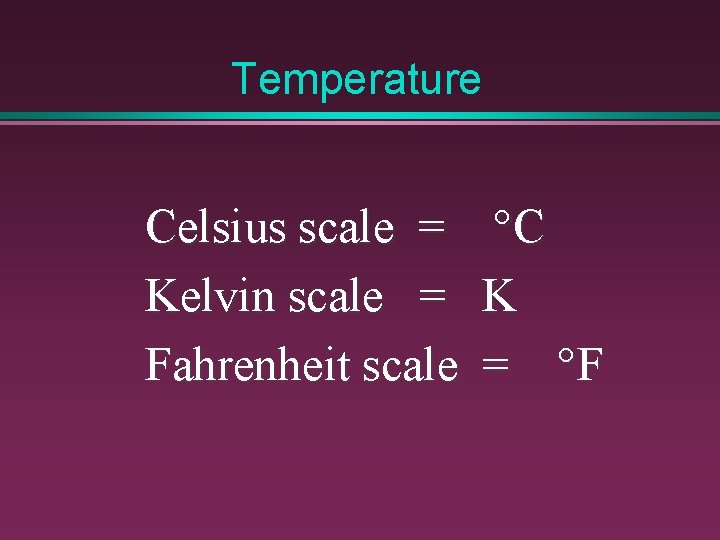
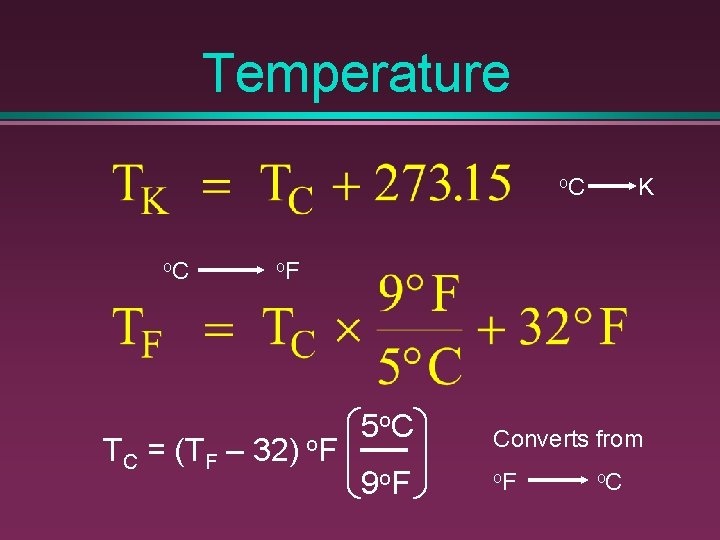
Temperature Celsius scale = Kelvin scale = Fahrenheit scale °C K = °F


Temperature o. C K o. F TC = (TF – 32) o. F 5 o. C 9 o. F Converts from o. F o. C

