Intro to Matter Matter EVERYTHING IN THE UNIVERSE






























- Slides: 43

Intro to Matter

Matter EVERYTHING IN THE UNIVERSE IS EITHER MATTER OR ENERGY

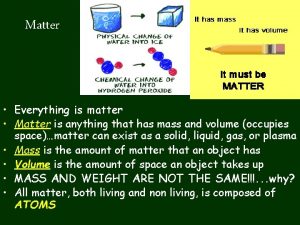
Matter MATTER – Anything that has mass and takes up space (volume).

Matter MATTER MAKES UP THINGS THAT ARE VERY LARGE & VERY SMALL.

LAW OF CONSERVATION OF MATTER CANNOT BE CREATED OR DESTROYED IT JUST CHANGES FROM ONE FORM TO ANOTHER. (MATTER GETS RECYCLED)

Physical and Chemical Properties All matter has different characteristics or properties. These properties can be either physical or chemical properties. A. Physical properties: properties that can be observed or measured. (color, mass, length, volume, density, state, etc) B. Chemical Properties: properties describe the ability of an object to change into a new substance. (flammability, reactivity)

Physical Properties Observable PHYSICAL properties of matter (You can sense them): COLOR STATE (PHASE) SHAPE ODOR (SMELL) TEXTURE HARDNESS

Chemical Properties: properties describes the ability of an object to change into a new substance. (flammability, reactivity)

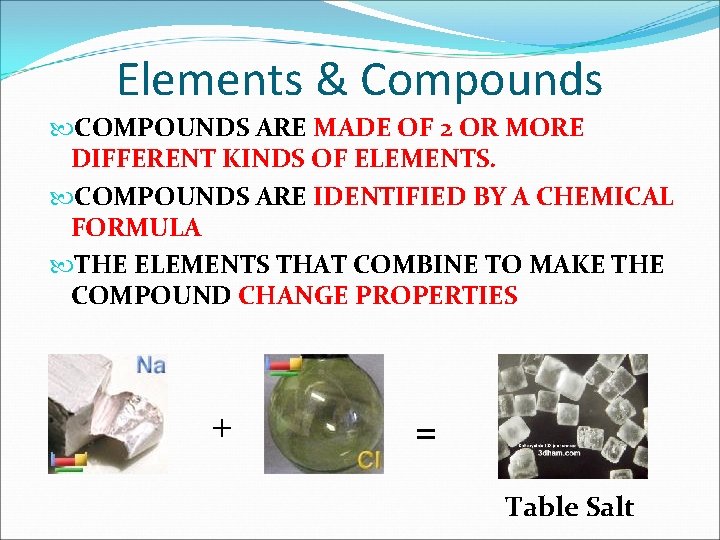
Elements & Compounds ELEMENTS & COMPOUNDS ARE THE BASIC BUILDING BLOCKS OF MATTER EACH ELEMENT & COMPOUND HAS SPECIFIC PROPERTIES ELEMENTS ARE MADE OF ONLY 1 KIND OF ATOM SOME COMMON ELEMENTS: HYDROGEN (H), HELIUM (He), OXYGEN (O), CARBON (C), IRON (Fe), CALCIUM (Ca), NITROGEN (N), SULFUR (S), SODIUM (NA) CHLORINE (Cl), SILICON (Si), POTASSIUM (K) GOLD (Au). MERCURY (Hg)

Elements & Compounds COMPOUNDS ARE MADE OF 2 OR MORE DIFFERENT KINDS OF ELEMENTS. COMPOUNDS ARE IDENTIFIED BY A CHEMICAL FORMULA THE ELEMENTS THAT COMBINE TO MAKE THE COMPOUND CHANGE PROPERTIES + = Table Salt

What is a Mixture? Mixture: is a combination of 2 or more elements or compounds that are not chemically combined. They are just mixed. Examples of Mixtures:

Characteristics of Mixtures Ø No chemical change occurs (the substances keep their identity). Ø THE SUBSTANCES CAN BE SEPARATED!!!!!

