MATTER PURE The universe is composed of matter











- Slides: 39

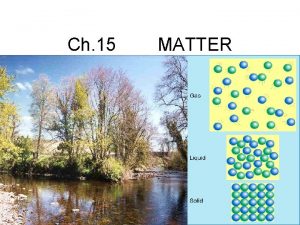
MATTER PURE

The universe is composed of matter energy either _______ or _______.

mass Matter is anything that has _______ space and occupies ____. trees air bear rocks soil water

Mass is a measure of the amount of matter ________ in an object. Grams , g The unit for mass is ________.

Compare the masses

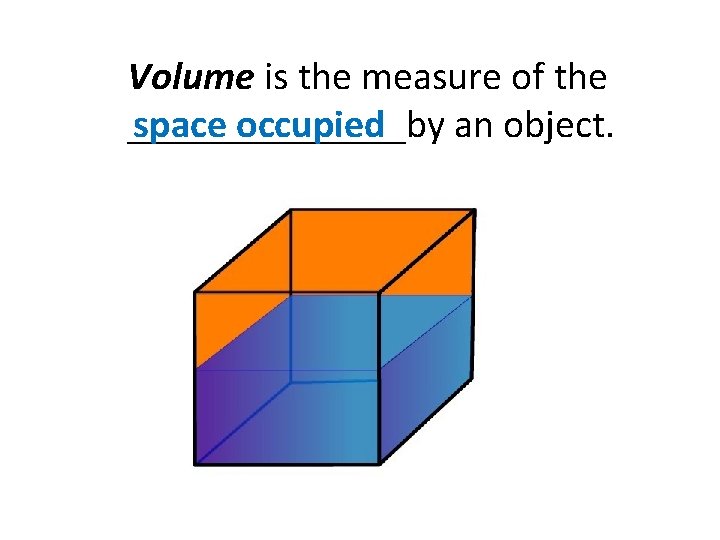
Volume is the measure of the space occupied _______by an object.

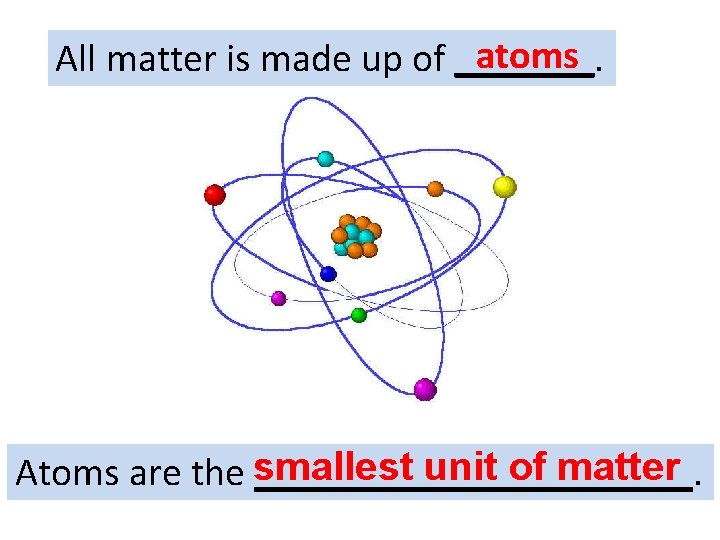
atoms All matter is made up of _______. smallest unit of matter Atoms are the ___________.

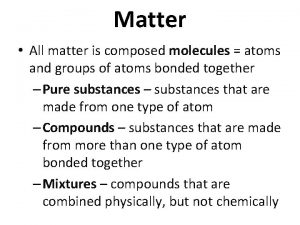
There are two types of matter: (1) Pure substance (2) Mixture

PURE SUBSTANCE Pure substances is matter that is composed of an element or a compound and cannot be separated by physical or chemical means.

