Composition of Matter Matter Everything in the universe



- Slides: 17

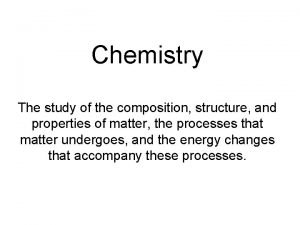
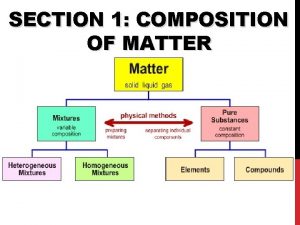
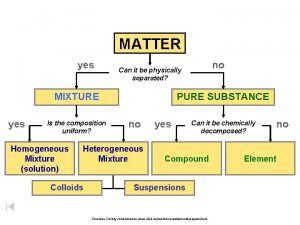
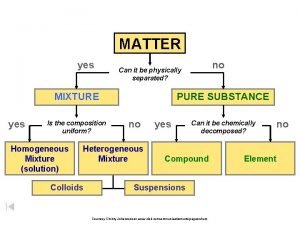
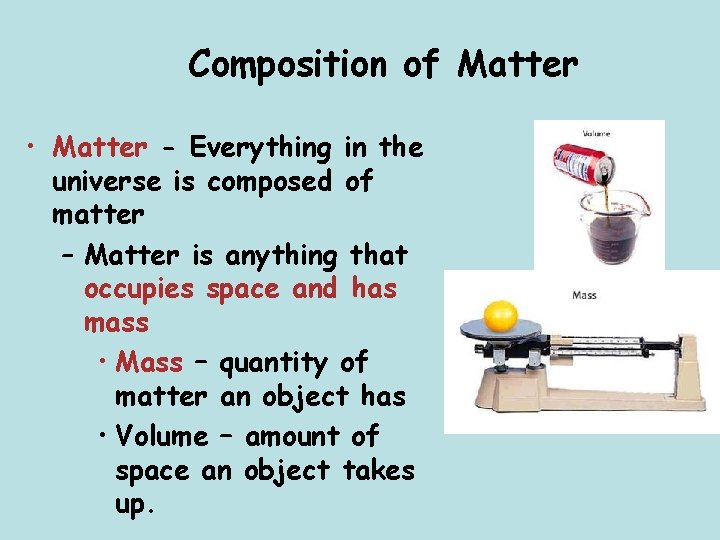
Composition of Matter • Matter - Everything in the universe is composed of matter – Matter is anything that occupies space and has mass • Mass – quantity of matter an object has • Volume – amount of space an object takes up.

Elements • Pure substances that cannot be broken down chemically into simpler kinds of matter • More than 100 elements (92 naturally occurring)

CHNOPS • 6 most common elements in living things are: – Carbon (C) – Hydrogen (H) – Nitrogen (N) – Oxygen (O) – Phosphorus (P) – Sulfur (S) • 90% or more of the mass of an organism is composed of C, H, N and O

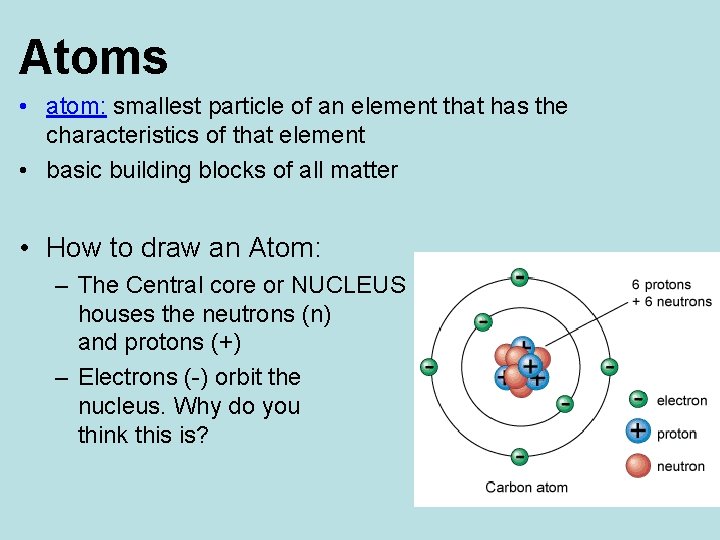
Atoms • atom: smallest particle of an element that has the characteristics of that element • basic building blocks of all matter • How to draw an Atom: – The Central core or NUCLEUS houses the neutrons (n) and protons (+) – Electrons (-) orbit the nucleus. Why do you think this is?