Separating Mixtures A. Filtration – filter out one substance to leave the other. Separating the soluble from the insoluble. B. Chromatography – used to separate out mixtures of dyes. Shows all the colors used to make certain dyes C. Evaporation – Used when you wish to collect a solute from a solution. Evaporate a LIQUID to leave the SOLID behind. D. Magnet – use a magnet to remove the metal substances from nonmetal substances.

Types of Mixtures Heterogeneous- the different substances are visible in the mixture. Homogeneous – The mixture looks the same and has the same characteristics throughout.

Physical Properties Some measurable PHYSICAL properties of matter (You have to have equipment to measure): - Volume - Mass - Weight - Density - Temperature - Length - Height - Speed

Measurable Physical Properties Measurable Properties – Properties that a tool is needed to measure. 1. Mass – the amount of matter in an object. This is different than weight!!!!! 2. Weight – is the amount of gravity pulling on an object For example: A human’s mass will never change. But if we traveled to the moon, our weight would change because there is less gravity than on Earth.

Measurable Properties of Matter VOLUME – is the amount of space an object takes up (no matter what object).

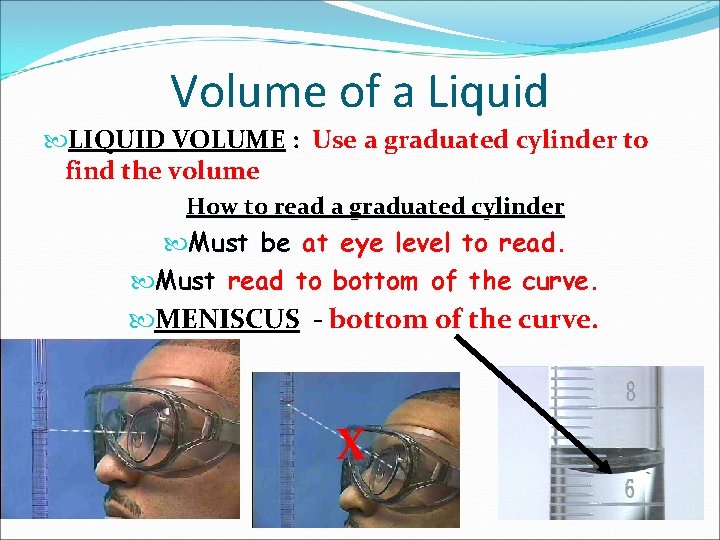
Volume of a Liquid LIQUID VOLUME : Use a graduated cylinder to find the volume How to read a graduated cylinder Must be at eye level to read. Must read to bottom of the curve MENISCUS - bottom of the curve. X

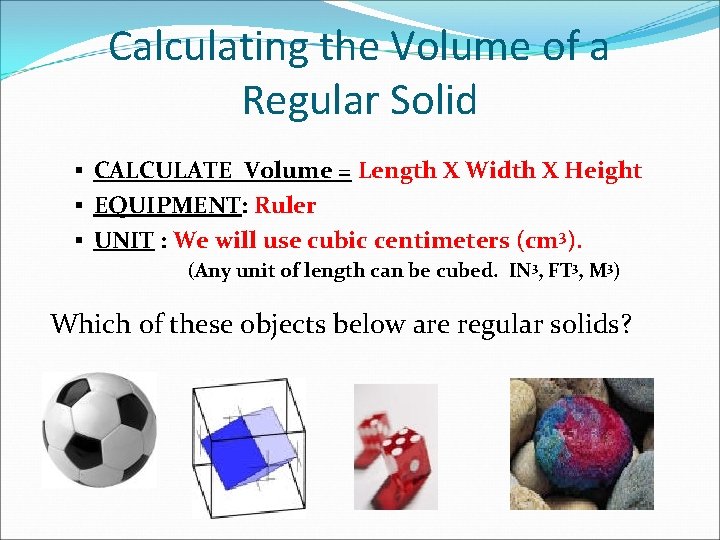
Calculating the Volume of a Regular Solid § CALCULATE Volume = Length X Width X Height § EQUIPMENT: Ruler § UNIT : We will use cubic centimeters (cm 3). (Any unit of length can be cubed. IN 3, FT 3, M 3) Which of these objects below are regular solids?