Pure substance

Pure substance

Pure substance

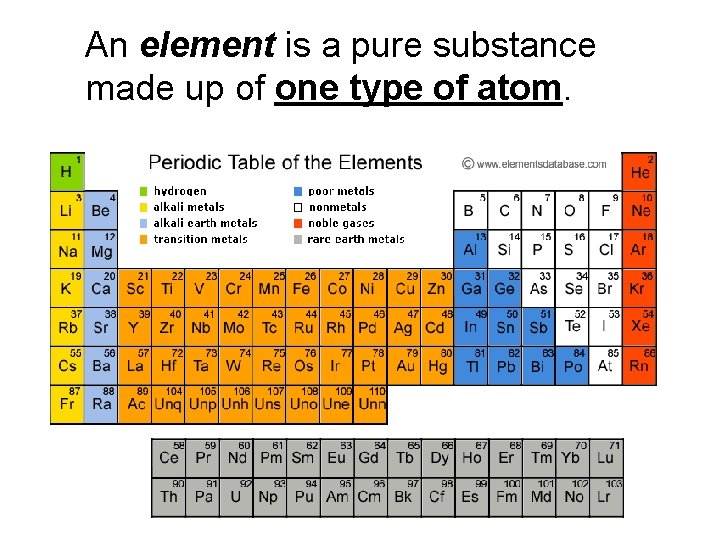
An element is a pure substance made up of one type of atom.

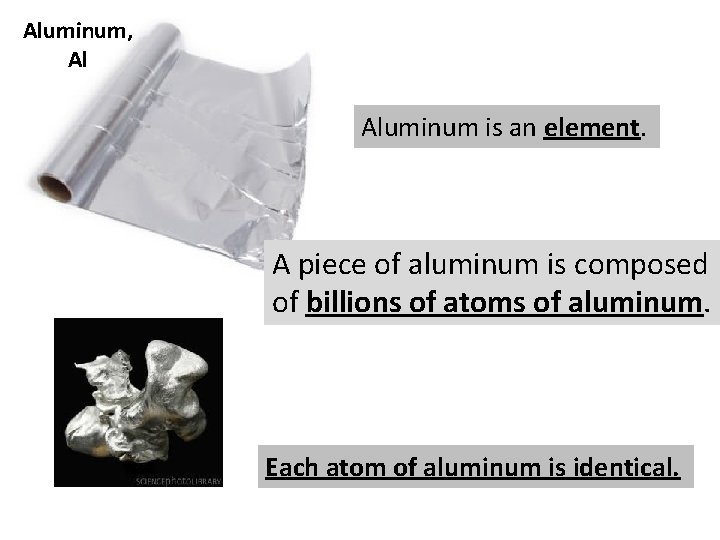
Aluminum, Al Aluminum is an element. A piece of aluminum is composed of billions of atoms of aluminum. Each atom of aluminum is identical.

There are 109 elements. Examples? ? ?

What makes one element different from another element? Their atoms!

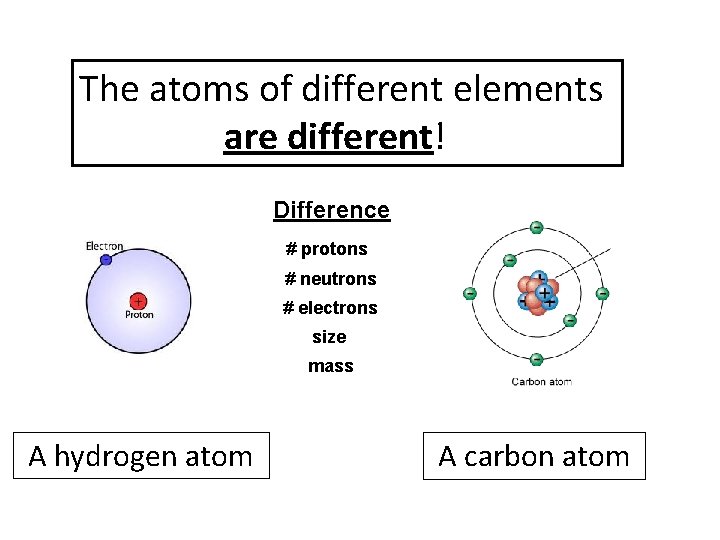
The atoms of different elements are different! Difference # protons # neutrons # electrons size mass A hydrogen atom A carbon atom

There are 109 different atoms.