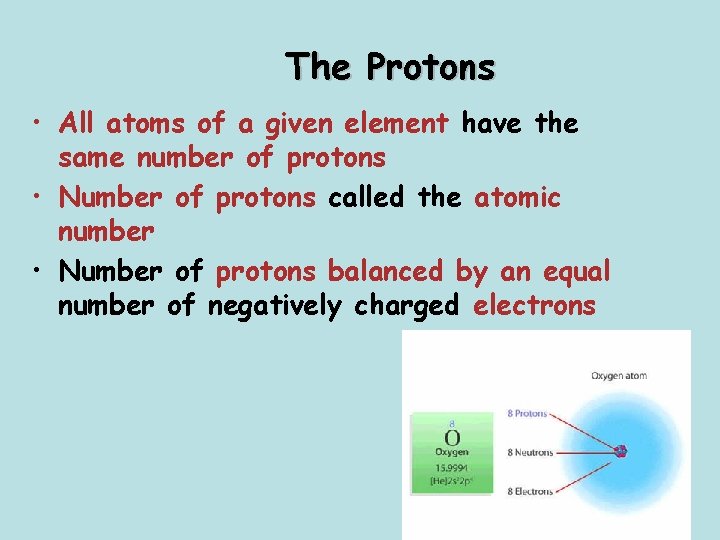
The Protons • All atoms of a given element have the same number of protons • Number of protons called the atomic number • Number of protons balanced by an equal number of negatively charged electrons

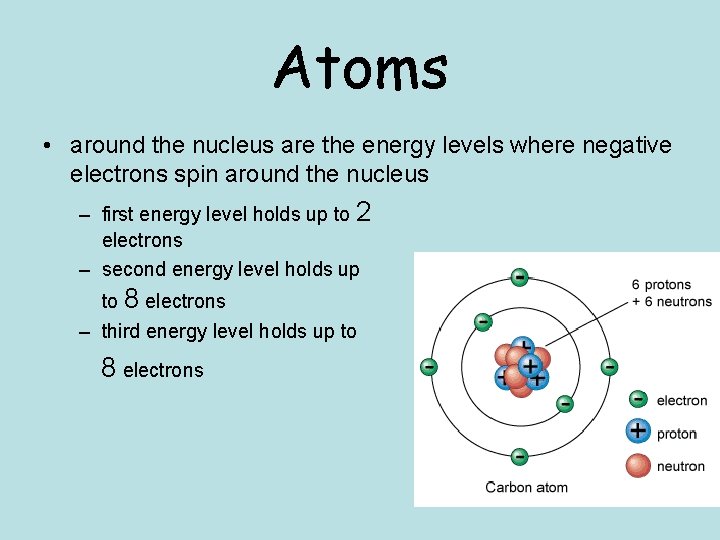
Atoms • around the nucleus are the energy levels where negative electrons spin around the nucleus – first energy level holds up to 2 electrons – second energy level holds up to 8 electrons – third energy level holds up to 8 electrons

How can you find the following? • What is the relationship between an atom and an element? Many atoms with the same # of protons make up elements • # of protons in an element? Atomic number • # of electrons in an element? # of protons • # of neutrons in an element? Atomic mass - # of protons