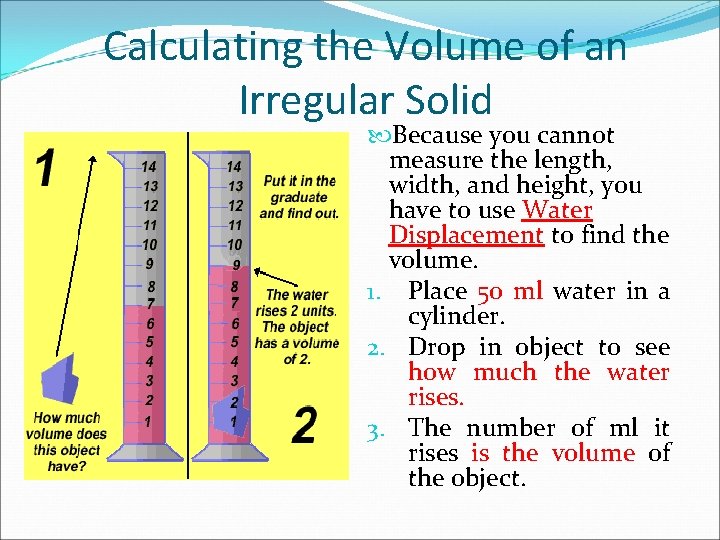
Calculating the Volume of an Irregular Solid Because you cannot measure the length, width, and height, you have to use Water Displacement to find the volume. 1. Place 50 ml water in a cylinder. 2. Drop in object to see how much the water rises. 3. The number of ml it rises is the volume of the object.

Density - the amount of matter in a given space or volume. Calculate : mass Density = -------volume (Mass divided by volume)

Density (physical property) Density is used to identify substances. All substances ALWAYS have the same density no matter how big or small the piece. The density of water is 1 g/ml. Anything with a density higher than 1 will sink in water. Anything with a density less than 1 will float in water. UNITS: g/cm 3 for solids, g/ml for liquids Density of gold = 19. 32 g/cm 3 Density of silver = 10. 50 g/cm 3 Density of ice =. 9 g/cm 3

BELL RINGER Calculate the density for the following objects: a) Mass= 10 g Volume= 5 ml 2 g/ml sinker 2 g/cm 3 sinker b) Mass= 16 g Volume= 8 cm c) Mass= 5 g Volume= 10 ml 0. 5 g/ml floater d) Mass= 12 g Volume= 12 cm 1 g/cm 3 water Which object is water? Which object will float in water? Which objects will sink in water?

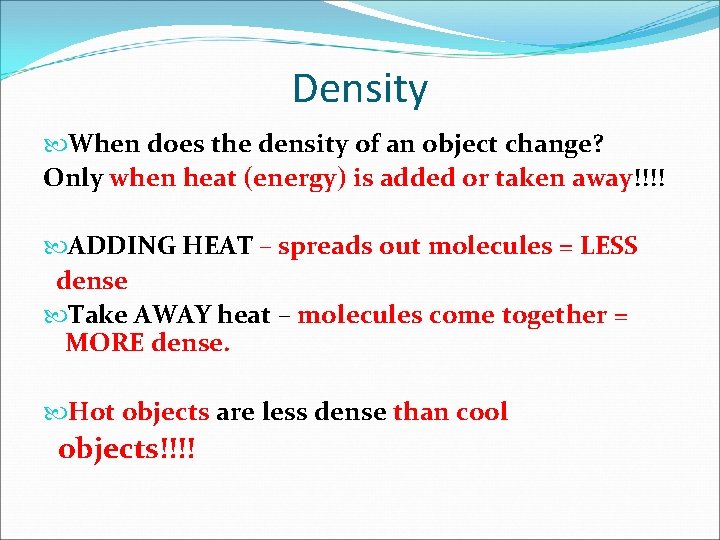
Density When does the density of an object change? Only when heat (energy) is added or taken away!!!! ADDING HEAT – spreads out molecules = LESS dense Take AWAY heat – molecules come together = MORE dense. Hot objects are less dense than cool objects!!!!