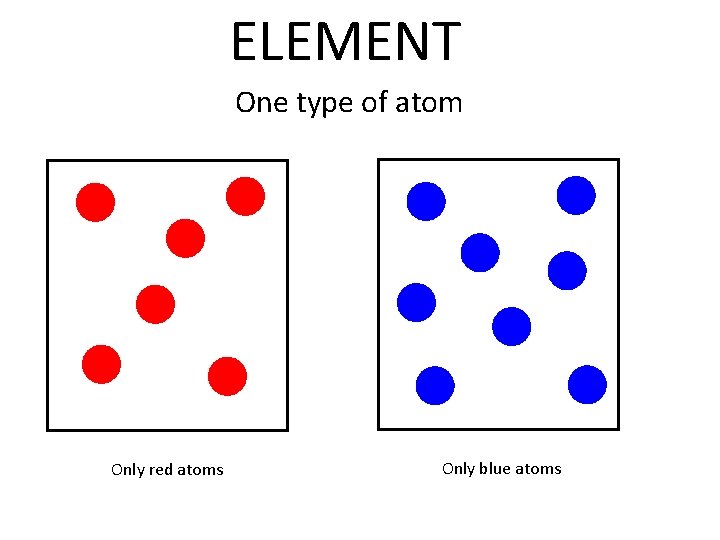
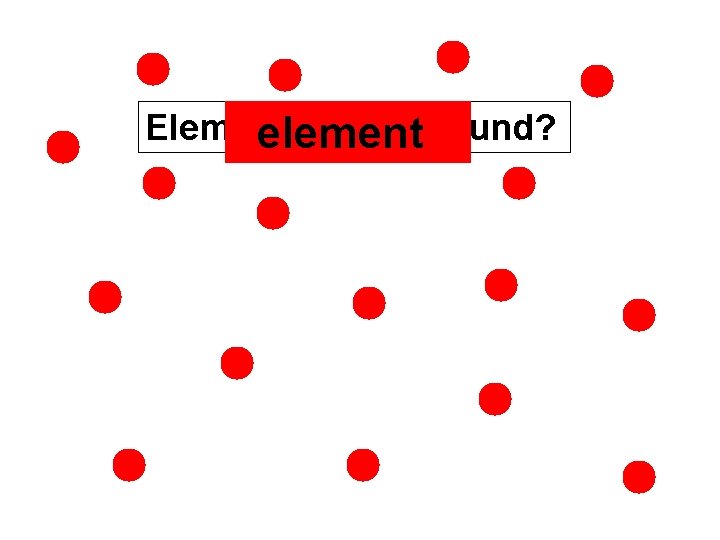
ELEMENT One type of atom Only red atoms Only blue atoms

Most elements do not exist alone. Elements combine to form compounds. Compound is a pure substance that is composed of atoms of two or more different elements(atoms) chemically joined.

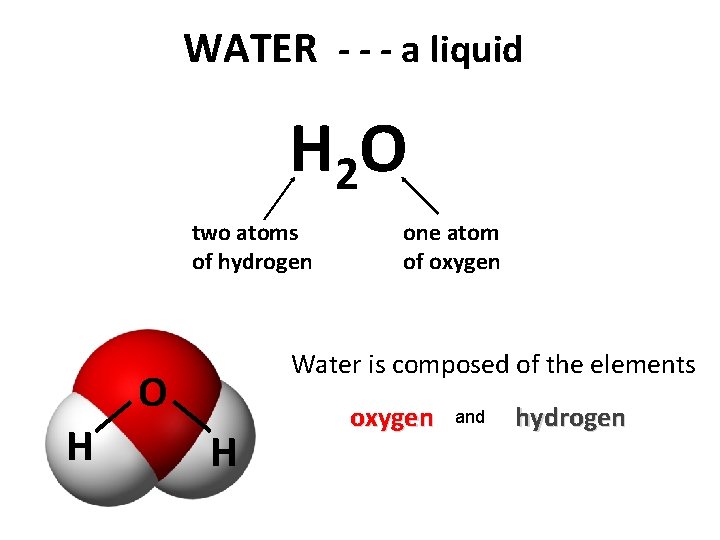
WATER - - - a liquid H 2 O two atoms of hydrogen Water is composed of the elements O H one atom of oxygen H oxygen and hydrogen