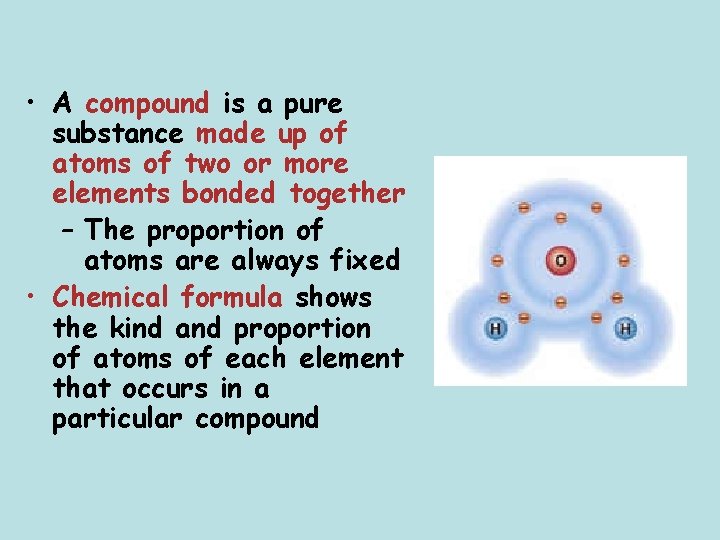
• A compound is a pure substance made up of atoms of two or more elements bonded together – The proportion of atoms are always fixed • Chemical formula shows the kind and proportion of atoms of each element that occurs in a particular compound

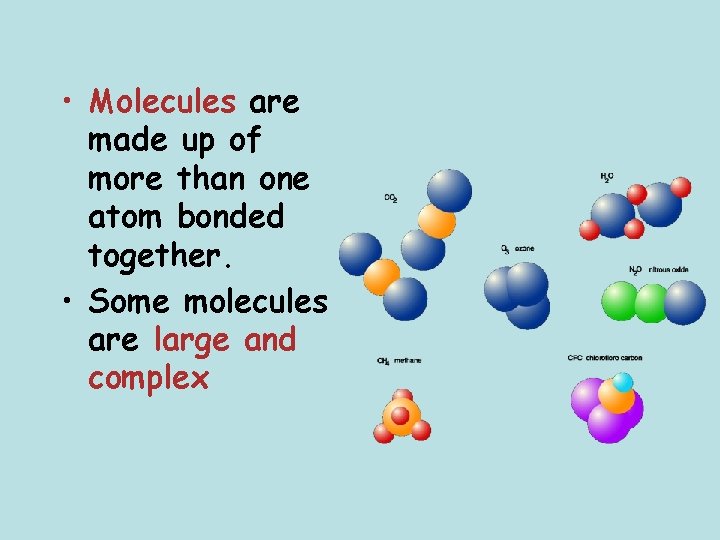
• Molecules are made up of more than one atom bonded together. • Some molecules are large and complex

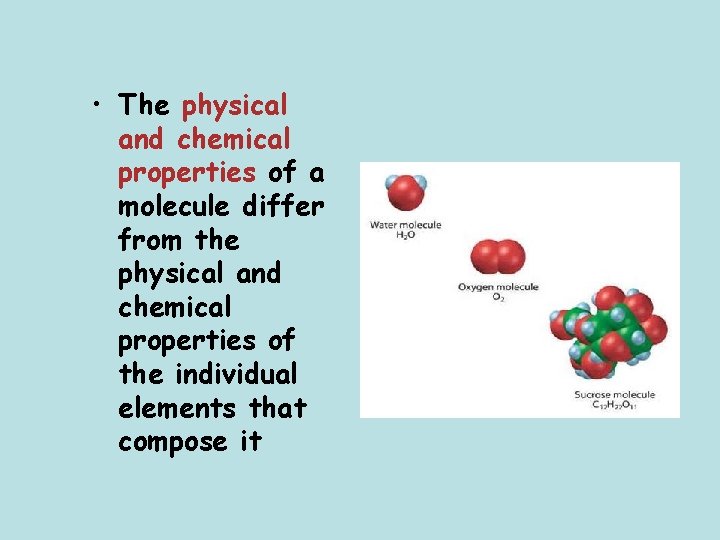
• The physical and chemical properties of a molecule differ from the physical and chemical properties of the individual elements that compose it