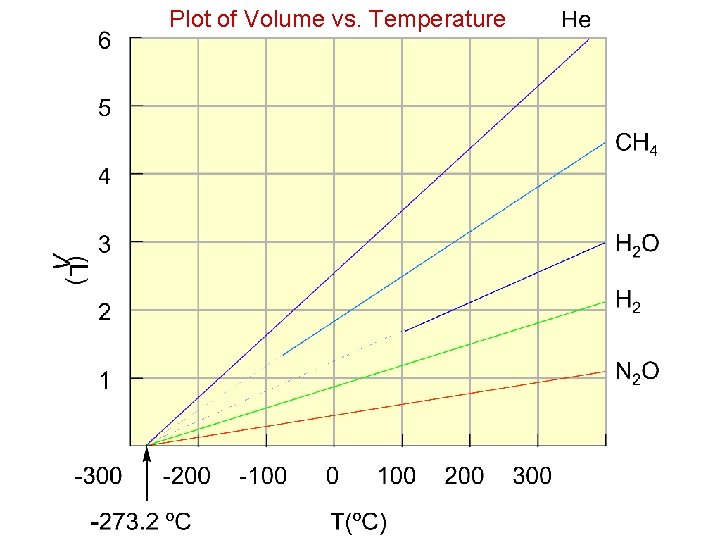
Plot of Volume vs. Temperature

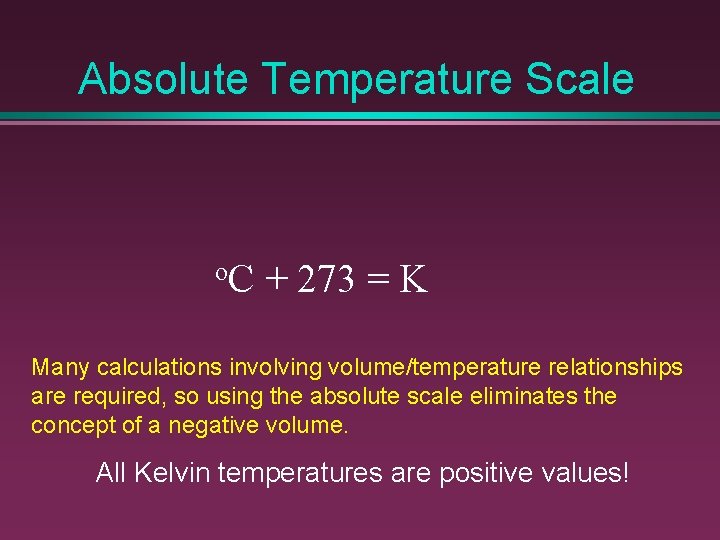
Absolute Temperature Scale o. C + 273 = K Many calculations involving volume/temperature relationships are required, so using the absolute scale eliminates the concept of a negative volume. All Kelvin temperatures are positive values!

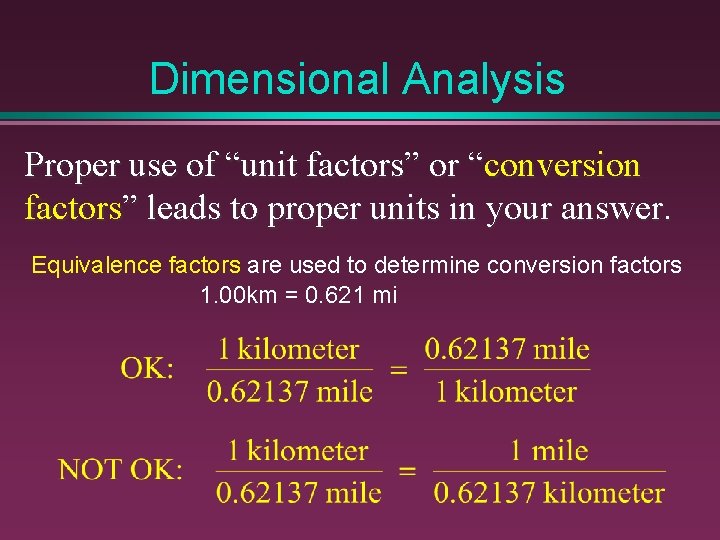
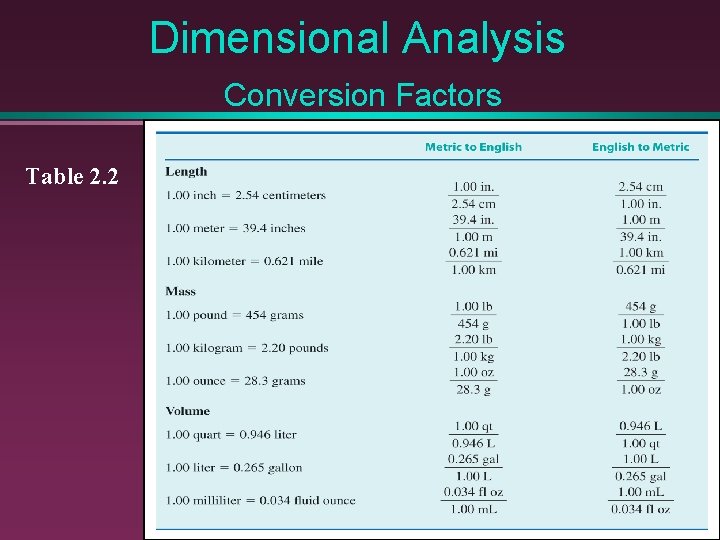
Dimensional Analysis Proper use of “unit factors” or “conversion factors” leads to proper units in your answer. Equivalence factors are used to determine conversion factors 1. 00 km = 0. 621 mi