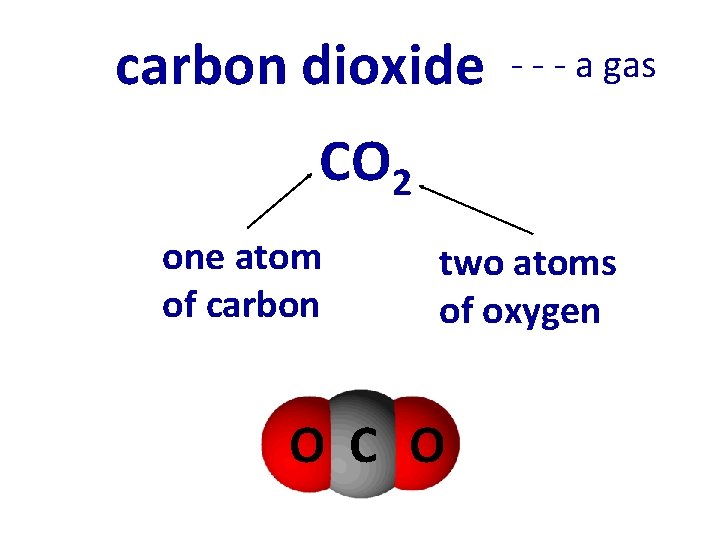
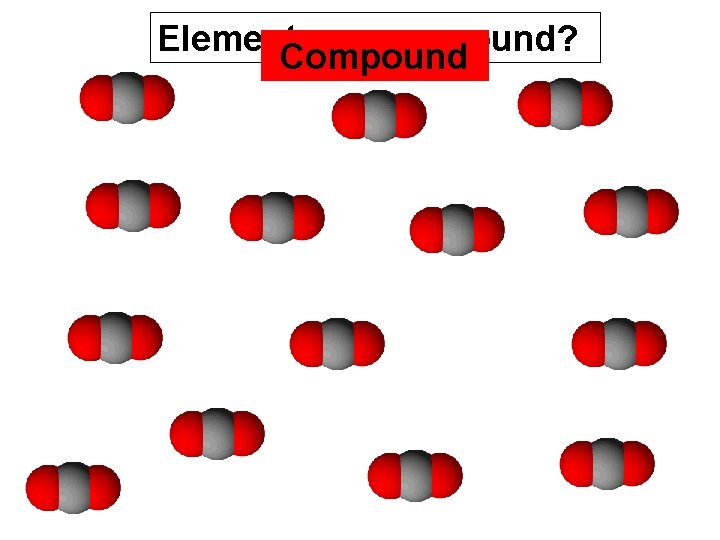
carbon dioxide - - - a gas CO 2 one atom of carbon two atoms of oxygen O C O

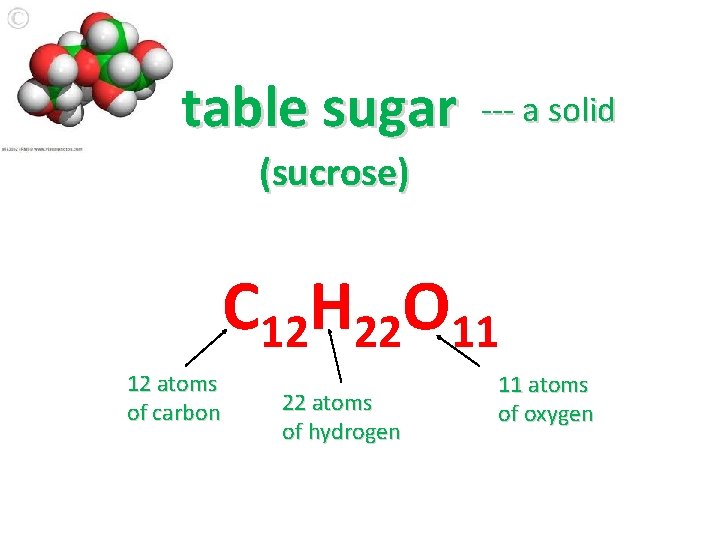
table sugar --- a solid (sucrose) C 12 H 22 O 11 12 atoms of carbon 22 atoms of hydrogen 11 atoms of oxygen