How do you draw an Atom to show its electrons will bond? Bohr Diagram: shows ALL electrons with a + (#) and n (#) in the nucleus Ex: Lewis Dot Structure: shows only the VALENCE electrons around the element symbol Ex:

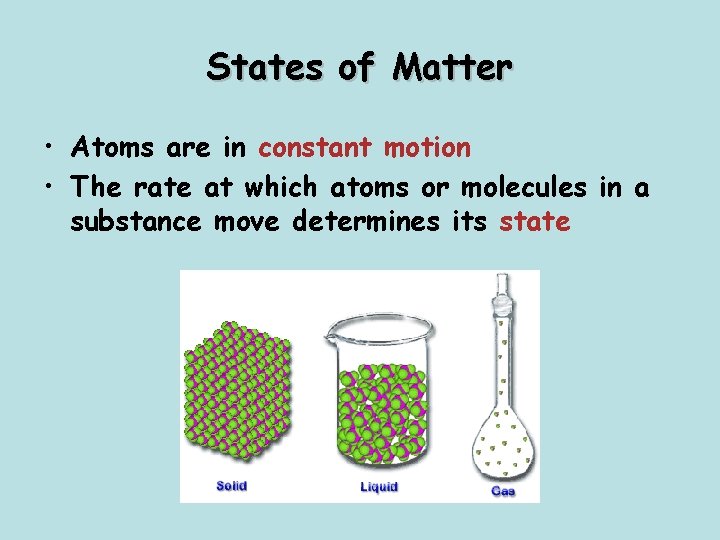
States of Matter • Atoms are in constant motion • The rate at which atoms or molecules in a substance move determines its state

Why do atoms bond? • For the same reasons WE do! – An Atom is typically unstable by itself – It will bond to other atoms so that it can fill up its outside shell of electrons. – Review: When an element bonds to become more stable it is then called a: Molecule

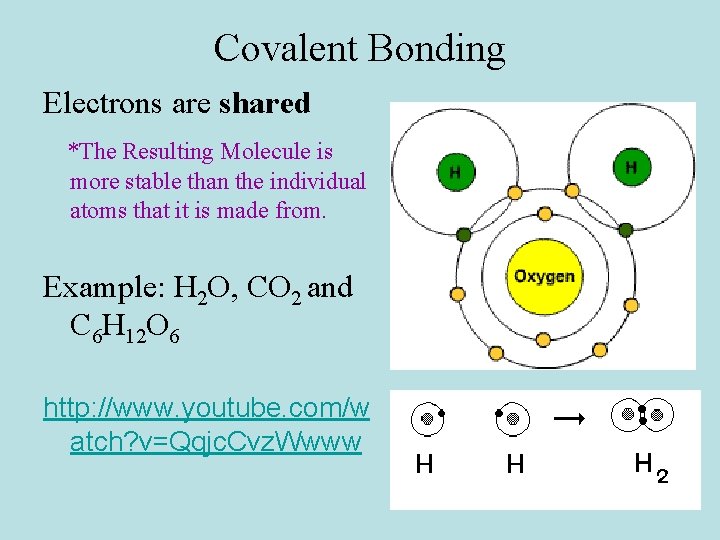
Covalent Bonding Electrons are shared *The Resulting Molecule is more stable than the individual atoms that it is made from. Example: H 2 O, CO 2 and C 6 H 12 O 6 http: //www. youtube. com/w atch? v=Qqjc. Cvz. Wwww