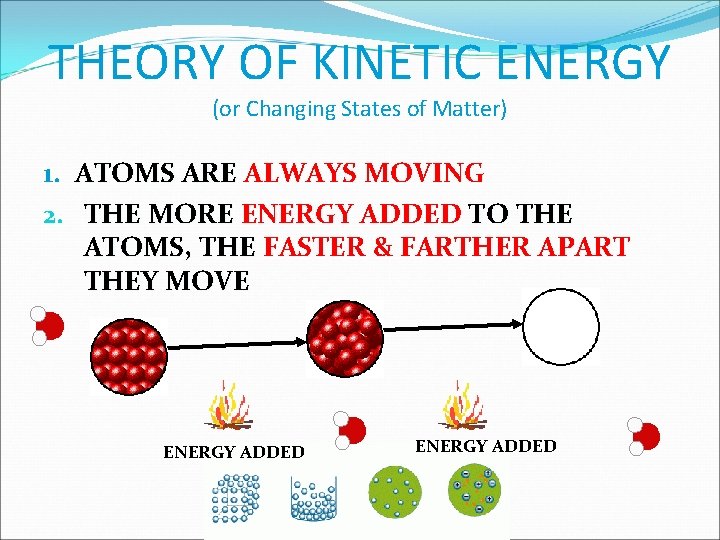
THEORY OF KINETIC ENERGY (or Changing States of Matter) 1. ATOMS ARE ALWAYS MOVING 2. THE MORE ENERGY ADDED TO THE ATOMS, THE FASTER & FARTHER APART THEY MOVE ENERGY ADDED

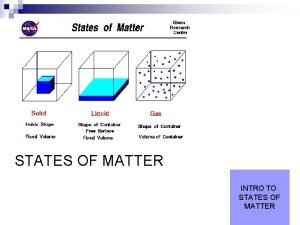
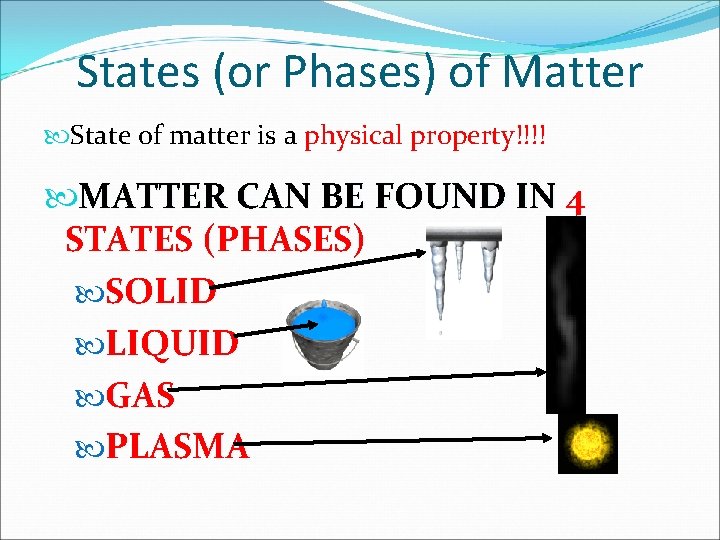
States (or Phases) of Matter State of matter is a physical property!!!! MATTER CAN BE FOUND IN 4 STATES (PHASES) SOLID LIQUID GAS PLASMA

Physical Changes I. Physical Changes: a property of matter that can be observed or measured without changing the identity of the matter. IN OTHER WORDS A CHANGE IN STATE A CHANGE IN SIZE, SHAPE, MASS DOES NOT CHANGE THE CHEMICAL MAKE UP OF THE MATTER YOU HAVE WHAT YOU STARTED WITH JUST IN A DIFFERENT FORM

Examples of Physical Change PAPER TORN INTO PIECES SUGAR DISSOLVED INTO TEA SALT DISSOLVED INTO WATER NAILS OR HAIR CUT

Physical Changes A change in phase is a physical change. Types of Phase Changes 1. Melting 2. Evaporation 3. Boiling 4. Condensation 5. Sublimation ALL PHASE CHANGES ARE CAUSED BY ADDING OR TAKING AWAY ENERGY!!!