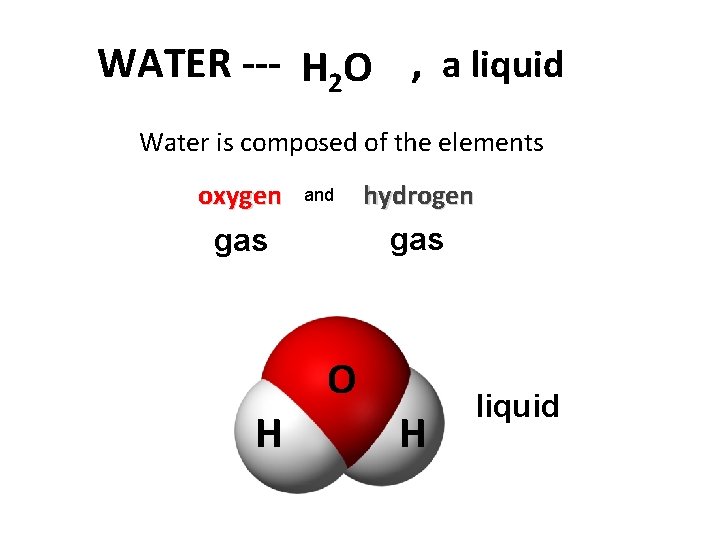
WATER --- H 2 O , a liquid Water is composed of the elements oxygen and hydrogen gas O H H liquid

Table salt - - - Na. Cl --- sodium chloride Sodium (Na) is a solid chlorine (Cl) is a poisonous gas, yet they combine to form table salt, an edible solid.

Compounds are completely different from the elements that make them up!!!! Na. Cl H 2 O

sodium + chlorine = sodium chloride Na + Cl = Na. Cl http: //www. youtube. com/watch? v=tb. Pxw. Di. X 1 NU

hydrogen + oxygen = H 2 + O 2 = water H 2 O http: //www. youtube. com/watch? v=DP 05 fx. R 4 o. Uk

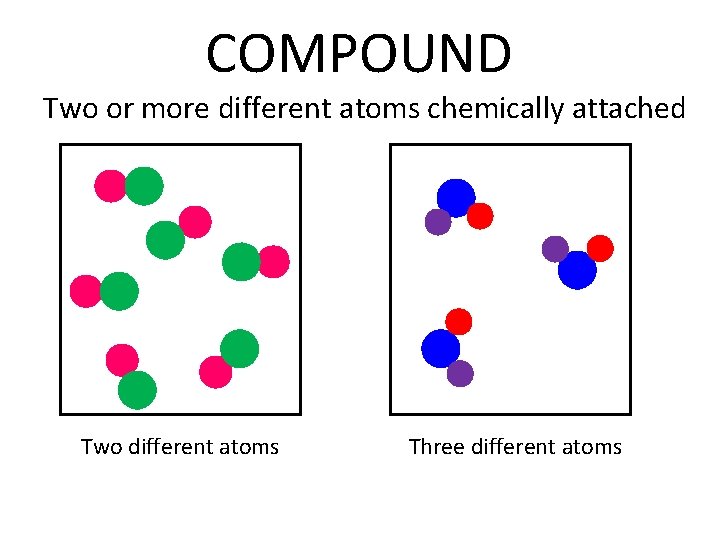
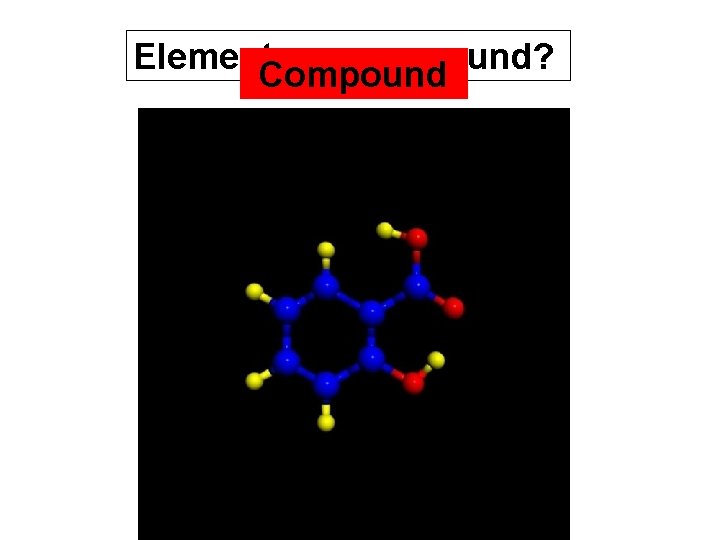
COMPOUND Two or more different atoms chemically attached Two different atoms Three different atoms