Dimensional Analysis Conversion Factors Table 2. 2

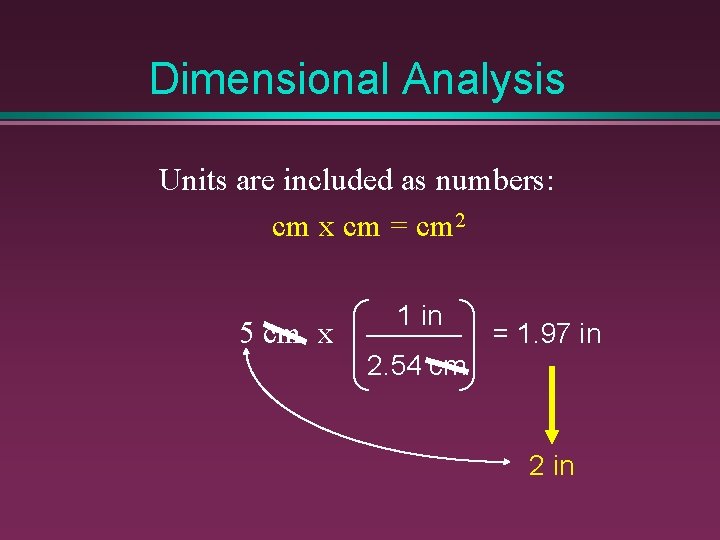
Dimensional Analysis Units are included as numbers: cm x cm = cm 2 5 cm x 1 in = 1. 97 in 2. 54 cm 2 in

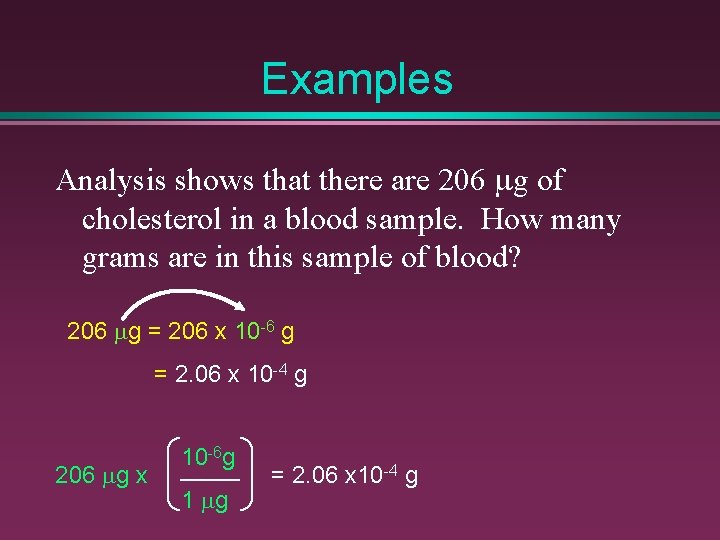
Examples Analysis shows that there are 206 mg of cholesterol in a blood sample. How many grams are in this sample of blood? 206 mg = 206 x 10 -6 g = 2. 06 x 10 -4 g 206 mg x 10 -6 g 1 mg = 2. 06 x 10 -4 g

Example #2 Your boss asks you to make 10 kg of aspirin. How many pounds of aspirin are in 10 kg? from Table 2. 2: 1. 00 kg = 2. 20 lbs 10 kg x 2. 2 lbs 1. 00 kg = 22 lbs