Remember… CHANGING STATES DOES NOT CHANGE THE CHEMICAL MAKE UP OF THE MATTER YOU HAVE WHAT YOU STARTED WITH JUST IN A DIFFERENT FORM ICE WATER VAPOR

Melting 1. MELTING – GOING FROM A SOLID TO A LIQUID Energy is added When more heat (energy) is added to matter the particles move faster till the bonds break.

Evaporation GOING FROM A LIQUID TO A GAS AT THE SURFACE ENERGY IS ADDED TO SURFACE OF LIQUID THE PARTICLES AT THE SURFACE MOVE FASTER BREAKING BONDS.

Boiling GOING FROM A LIQUID TO A GAS ALL THROUGHOUT THE LIQUID ENERGY IS ADDED TO ALL OF LIQUID PARTICLES MOVE FASTER ALL THROUGH THE LIQUID SOME PARTICLES MOVE FAST ENOUGH TO CHANGE TO GAS WHILE THEY ARE IN THE MIDDLE OF THE LIQUID Water boils at 212 F, 100 C. EX: pan of liquid on the stove

Condensation CHANGING FROM A GAS TO A LIQUID ENERGY IS REMOVED THE PARTICLES MOVE SLOWER THE ATTRACTION BETWEEN THE PARTICLES BEGAN TO PULL THE PARTICLES CLOSER TOGETHER & THE MATTER TURNS INTO A LIQUID Ex: dew in the lawn, water running down mirror, ring on table under glass

Freezing – CHANGING FROM A LIQUID TO A SOLID – ENERGY IS REMOVED • THE PARTICLES MOVE EVEN SLOWER • THE ATTRACTION BETWEEN THE PARTICLES BEGAN TO PULL THE PARTICLES CLOSER TOGETHER & THE MATTER TURNS INTO A SOLID Water freezes at 32 F, 0 C EX: water to ice, wax or chocolate hardening

Sublimation Substance goes straight from a solid to a gas or from gas to solid. DRY ICE!!!! • Heat added or taken away rapidly • Molecules speed up and spread out Examples of Sublimation: Snowflakes, dry ice, frost

Chemical Change CHEMICAL CHANGE – occurs when 2 or more substances are combined into entirely new substance with all new properties. CANNOT CHANGE IT BACK!!!!!

Signs of a Chemical Change FIZZES OR BUBBLES COLOR CHANGE HEAT GIVEN OFF LIGHT GIVEN OFF ODOR HEAT REQUIRED FOR REACTION NEW SUBSTANCE FORMED Precipitate (solid) Gas (bubbles)

Examples of a Chemical Change BAKING BREAD – NO LONGER HAVE FLOUR, EGGS, MILK, YEAST ALKA-SELTZER BAKING SODA & VINEGAR RUST TARNISH

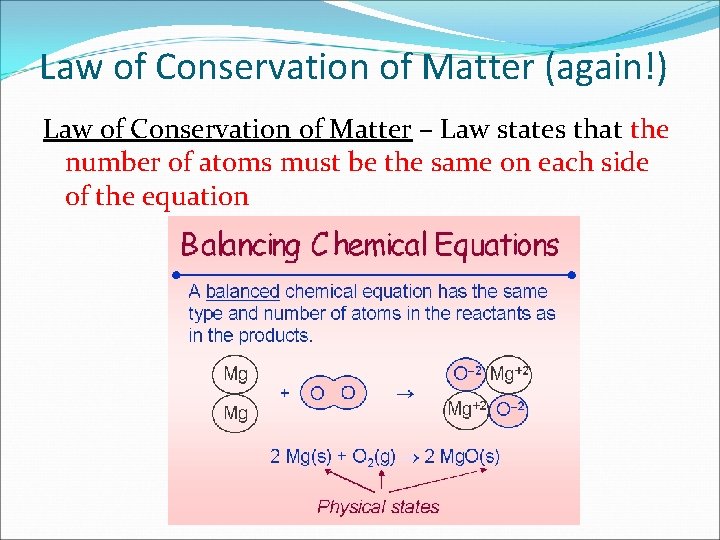
Law of Conservation of Matter (again!) Law of Conservation of Matter – Law states that the number of atoms must be the same on each side of the equation