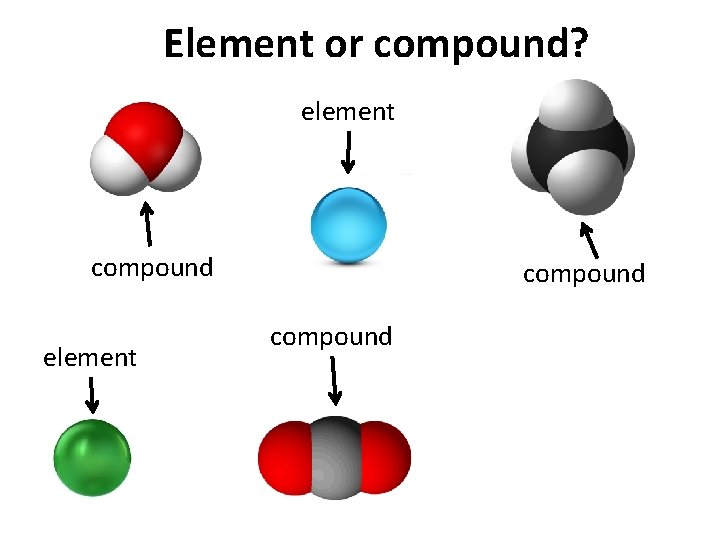
Element or compound? element compound

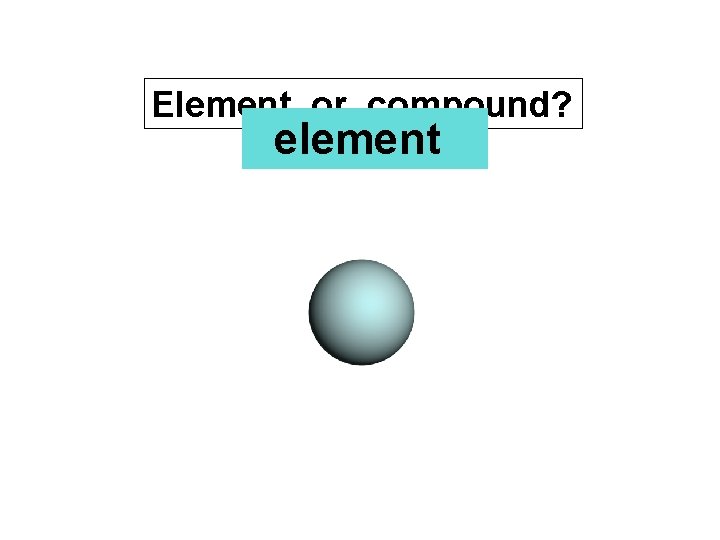
Element or compound? element

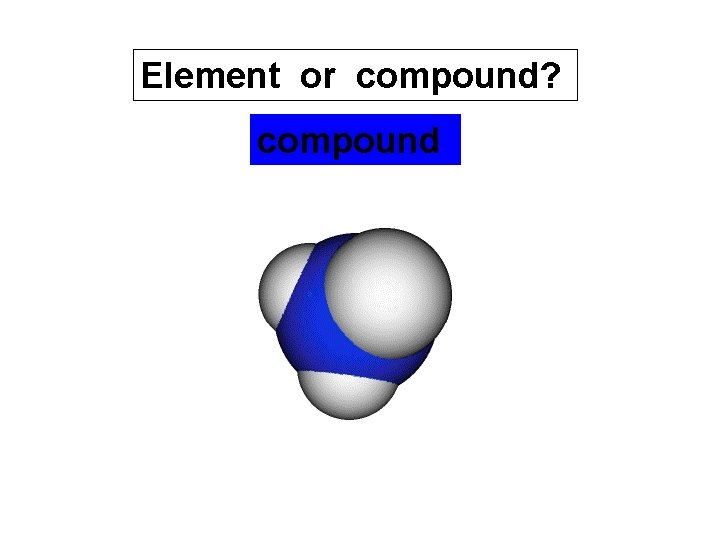
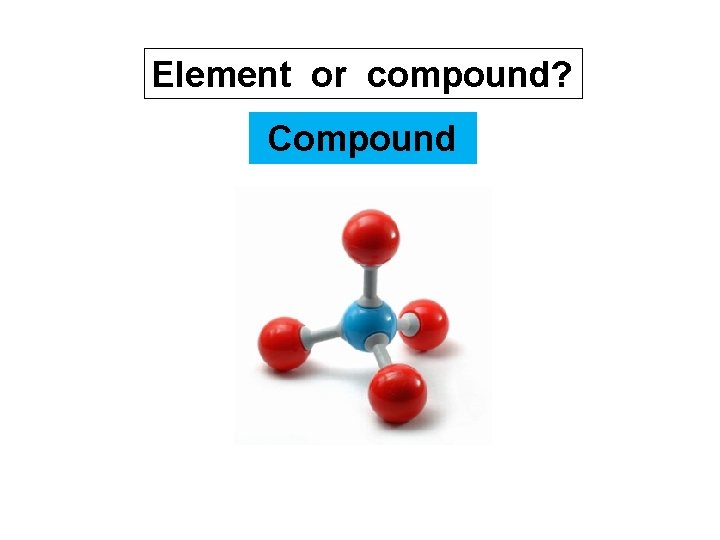
Element or compound? compound