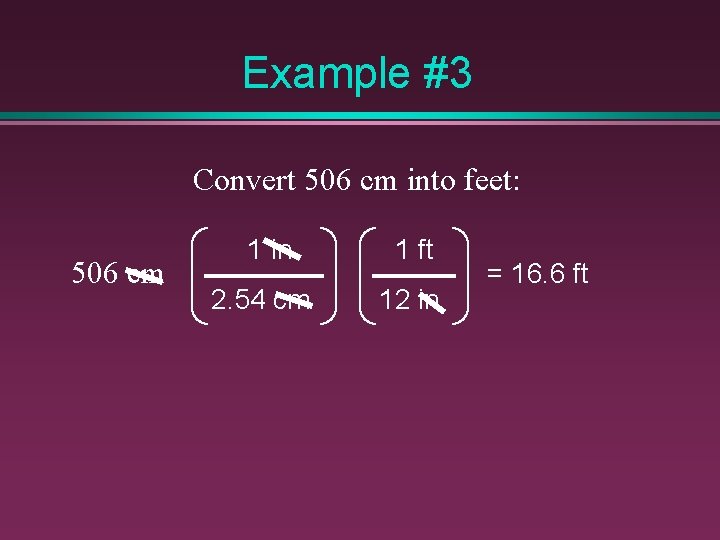
Example #3 Convert 506 cm into feet: 506 cm 1 in 2. 54 cm 1 ft 12 in = 16. 6 ft

End of Chapter 1 Learning objectives and important terms are listed on pages 29 -30 in your text. Homework is found on OWL

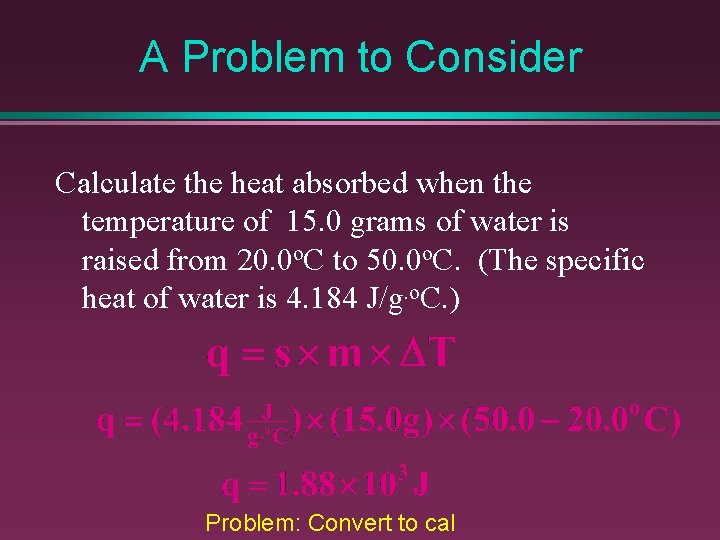
Heat Energy and Specific Heat When heat capacities are compared for one gram amounts of substance it is referred to as the specific heat capacity (or “specific heat”) is the heat required to raise the temperature of one gram of a substance by one degree Celsius. – To find the heat required (q) you must multiply the specific heat, s, of the substance times its mass in grams, m, and the temperature change, DT.

A Problem to Consider Calculate the heat absorbed when the temperature of 15. 0 grams of water is raised from 20. 0 o. C to 50. 0 o. C. (The specific heat of water is 4. 184 J/g. o. C. ) Problem: Convert to cal

Chapter 2 Study Problems • Review all terms • Problems: 1, 3, 5, 11, 13, 15, 21, 25, 27, 35, 37, 47, 55, 57, 59 61, 69, 71, 72, 81 Try Practice Tests for Chpts 1 and 2
 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Cell and molecular biology lectures
Cell and molecular biology lectures Enteroendocrine cell
Enteroendocrine cell Molecular cell biology lecture
Molecular cell biology lecture Covalent bond boiling point
Covalent bond boiling point Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Real power and reactive power
Real power and reactive power Informsu
Informsu Point point power
Point point power Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Ap chemistry molecular geometry
Ap chemistry molecular geometry Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Power system analysis lecture notes
Power system analysis lecture notes Power semiconductor devices lecture notes
Power semiconductor devices lecture notes Switch mode power supply lecture notes
Switch mode power supply lecture notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Customs in things fall apart
Customs in things fall apart Nature and nature's law lay hid in night
Nature and nature's law lay hid in night Nature nature controversy
Nature nature controversy Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa sống lại
Chúa sống lại Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Các số nguyên tố là gì
Các số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Comparing kamikaze and the prelude
Comparing kamikaze and the prelude Nature and power of prejudice
Nature and power of prejudice Ozymandias quotations
Ozymandias quotations Storm on the island power of nature
Storm on the island power of nature