Matter What is matter Matter is everything around


























- Slides: 27

Matter

What is matter? • Matter is everything around you, including you! • Matter is what all things are made of.

Matter has 3 forms. 1. Solid 2. Liquid 3. Gas

Solids • A solid has its own shape. • A solid does not change unless you cut, bend, or break it. • Solids take up space and have mass.

Liquids • Liquids do not have their own shape. • Liquids take the shape of their container. • Liquids take up space and have mass.

Gases • Gases have no definite size or shape. • Gases take the shape of its container. • A gas will fill all the space inside a container. • Gases take up space and have mass.

Mixtures: • A mixture is a combination of two or more substances in which each pure substance retains its individual chemical properties • This is a physical blending, not a chemical blending. • Composition of the mixture can vary • Can be physically separated

Heterogeneous & Homogeneous Mixtures: • Heterogeneous Mixture- one that does not blend smoothly throughout and which the individual substances remain distinct • Examples: Sand Water, Oil and Water, Cement • Homogeneous Mixture (Solutions)- has constant composition throughout; it always has a single phase • Examples: salt water, vinegar, alloys














METHODS OF SEPARATING MIXTURES • Magnet • Filter • Decant • Evaporation • Centrifuge • Chromatography • Distillation

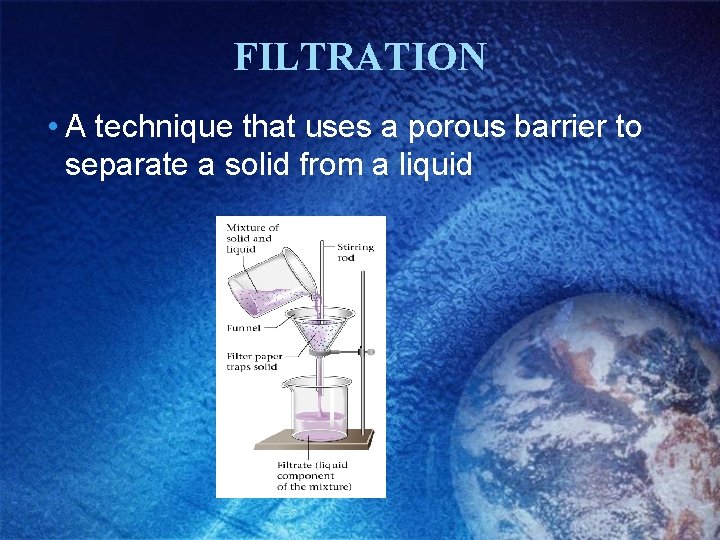
FILTRATION • A technique that uses a porous barrier to separate a solid from a liquid

DISTILLATION • A technique that is based on differences in the boiling points of substances

CRYSTALLIZATION • A technique that results in the formation of pure solid particles of a substance from a solution containing the dissolved substance

CHROMATOGRAPHY • A technique that separates the components of a mixture on the basis of the tendency of each to travel or be drawn across the surface of another material

ELEMENTS AND COMPOUNDS • Elements- are the simplest form of matter that can exist under NORMAL laboratory conditions – Cannot be separated into simpler substances by chemical means – Are the building blocks for all other -substances • Compounds-are substances that can be separated into simpler substances by chemical means