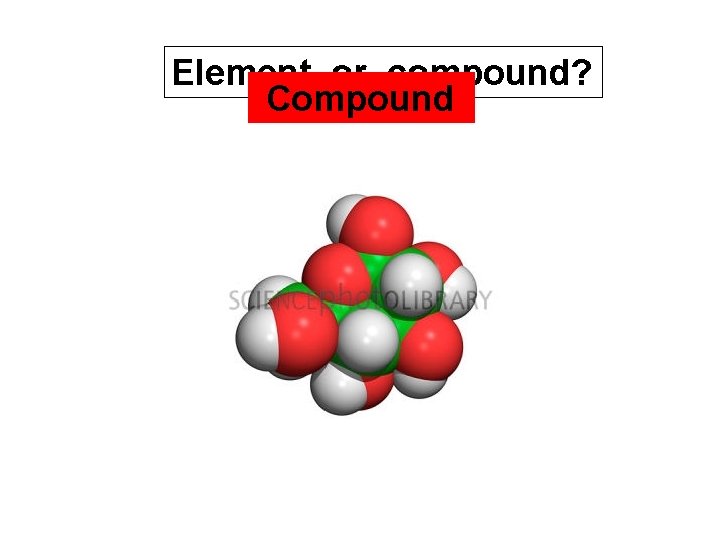
Element or compound? Compound

Element or compound? element

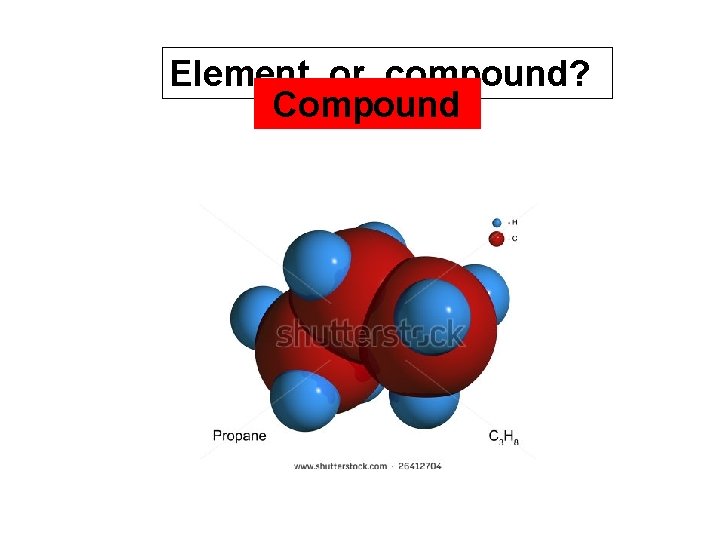
Element or compound? Compound

Element or compound? Compound

Element. Compound or compound?

Element or compound? Compound

Element or compound? element
 Universe is composed of
Universe is composed of All life is composed of matter
All life is composed of matter Mecanical mixture
Mecanical mixture đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Cái miệng nó xinh thế
Cái miệng nó xinh thế Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Tư thế ngồi viết
Tư thế ngồi viết Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Gấu đi như thế nào
Gấu đi như thế nào Thể thơ truyền thống
Thể thơ truyền thống Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Frameset trong html5
Frameset trong html5 Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Thang điểm glasgow
Thang điểm glasgow đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dot
Dot Bổ thể
Bổ thể Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 độ dài liên kết
độ dài liên kết Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Phối cảnh
Phối cảnh Hát lên người ơi alleluia
Hát lên người ơi alleluia