Polarity • Molecules with partial charges on opposite ends ex: H 2 O • Polar Covalent Bonding: electrons are shared unequally, so the electrical charge is not balanced (ex: sugar) • Non- Polar Covalent Bonding: electrons are shared equally by united atoms with a balanced electrical charge (ex: oil, grease, wax)

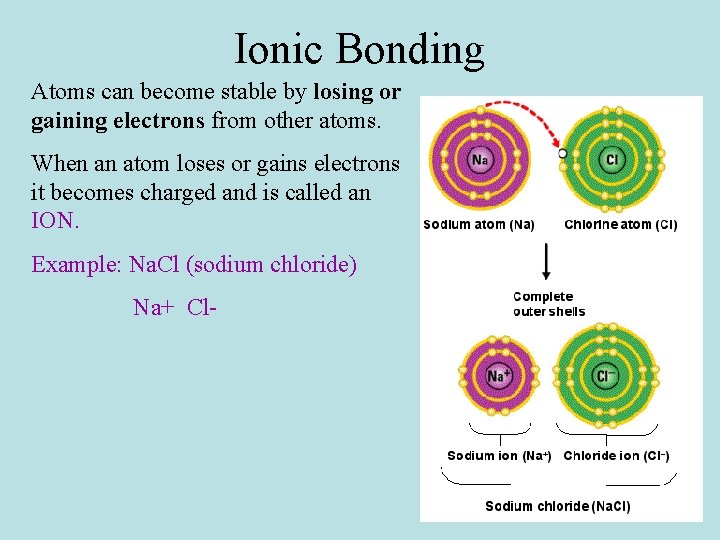
Ionic Bonding Atoms can become stable by losing or gaining electrons from other atoms. When an atom loses or gains electrons it becomes charged and is called an ION. Example: Na. Cl (sodium chloride) Na+ Cl-

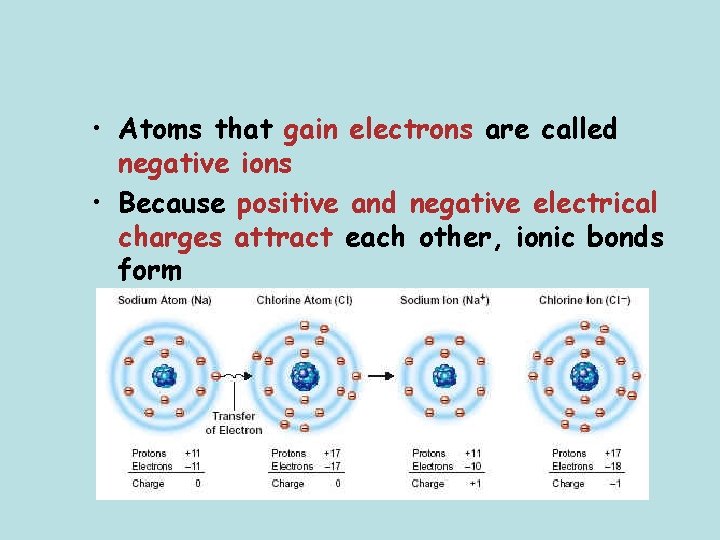
• Atoms that gain electrons are called negative ions • Because positive and negative electrical charges attract each other, ionic bonds form
 What is the answer to life the universe and everything
What is the answer to life the universe and everything Section 1 composition of matter
Section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Everything around us is made up of matter
Everything around us is made up of matter States of matter flowchart
States of matter flowchart Composition of matter notes
Composition of matter notes Composition of matter flow chart
Composition of matter flow chart The study of composition structure and properties of matter
The study of composition structure and properties of matter What is composition in matter
What is composition in matter Properties of solid liquid and gas
Properties of solid liquid and gas Section 1 composition of matter
Section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Matter with uniform compositions.
Matter with uniform compositions. Mixture
Mixture Compounds vs mixtures
Compounds vs mixtures Concept map about matter
Concept map about matter Composition of matter flow chart
Composition of matter flow chart