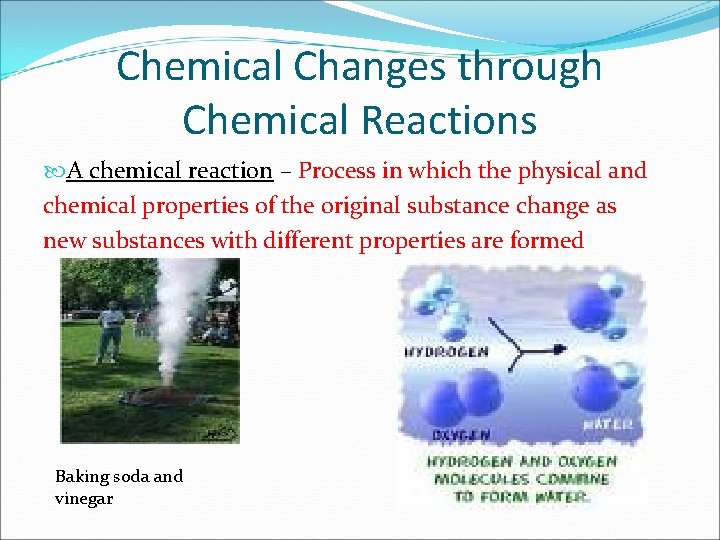
Chemical Changes through Chemical Reactions A chemical reaction – Process in which the physical and chemical properties of the original substance change as new substances with different properties are formed Baking soda and vinegar

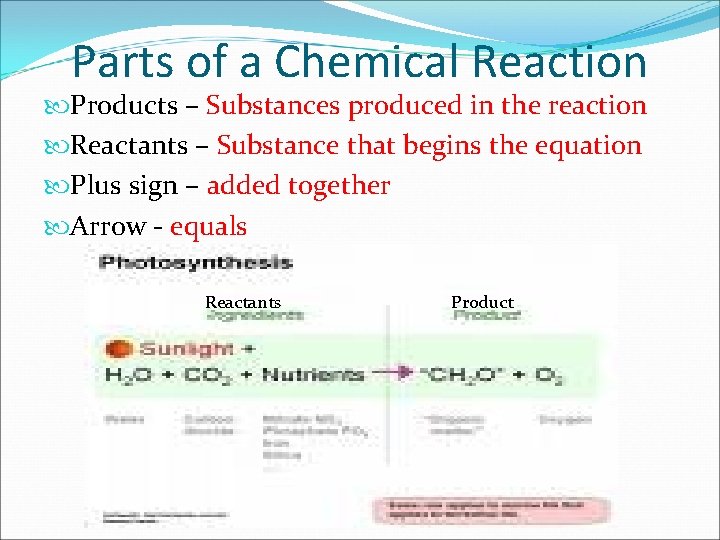
Parts of a Chemical Reaction Products – Substances produced in the reaction Reactants – Substance that begins the equation Plus sign – added together Arrow - equals Reactants Product

 What is the answer to life the universe and everything
What is the answer to life the universe and everything Everything around us is made of
Everything around us is made of All matter is in constant motion
All matter is in constant motion Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dot
Dot Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Bổ thể
Bổ thể Phản ứng thế ankan
Phản ứng thế ankan Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Hát lên người ơi alleluia
Hát lên người ơi alleluia điện thế nghỉ
điện thế nghỉ Tia chieu sa te
Tia chieu sa te Một số thể thơ truyền thống
Một số thể thơ truyền thống Hệ hô hấp
Hệ hô hấp Công của trọng lực
Công của trọng lực Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Tư thế ngồi viết
Tư thế ngồi viết Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào V cc cc
V cc cc Thể thơ truyền thống
Thể thơ truyền thống Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Phép trừ bù
Phép trừ bù Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Thang điểm glasgow
Thang điểm glasgow đại từ thay thế
đại từ thay thế Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể