CHEMISTRY Composition of Matter n Matter Everything in
















































- Slides: 119

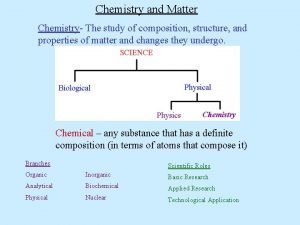
CHEMISTRY

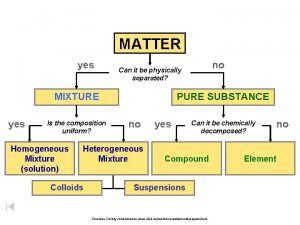
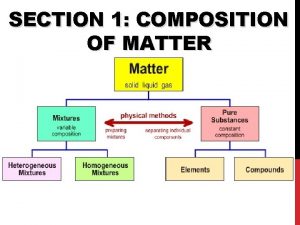
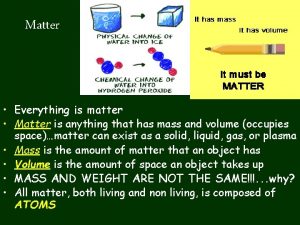
Composition of Matter n Matter - Everything in universe is composed of matter n Matter is anything that occupies space or has mass n Mass – quantity of matter an object has n Weight – pull of gravity on an object

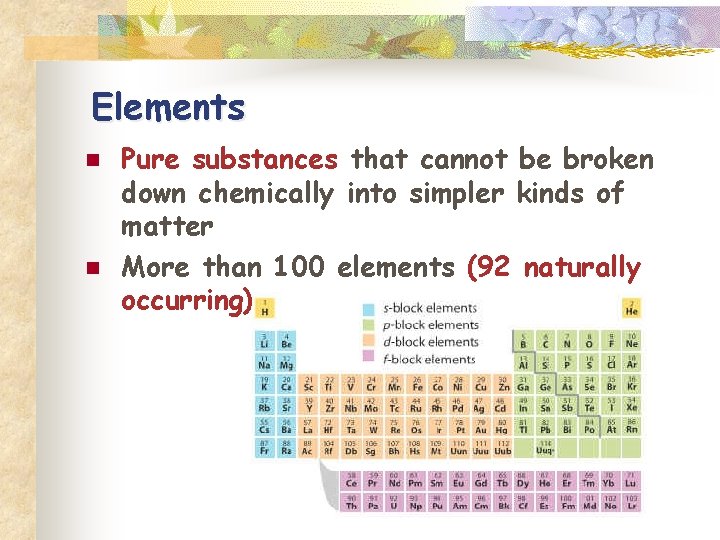
Elements n n Pure substances that cannot be broken down chemically into simpler kinds of matter More than 100 elements (92 naturally occurring)

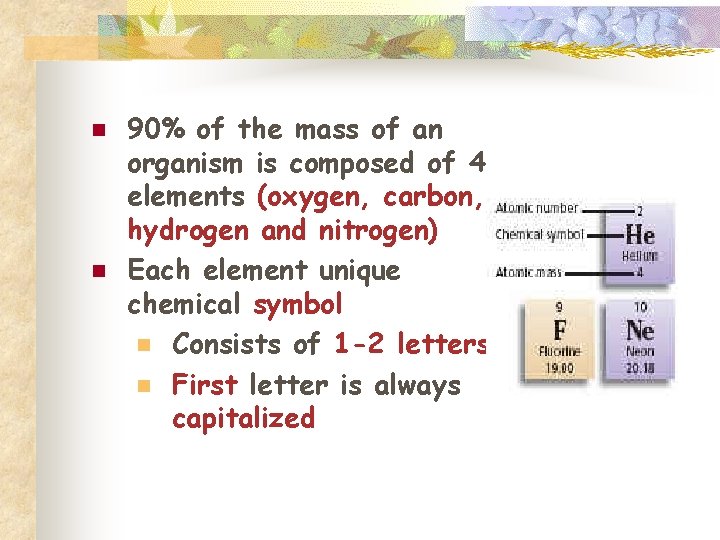
n n 90% of the mass of an organism is composed of 4 elements (oxygen, carbon, hydrogen and nitrogen) Each element unique chemical symbol n Consists of 1 -2 letters n First letter is always capitalized

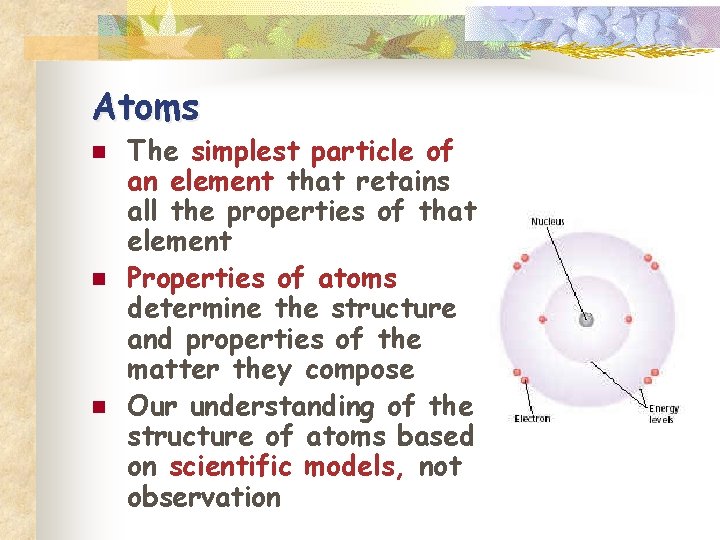
Atoms n n n The simplest particle of an element that retains all the properties of that element Properties of atoms determine the structure and properties of the matter they compose Our understanding of the structure of atoms based on scientific models, not observation

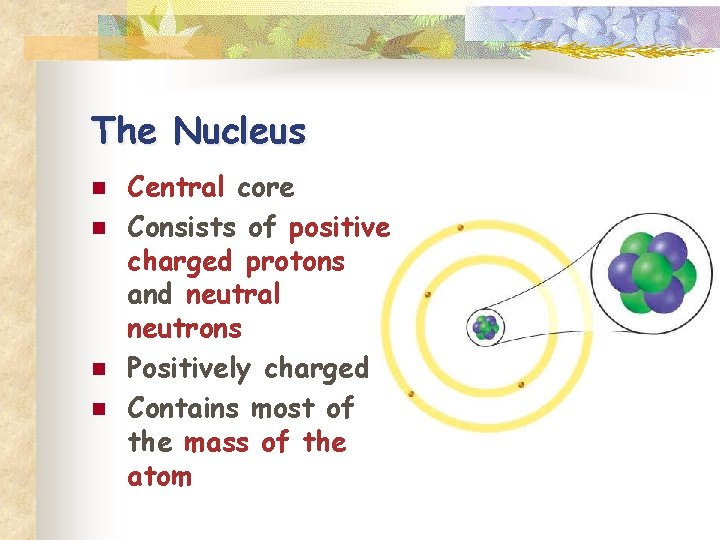
The Nucleus n n Central core Consists of positive charged protons and neutral neutrons Positively charged Contains most of the mass of the atom

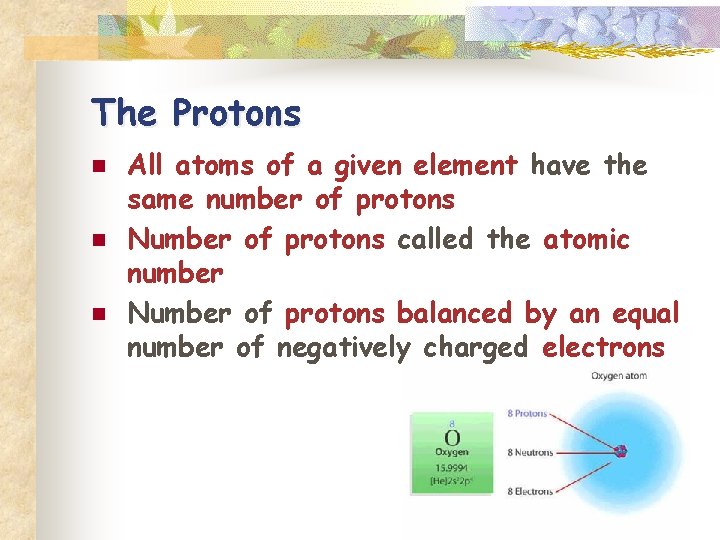
The Protons n n n All atoms of a given element have the same number of protons Number of protons called the atomic number Number of protons balanced by an equal number of negatively charged electrons

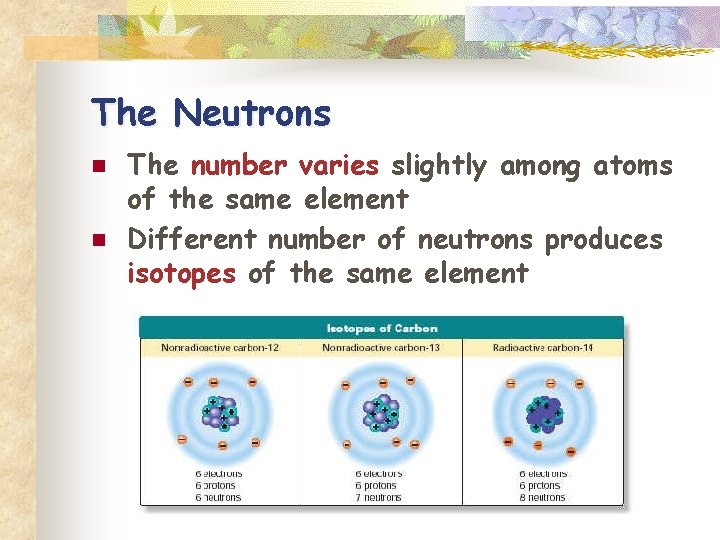
The Neutrons n n The number varies slightly among atoms of the same element Different number of neutrons produces isotopes of the same element

Atomic Mass n n n Protons & neutrons are found in the nucleus of an atom Protons and neutrons each have a mass of 1 amu (atomic mass unit) The atomic mass of an atom is found by adding the number of protons & neutrons in an atom

The Electrons n n Negatively charged high energy particles with little or no mass Travel at very high speeds at various distances (energy levels) from the nucleus

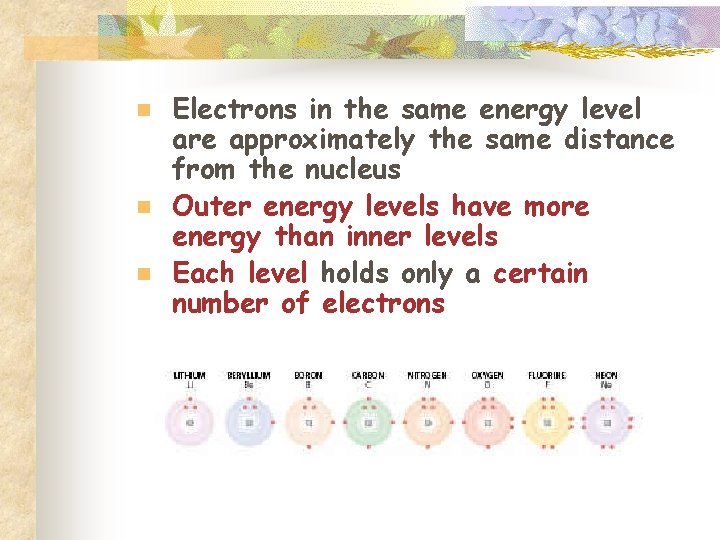
n n n Electrons in the same energy level are approximately the same distance from the nucleus Outer energy levels have more energy than inner levels Each level holds only a certain number of electrons

Energy Levels n n Atoms have 7 energy levels The levels are K (closest to the nucleus), L, M, N, O, P, Q (furthest from the nucleus) The K level can only hold 2 electrons Levels L – Q can hold 8 electrons (octet rule)

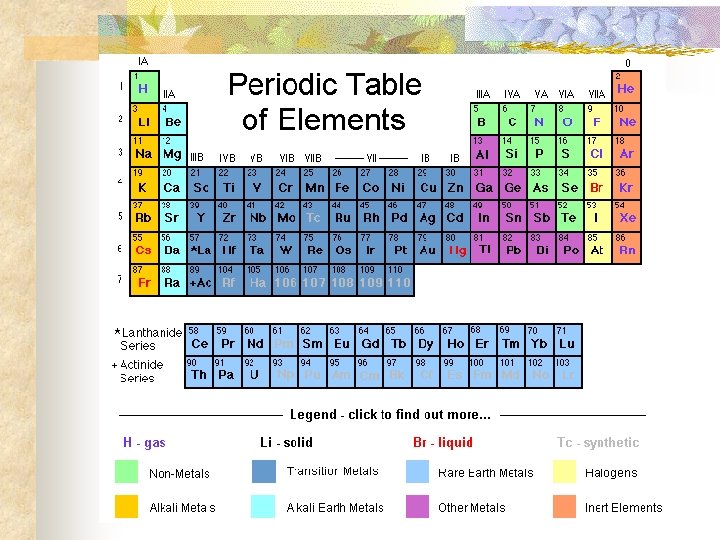
Periodic Table n n n Elements are arranged by their atomic number on the Periodic Table The horizontal rows are called Periods & tell the number of energy levels Vertical groups are called Families & tell the outermost number of electrons


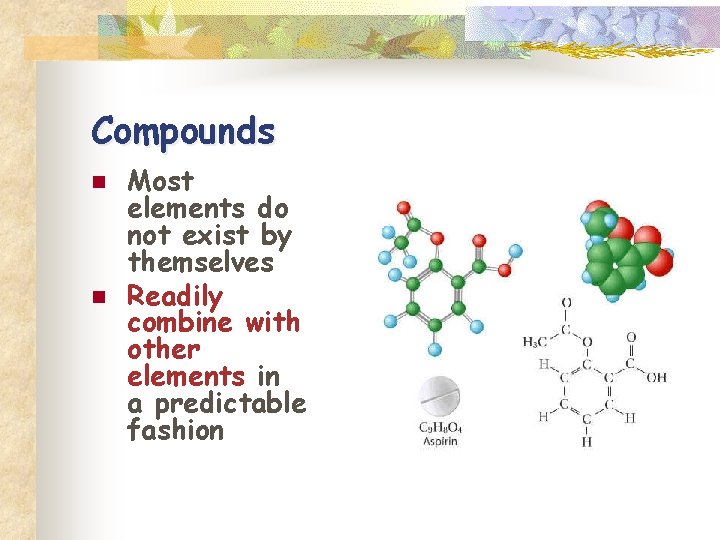
Compounds n n Most elements do not exist by themselves Readily combine with other elements in a predictable fashion

n n A compound is a pure substance made up of atoms of two or more elements n The proportion of atoms are always fixed Chemical formula shows the kind and proportion of atoms of each element that occurs in a particular compound

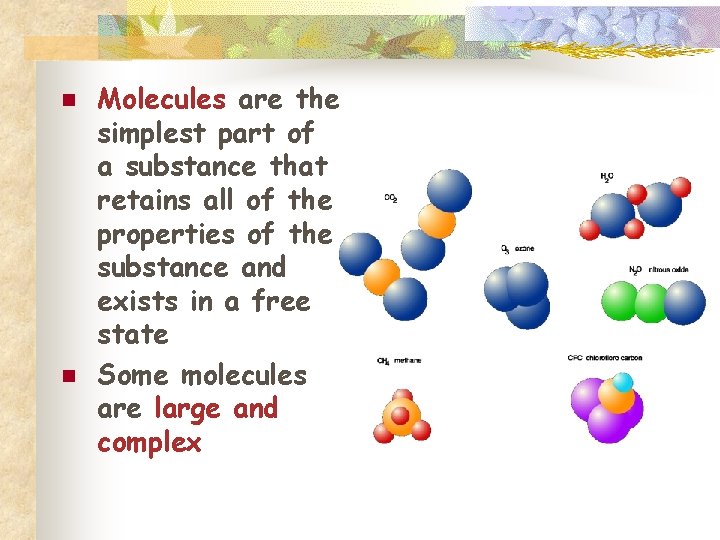
n n Molecules are the simplest part of a substance that retains all of the properties of the substance and exists in a free state Some molecules are large and complex

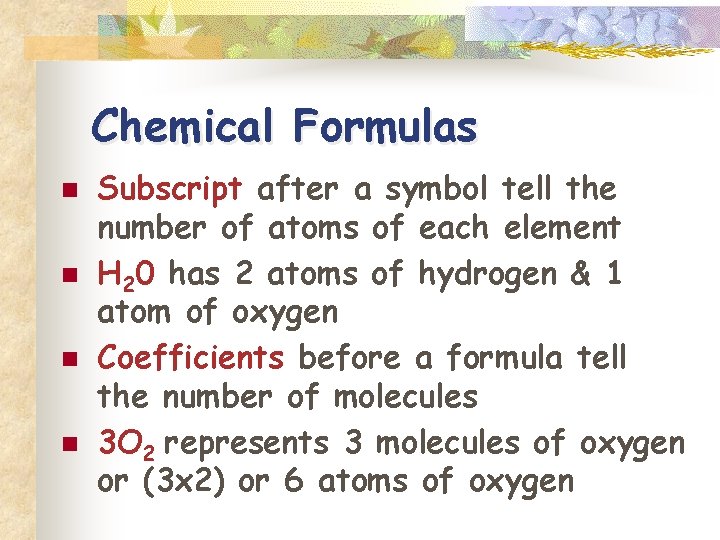
Chemical Formulas n n Subscript after a symbol tell the number of atoms of each element H 20 has 2 atoms of hydrogen & 1 atom of oxygen Coefficients before a formula tell the number of molecules 3 O 2 represents 3 molecules of oxygen or (3 x 2) or 6 atoms of oxygen

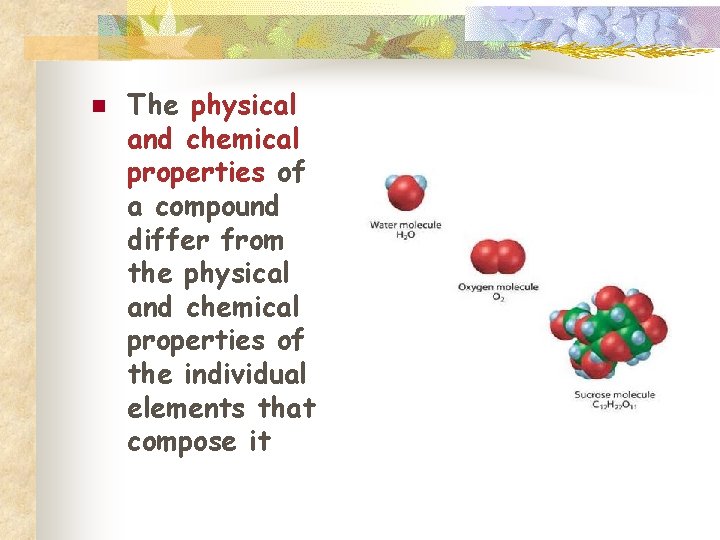
n The physical and chemical properties of a compound differ from the physical and chemical properties of the individual elements that compose it

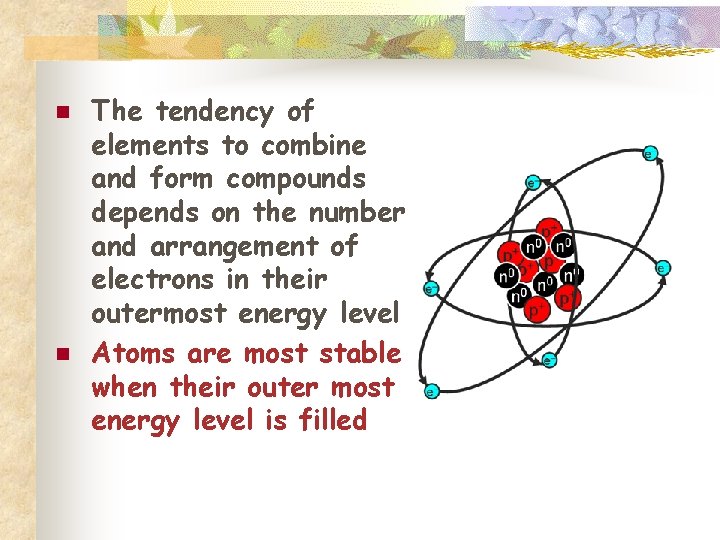
n n The tendency of elements to combine and form compounds depends on the number and arrangement of electrons in their outermost energy level Atoms are most stable when their outer most energy level is filled

n n n Most atoms are not stable in their natural state Tend to react (combine) with other atoms in order to become more stable (undergo chemical reactions) In chemical reactions bonds are broken; atoms rearranged and new chemical bonds are formed that store energy

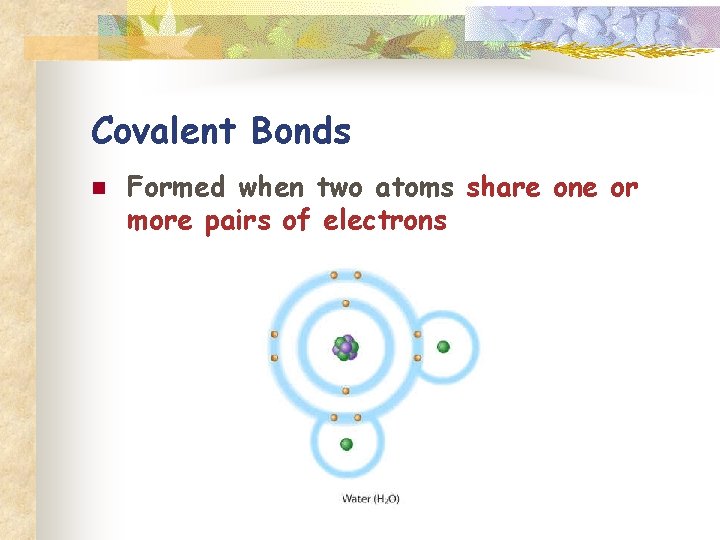
Covalent Bonds n Formed when two atoms share one or more pairs of electrons

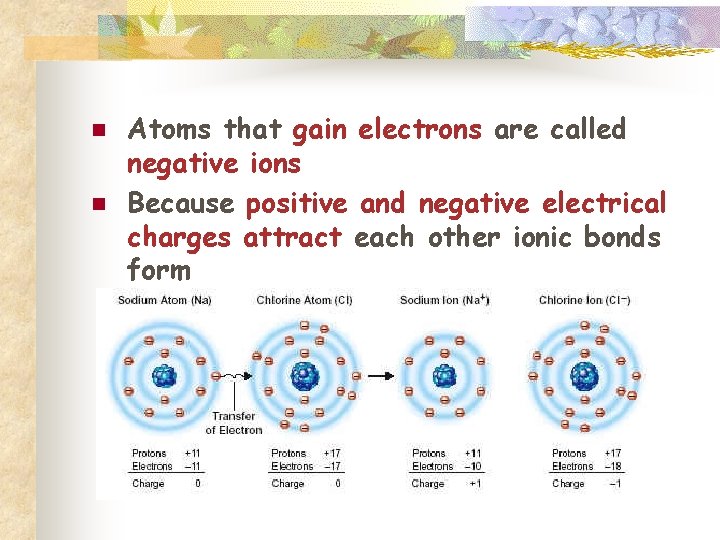
Ionic Bonds n n Some atoms become stable by losing or gaining electrons Atoms that lose electrons are called positive ions

n n Atoms that gain electrons are called negative ions Because positive and negative electrical charges attract each other ionic bonds form

Energy and Matter n Energy n The ability to do work or cause change n Occurs in various forms n Can be converted to another form n Forms important to biological systems are chemical, thermal, electrical and mechanical energy n Free energy is the energy in a system that is available for work

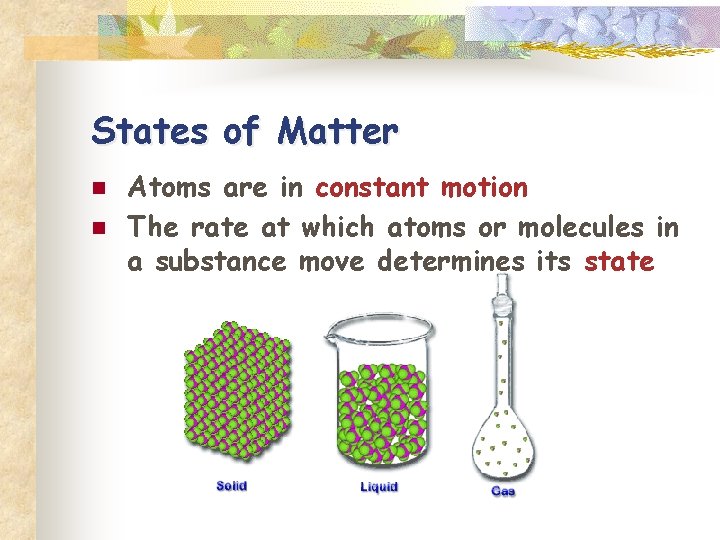
States of Matter n n Atoms are in constant motion The rate at which atoms or molecules in a substance move determines its state

n Solid n Molecules tightly linked together in a definite shape n Vibrate in place n Fixed volume and shape

n Liquids n Molecules not as tightly linked as a solid n Maintain fixed volume n Able to flow and conform to shape of container

n. Gas Molecules have little or no attraction to each other n Fill the volume of the occupied container n Move most rapidly n To cause a substance to change state, thermal energy (heat) must be added to or removed from a substance n

Energy and Chemical Reactions n Living things undergo thousands of chemical reactions as part of the life process

n n Many are very complex involving multistep sequences called biochemical pathways Chemical equations represent chemical reactions Reactants are shown on the left side of the equation Products are shown on the right side

n n The number of each kind of atom must be the same on either side of the arrow (equation must be balanced) Bonds may be broken or made forming new compounds

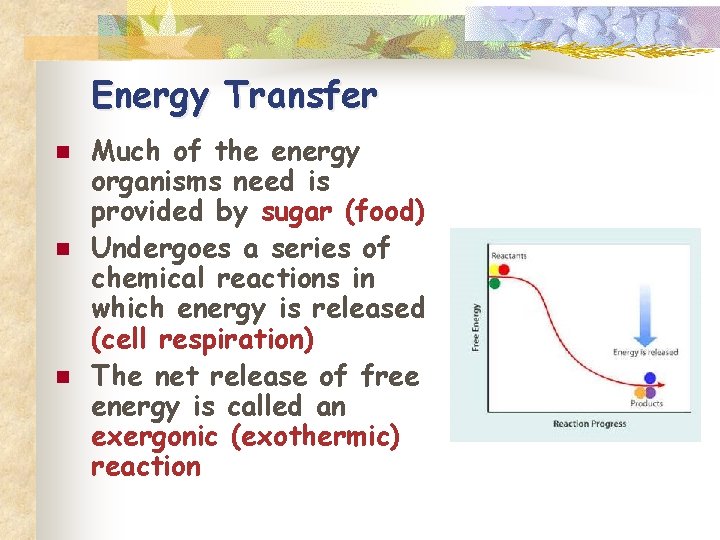
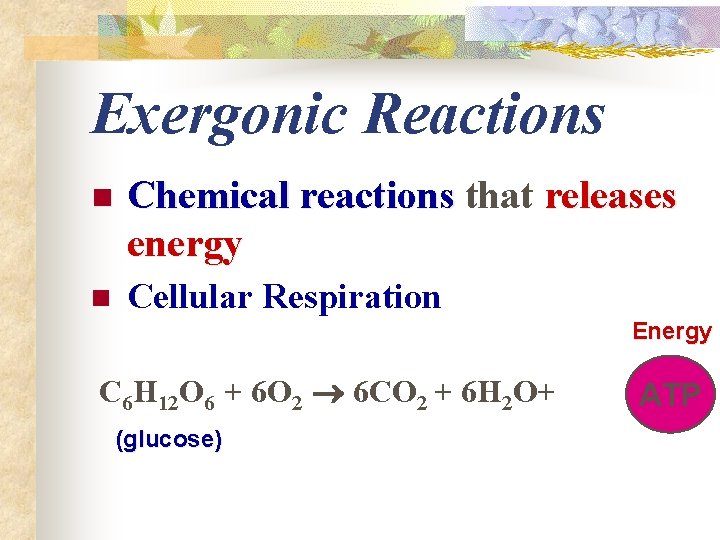
Energy Transfer n n n Much of the energy organisms need is provided by sugar (food) Undergoes a series of chemical reactions in which energy is released (cell respiration) The net release of free energy is called an exergonic (exothermic) reaction

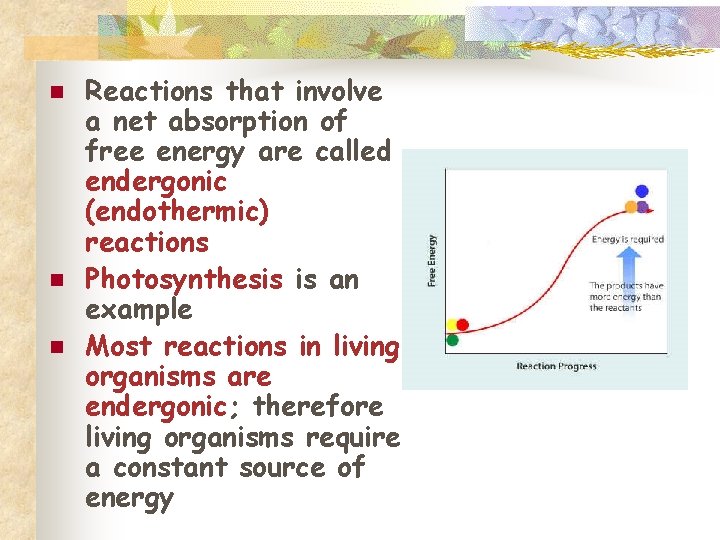
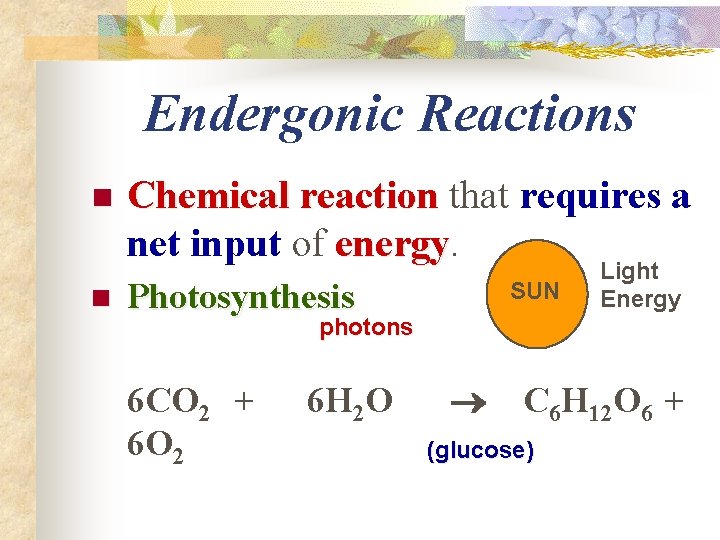
n n n Reactions that involve a net absorption of free energy are called endergonic (endothermic) reactions Photosynthesis is an example Most reactions in living organisms are endergonic; therefore living organisms require a constant source of energy

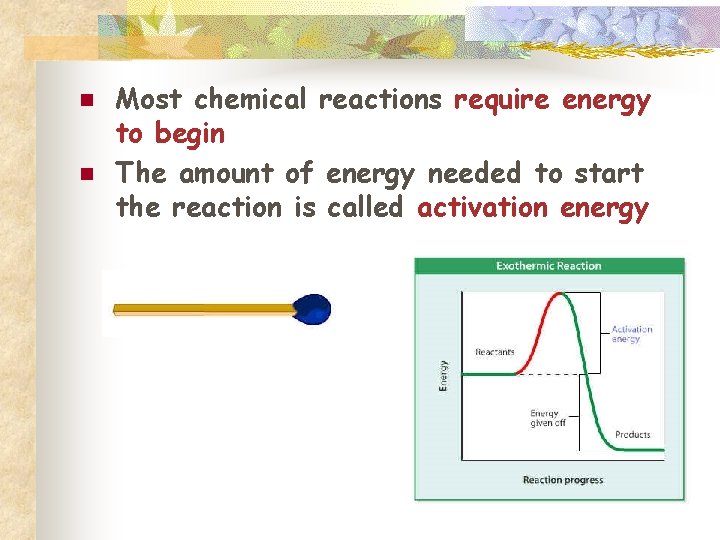
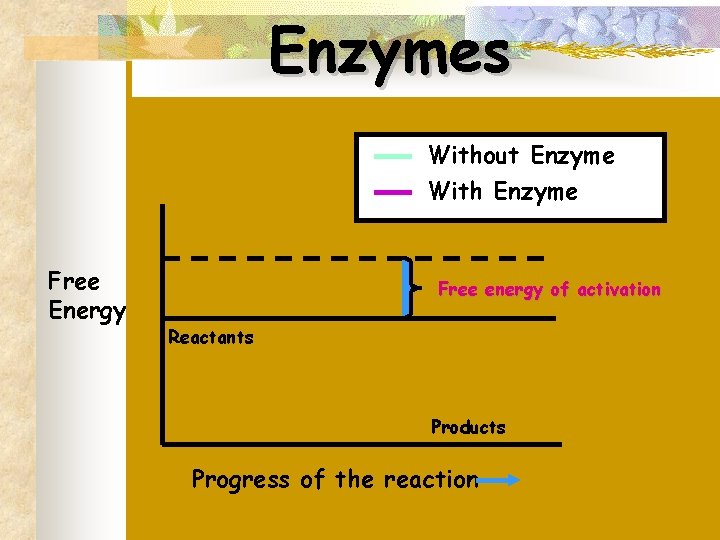
n n Most chemical reactions require energy to begin The amount of energy needed to start the reaction is called activation energy

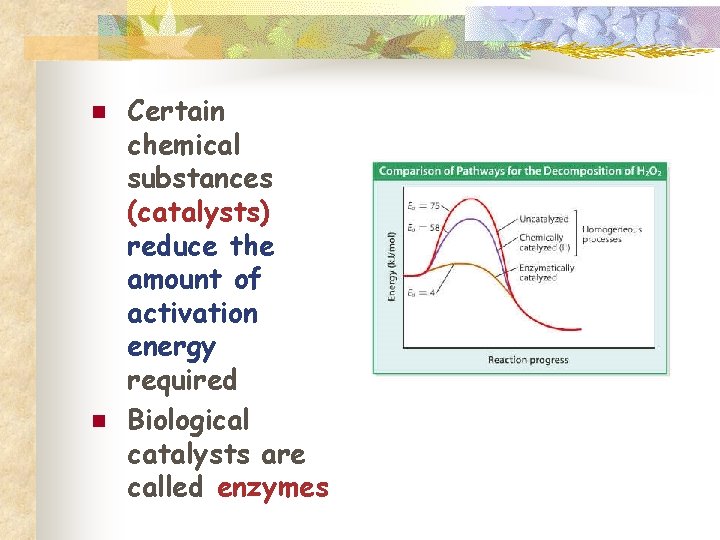
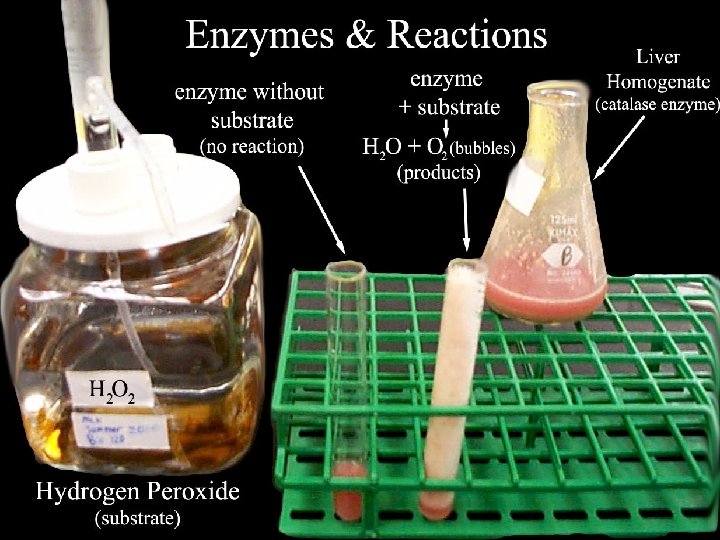
n n Certain chemical substances (catalysts) reduce the amount of activation energy required Biological catalysts are called enzymes

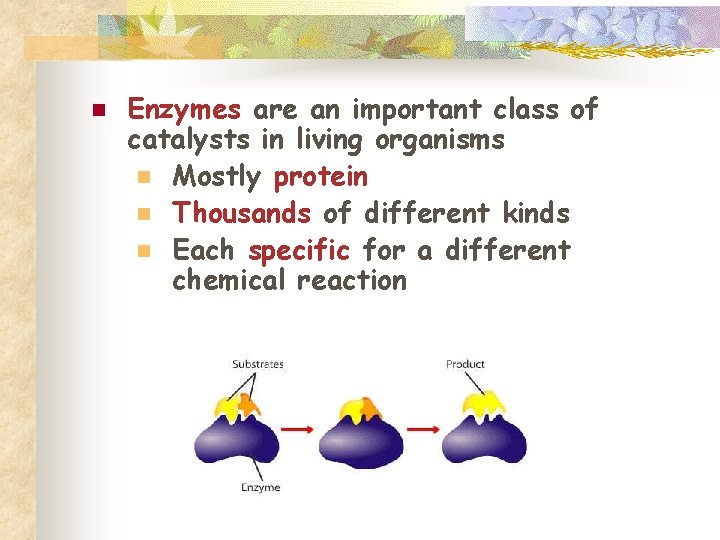
n Enzymes are an important class of catalysts in living organisms n Mostly protein n Thousands of different kinds n Each specific for a different chemical reaction

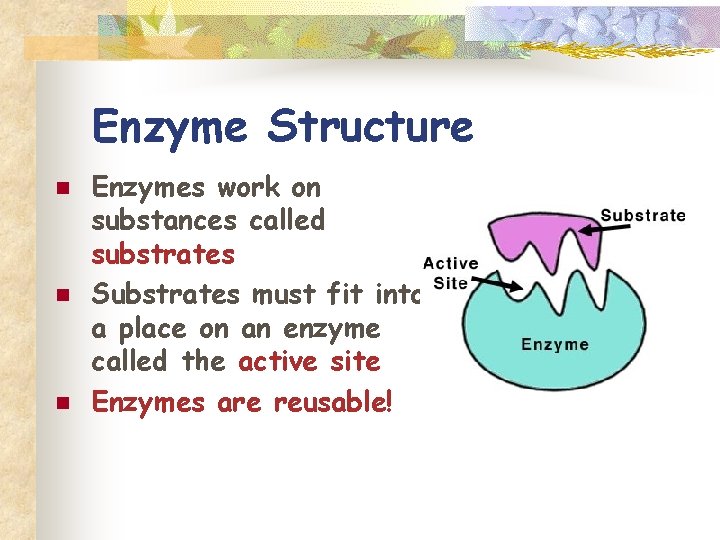
Enzyme Structure n n n Enzymes work on substances called substrates Substrates must fit into a place on an enzyme called the active site Enzymes are reusable!

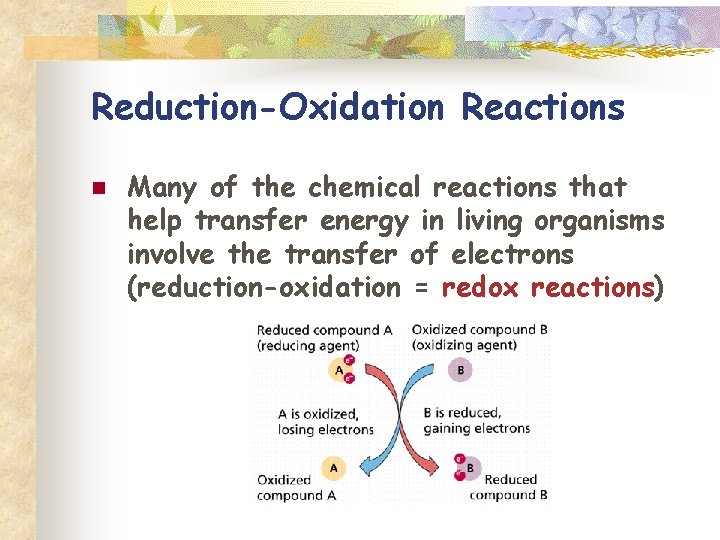
Reduction-Oxidation Reactions n Many of the chemical reactions that help transfer energy in living organisms involve the transfer of electrons (reduction-oxidation = redox reactions)

n Oxidation reaction – reactant loses electron(s) becoming more positive

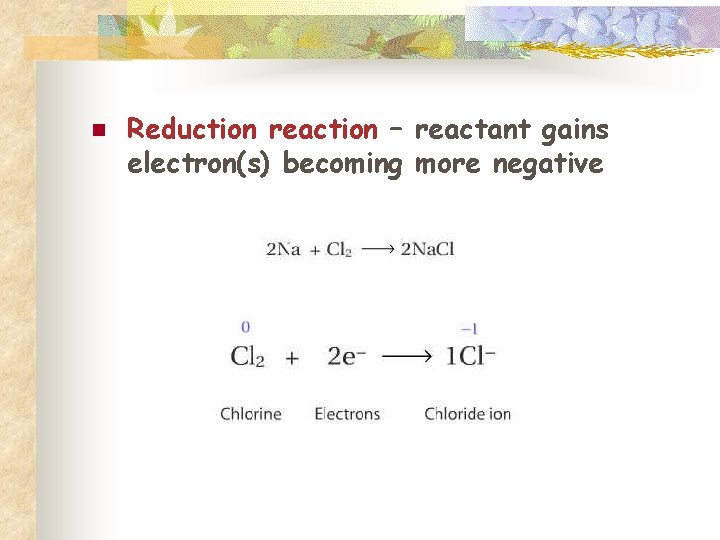
n Reduction reaction – reactant gains electron(s) becoming more negative

Solutions

Solutions n A solution is a mixture in which 2 or more substances are uniformly distributed in another substance

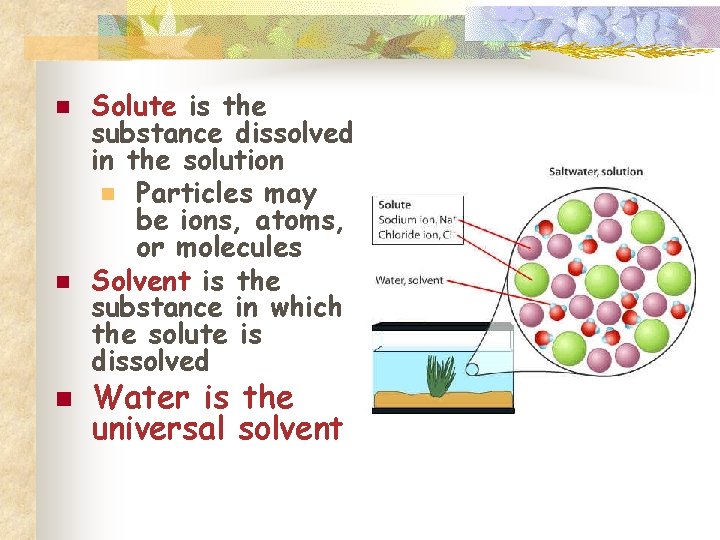
n n n Solute is the substance dissolved in the solution n Particles may be ions, atoms, or molecules Solvent is the substance in which the solute is dissolved Water is the universal solvent

n n n Solutions can be composed of varying proportions of a given solute in a given solvent --- vary in concentration (measurement of the amount of solute) A saturated solution is one in which no more solute can be dissolved Aqueous solution (water) are universally important to living things

n Dissociation of water n Breaking apart of the water molecule into two ions of opposite charge (due to strong attraction of oxygen atom of one molecule for H atom of another water molecule) n H 2 O H+ (hydrogen ion) + OH- (hydroxide ion) n H + + H 2 O H 3 O (hydronium ion)

Acids and Bases n One of the most important aspects of a living system is the degree of acidity or alkalinity

Acids n Number of hydronium ions in solutions is greater than the number of hydroxide ions n HCl H+ + Cl-

Bases n Number of hydroxide ions in solution is greater than the number of hydronium ions n Na. OH Na+ + OH-

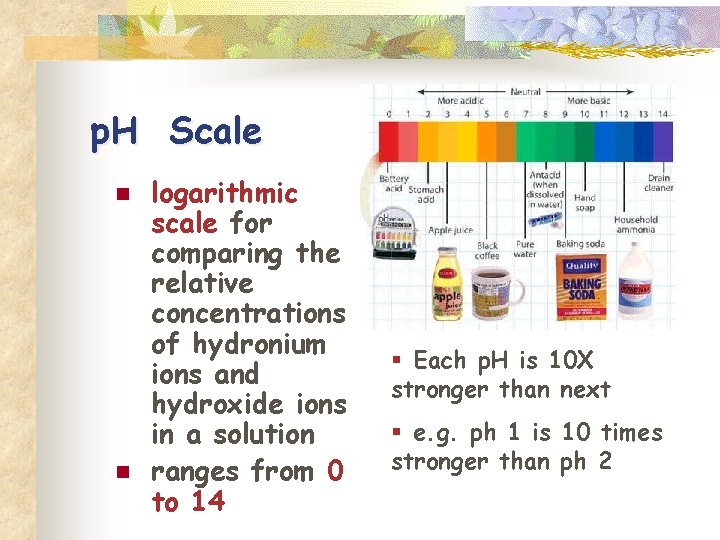
p. H Scale n n logarithmic scale for comparing the relative concentrations of hydronium ions and hydroxide ions in a solution ranges from 0 to 14 § Each p. H is 10 X stronger than next § e. g. ph 1 is 10 times stronger than ph 2

n n n the lower the p. H the stronger the acid the higher the p. H the stronger the base p. H 7. 0 is neutral

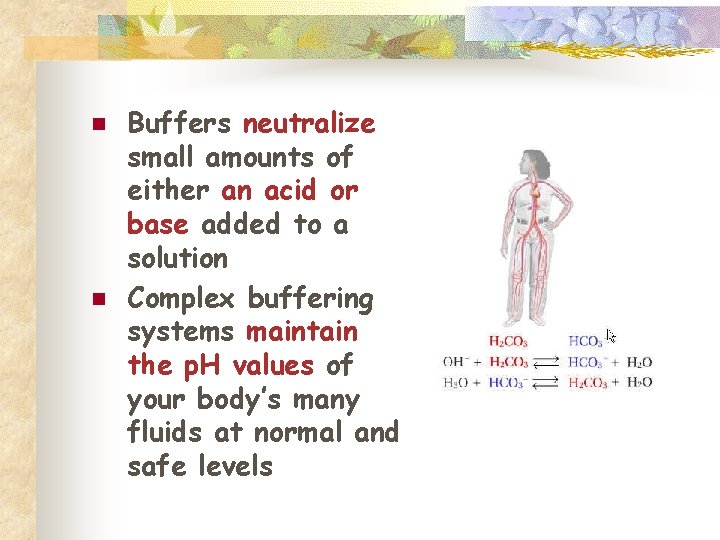
Buffers n n Control of p. H is very important Most enzymes function only within a very narrow p. H Control is accomplished with buffers made by the body Buffers keep a neutral p. H (p. H 7)

n n Buffers neutralize small amounts of either an acid or base added to a solution Complex buffering systems maintain the p. H values of your body’s many fluids at normal and safe levels

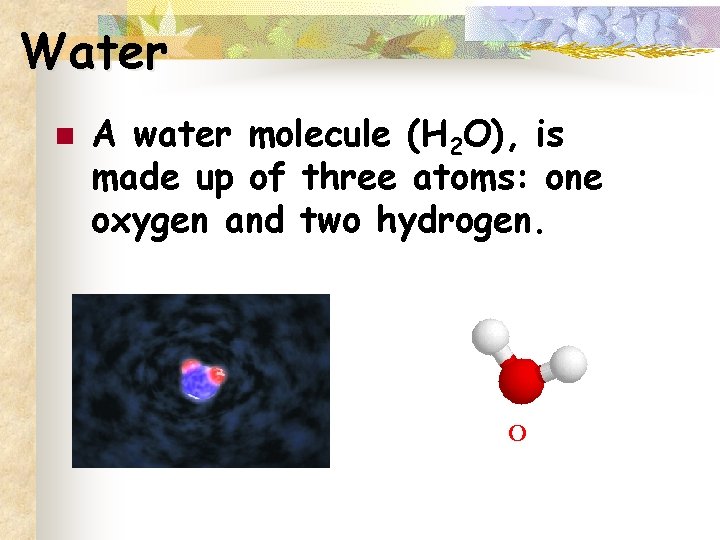
Water n A water molecule (H 2 O), is made up of three atoms: one oxygen and two hydrogen. H O H

Hydrogen Bonds -formed between a highly electronegative atom of a polar molecule and a Hydrogen -one hydrogen bond is weak , but many hydrogen bonds are strong


Properties of Water • At sea level, pure water boils at 100 °C and freezes at 0 °C. • The boiling temperature of water decreases at higher elevations (lower atmospheric pressure). • For this reason, an egg will take longer to boil at higher altitudes

Properties of Water n What are they?

Properties of Water n Cohesion

Properties of Water n Cohesion n Adhesion

Properties of Water n Cohesion n Adhesion n High Specific Heat

Properties of Water n Cohesion n Adhesion n High Specific Heat n High Heat of Vaporization

Properties of Water n n n Cohesion Adhesion High Specific Heat High Heat of Vaporization Less Dense as a Solid

Cohesion • Attraction between particles of the same substance - why water is attracted to itself Results in: Surface tension (a measure of the strength of water’s surface) • surface film on water -allows insects to walk on the surface of water

Adhesion • Attraction between two different substances. - water will make hydrogen bonds with other surfaces such as glass, soil, plant tissues, and cotton. • Capillary action-water molecules will “tow” each other along when in a thin glass tube. - i. e. transpiration process which plants and trees remove water from the soil, and paper towels soak up water.

High Specific Heat Amount of heat needed to raise or lower 1 g of a substance 1° C. • Water resists temperature change, both for heating and cooling. • Water can absorb or release large amounts of heat energy with little change in actual temperature.

High Heat of Vaporization Amount of energy to convert 1 g or a substance from a liquid to a gas • In order for water to evaporate, hydrogen bonds must be broken. As water evaporates, it removes a lot of heat with it.

“Water vapor forms a kind of global ‘blanket’ which helps to keep the earth warm. Heat radiated from the sun-warmed surface of the earth is absorbed and held by the vapor. ” n

Water is Less Dense as a Solid • Ice is less dense as a solid than as a liquid (ice floats) Liquid water has hydrogen bonds that are constantly being broken and reformed. Frozen water forms a crystal-like lattice whereby molecules are set at fixed distances.

Water is Less Dense as a Solid • Which is ice and which is water?

Water is Less Dense as a Solid Water Ice

Homeostasis Ability to maintain a steady state despite changing conditions n Water is important to this process because: a. Makes a good insulator b. Resists temperature change c. Universal solvent d. Coolant e. Ice protects against temperature extremes n

CARBOHYDRATES

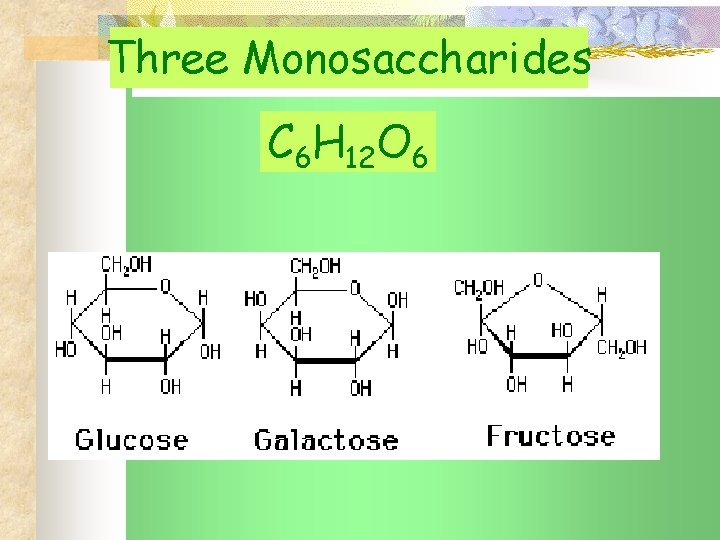
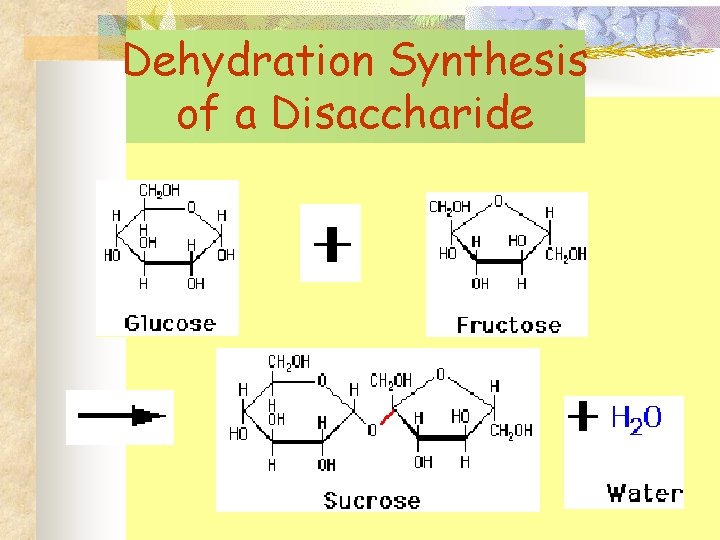
Characteristics of Carbohydrates n n n Consist of carbon, hydrogen, & oxygen Energy containing molecules Some provide structure Basic building block is a monosaccharide (CH 2 O)n ; n = 3, 5, 6 Two monosaccharides form a disaccharide

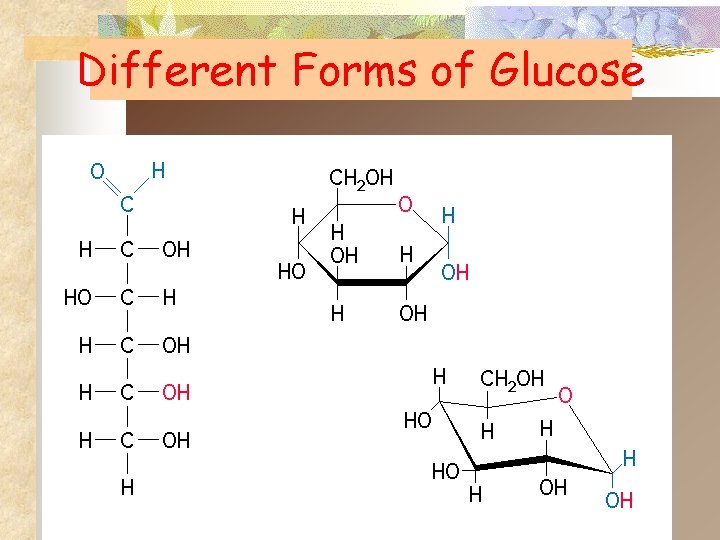
Different Forms of Glucose

Three Monosaccharides C 6 H 12 O 6

Dehydration Synthesis of a Disaccharide

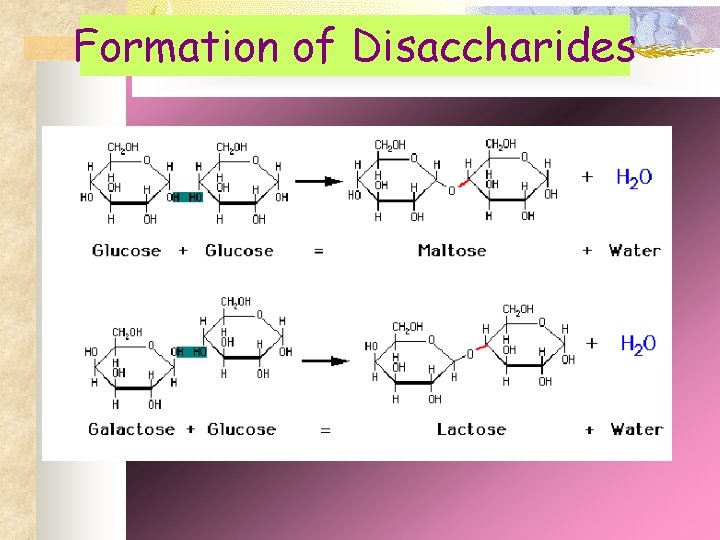
Formation of Disaccharides

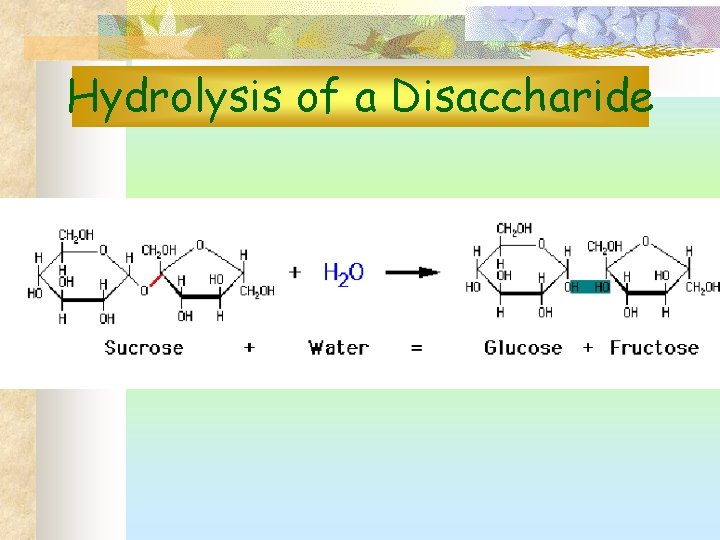
Hydrolysis of a Disaccharide

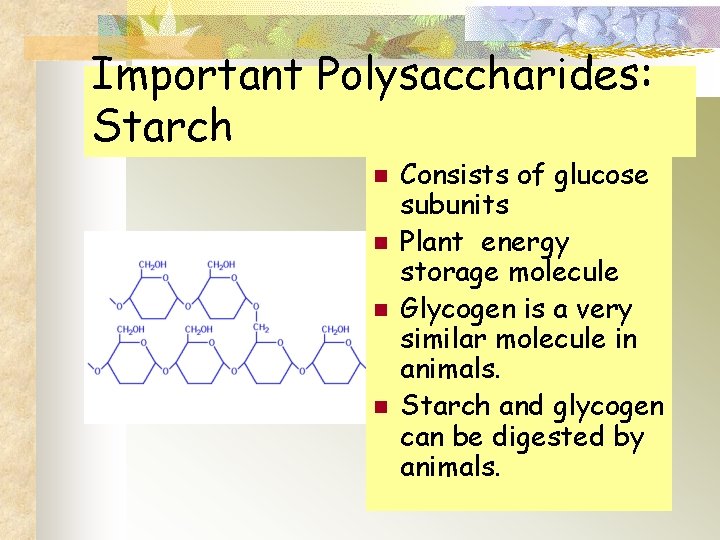
Important Polysaccharides: Starch n n Consists of glucose subunits Plant energy storage molecule Glycogen is a very similar molecule in animals. Starch and glycogen can be digested by animals.

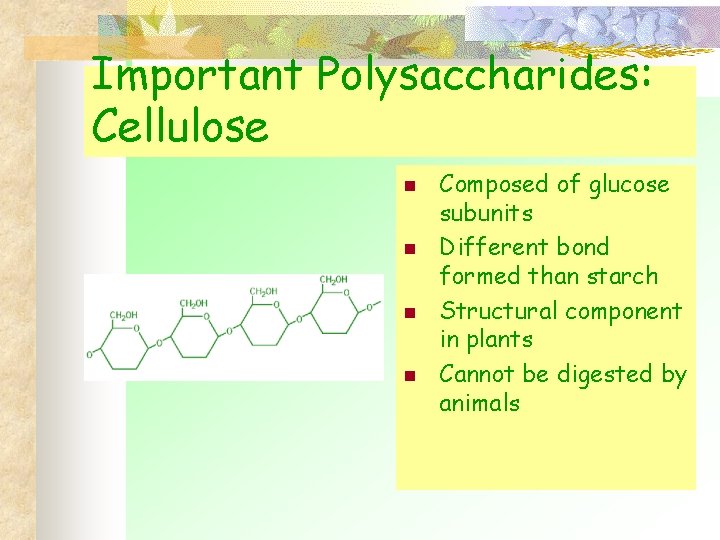
Important Polysaccharides: Cellulose n n Composed of glucose subunits Different bond formed than starch Structural component in plants Cannot be digested by animals

BIOENERGETICS

What is Bioenergetics? The study of energy in living systems (environments) and the organisms (plants and animals) that utilize them

Energy n n Required by all organisms May be Kinetic or Potential energy

Kinetic Energy n n Energy of Motion Heat and light energy are examples

Potential Energy n n Energy of position Includes energy stored in chemical bonds

Two Types of Energy Reactions

Endergonic Reactions n n Chemical reaction that requires a net input of energy Photosynthesis SUN Light Energy photons 6 CO 2 + 6 O 2 6 H 2 O C 6 H 12 O 6 + (glucose)

Exergonic Reactions n Chemical reactions that releases energy n Cellular Respiration Energy C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O+ (glucose) ATP

Metabolic Reactions of Cells

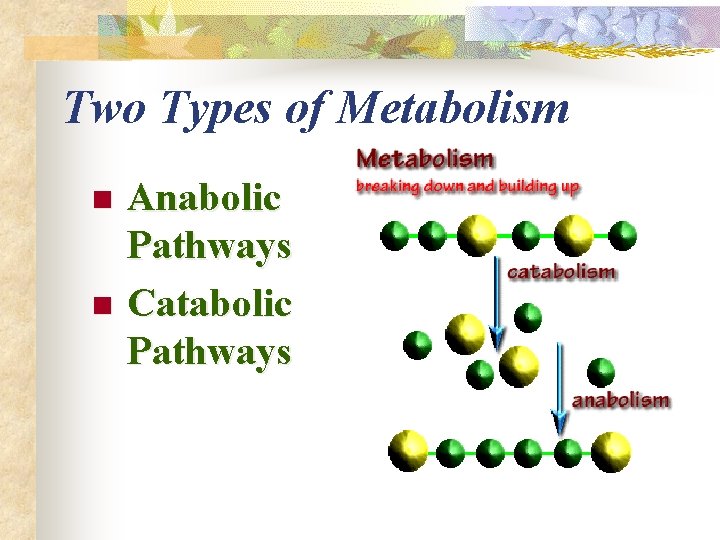
What is Metabolism? n The sum total of the chemical activities of all cells

Two Types of Metabolism n n Anabolic Pathways Catabolic Pathways

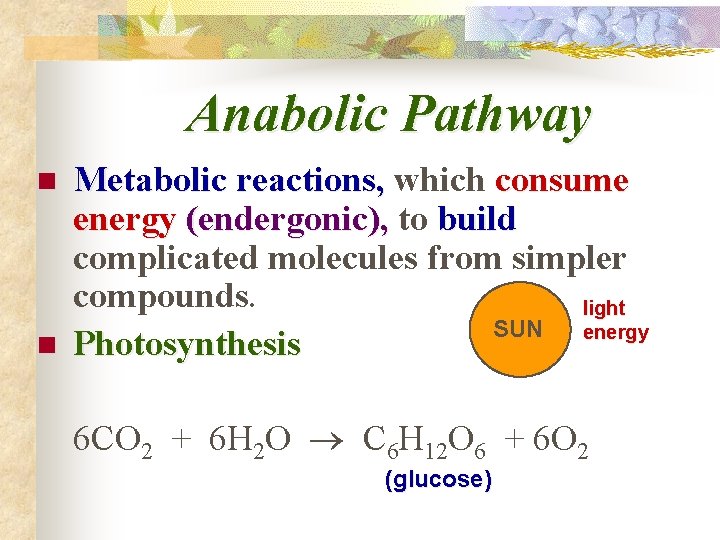
Anabolic Pathway n n Metabolic reactions, which consume energy (endergonic), to build complicated molecules from simpler compounds. light SUN energy Photosynthesis 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 (glucose)

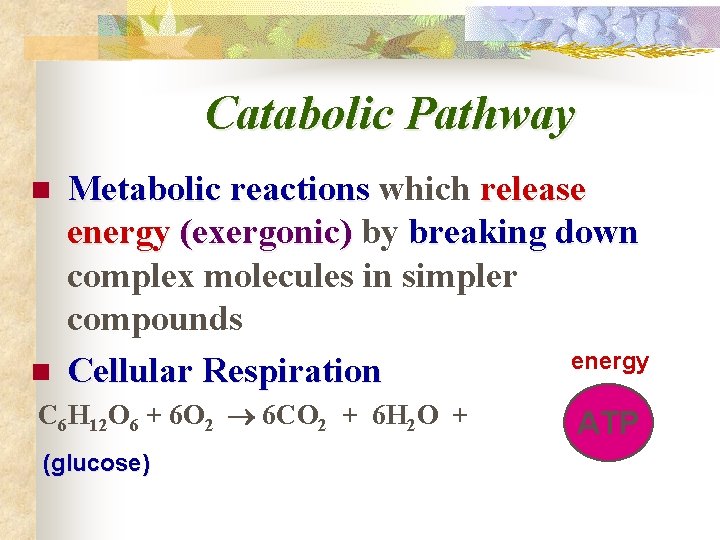
Catabolic Pathway n Metabolic reactions which release energy (exergonic) by breaking down complex molecules in simpler compounds energy Cellular Respiration n C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + (glucose) ATP

Cellular Energy - ATP

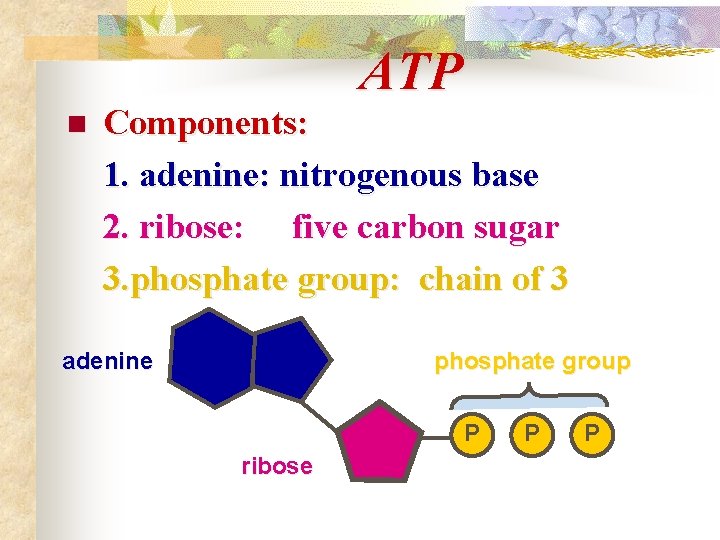
ATP n Components: 1. adenine: nitrogenous base 2. ribose: five carbon sugar 3. phosphate group: chain of 3 adenine phosphate group P ribose P P

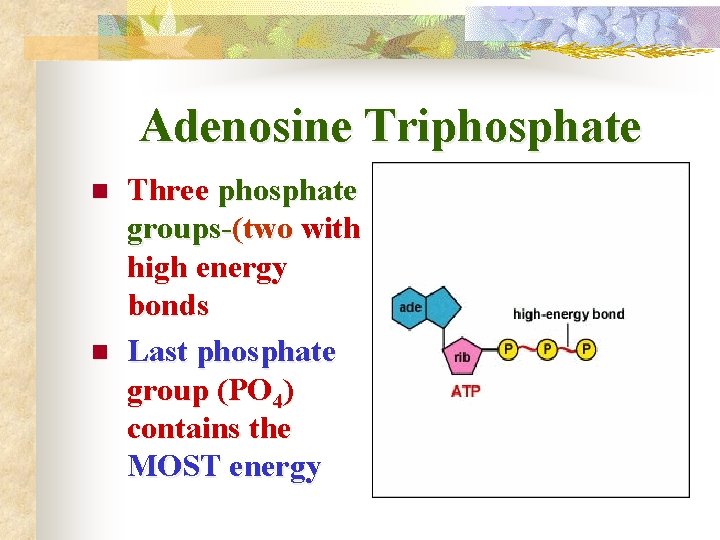
Adenosine Triphosphate n n Three phosphate groups-(two with high energy bonds Last phosphate group (PO 4) contains the MOST energy

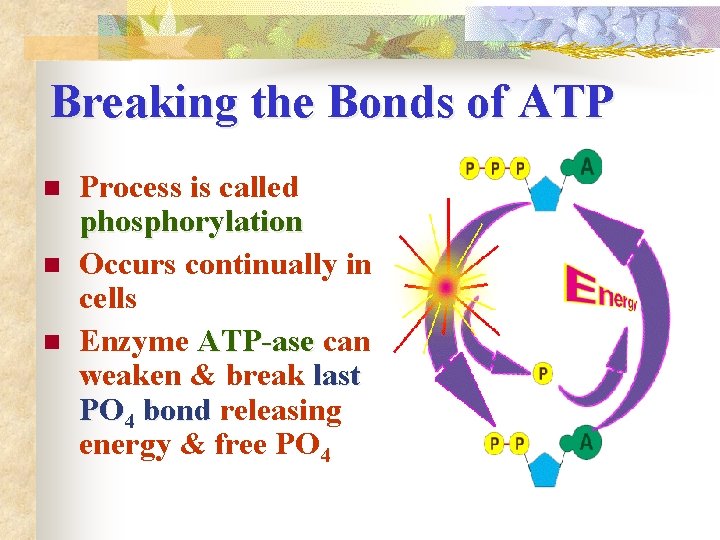
Breaking the Bonds of ATP n n n Process is called phosphorylation Occurs continually in cells Enzyme ATP-ase can weaken & break last PO 4 bond releasing energy & free PO 4

How does ATP work ? n n Organisms use enzymes to break down energy-rich glucose to release its potential energy This energy is trapped and stored in the form of adenosine triphosphate(ATP)

How Much ATP Do Cells Use? n It is estimated that each cell will generate and consume approximately 10, 000 molecules of ATP per second

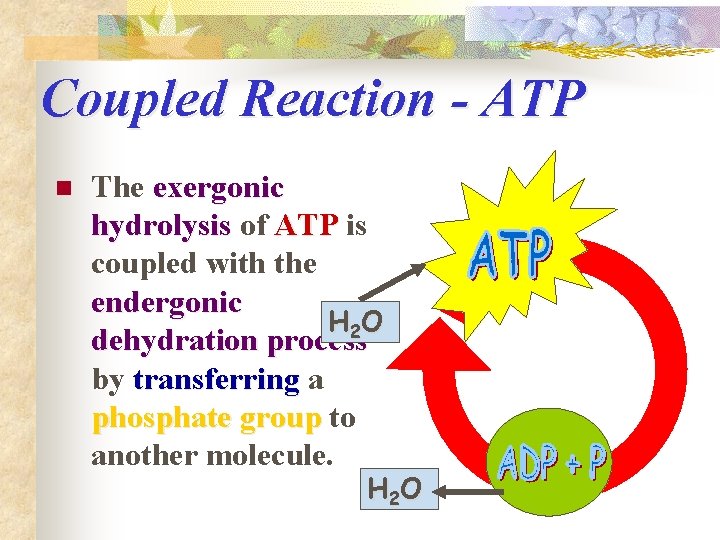
Coupled Reaction - ATP n The exergonic hydrolysis of ATP is coupled with the endergonic H 2 O dehydration process by transferring a phosphate group to another molecule. H 2 O

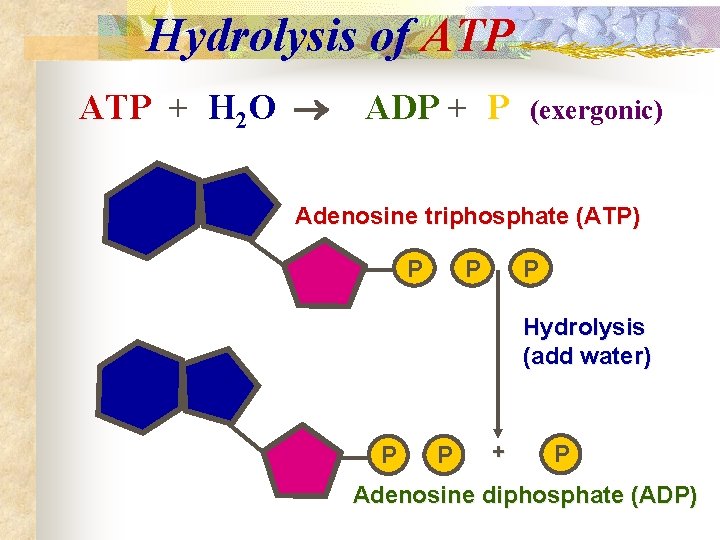
Hydrolysis of ATP + H 2 O ADP + P (exergonic) Adenosine triphosphate (ATP) P P P Hydrolysis (add water) P P + P Adenosine diphosphate (ADP)

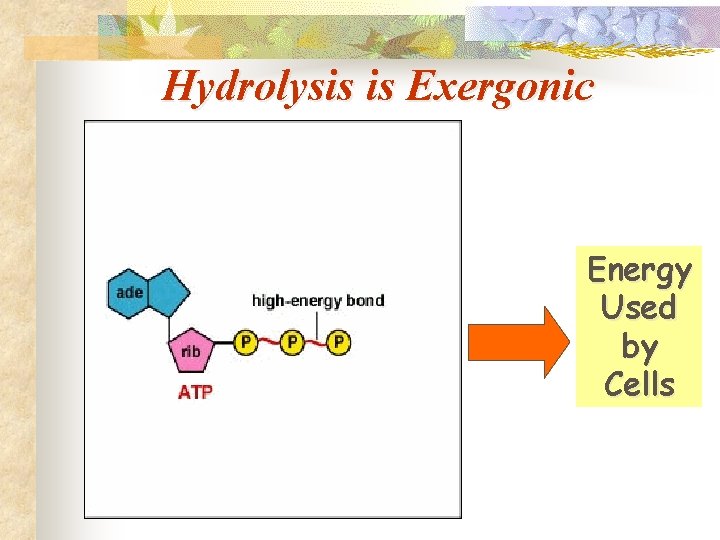
Hydrolysis is Exergonic Energy Used by Cells

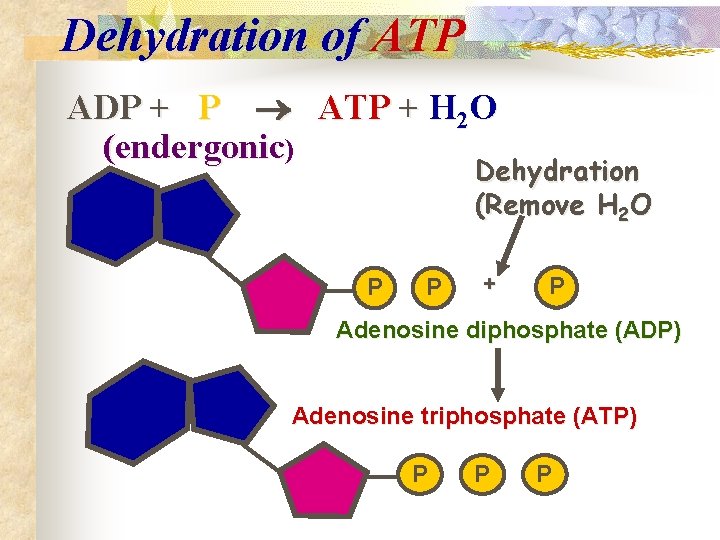
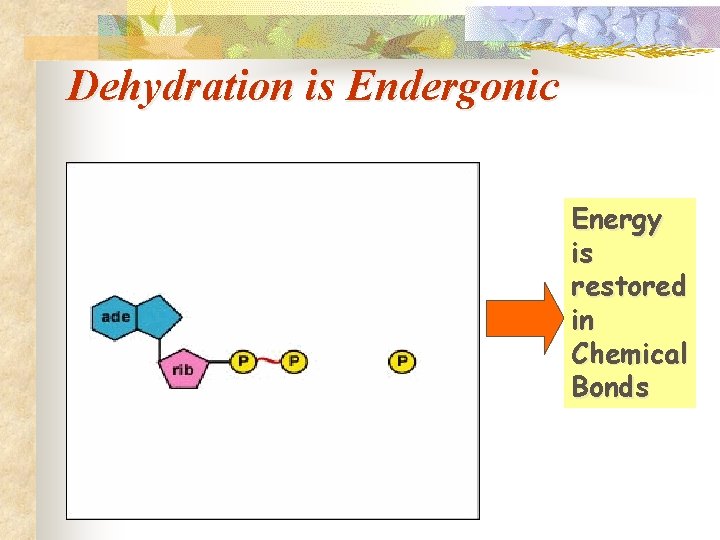
Dehydration of ATP ADP + P ATP + H 2 O (endergonic) Dehydration (Remove H 2 O P P + P Adenosine diphosphate (ADP) Adenosine triphosphate (ATP) P P P

Dehydration is Endergonic Energy is restored in Chemical Bonds

Enzymes

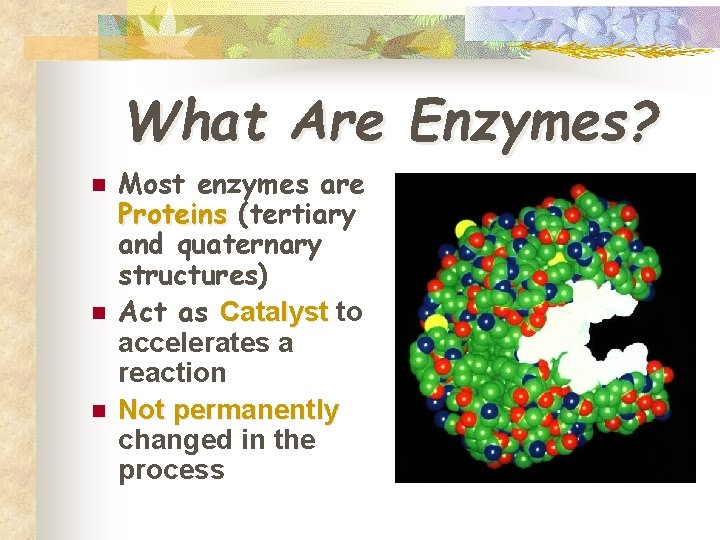
What Are Enzymes? n n n Most enzymes are Proteins (tertiary and quaternary structures) Act as Catalyst to accelerates a reaction Not permanently changed in the process

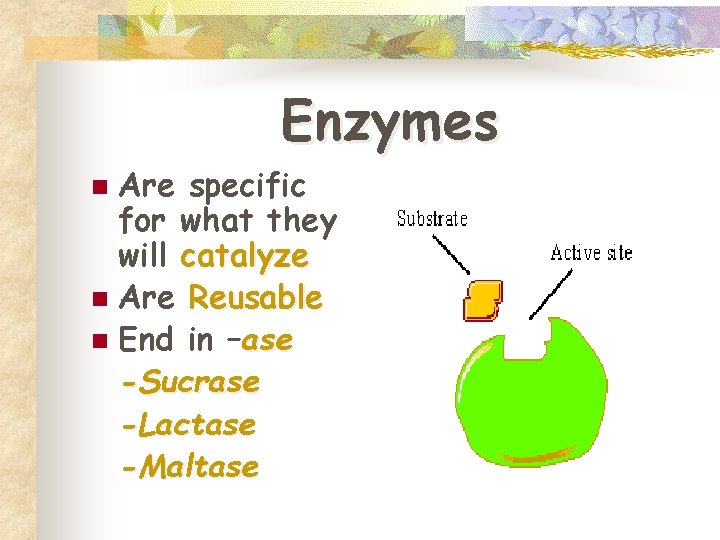
Enzymes Are specific for what they will catalyze n Are Reusable n End in –ase -Sucrase -Lactase -Maltase n

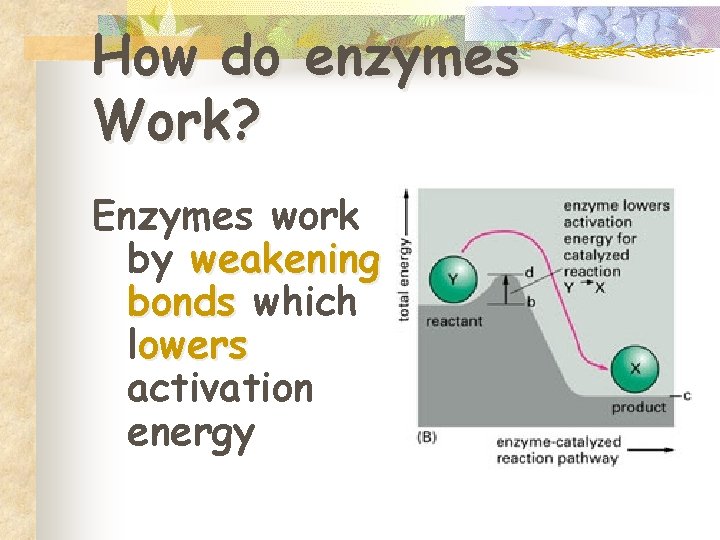
How do enzymes Work? Enzymes work by weakening bonds which lowers activation energy

Enzymes Without Enzyme With Enzyme Free Energy Free energy of activation Reactants Products Progress of the reaction


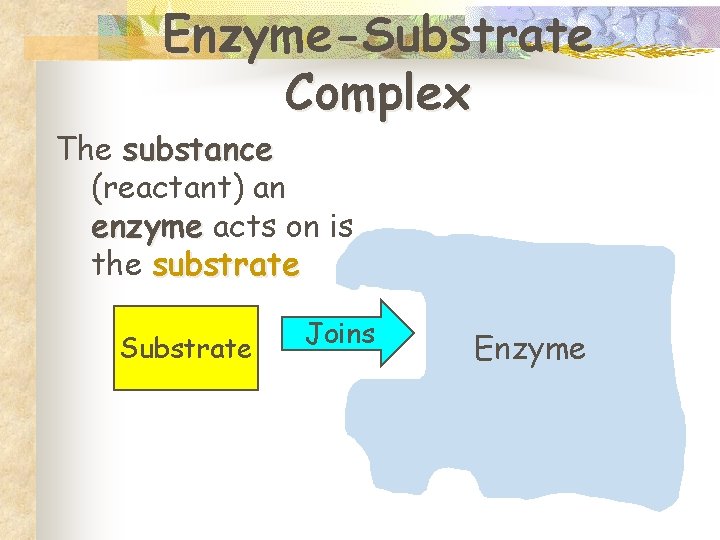
Enzyme-Substrate Complex The substance (reactant) an enzyme acts on is the substrate Substrate Joins Enzyme

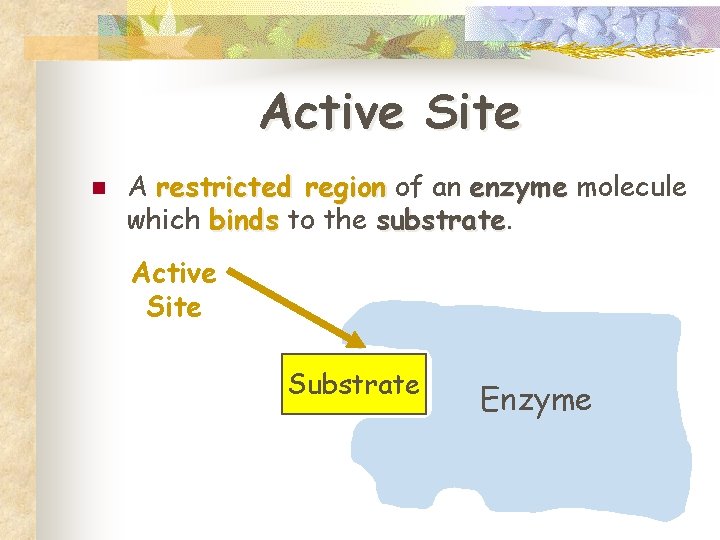
Active Site n A restricted region of an enzyme molecule which binds to the substrate Active Site Substrate Enzyme

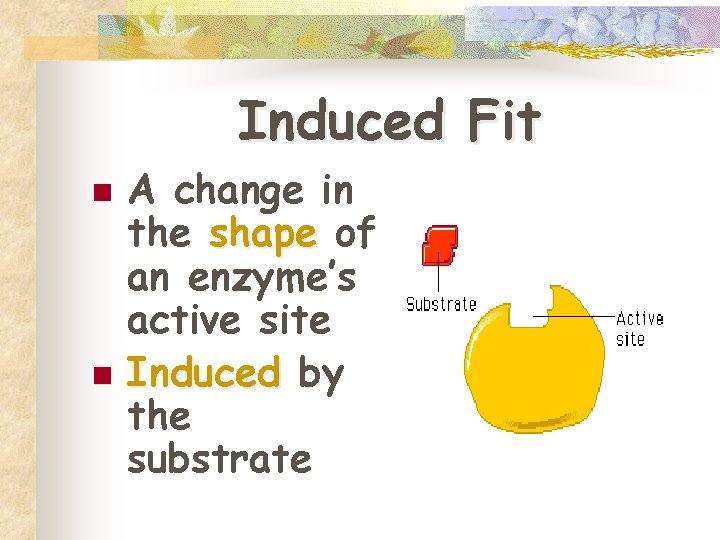
Induced Fit n n A change in the shape of an enzyme’s active site Induced by the substrate

Factors Affecting Enzyme Activity n n Temperature p. H Cofactors & Coenzymes Inhibitors

Temperature & p. H n n n High temperatures are the most dangerous reactions & denature enzymes (Most like normal Body temperatures) temperatures Most enzymes like near neutral p. H (6 to 8) Denatured (unfolded) by ionic salts

Cofactors and Coenzymes n n Inorganic substances (zinc, iron) and vitamins (respectively) are sometimes need for proper enzymatic activity Example: Iron must be present in the quaternary structure of hemoglobin in order for it to pick up oxygen

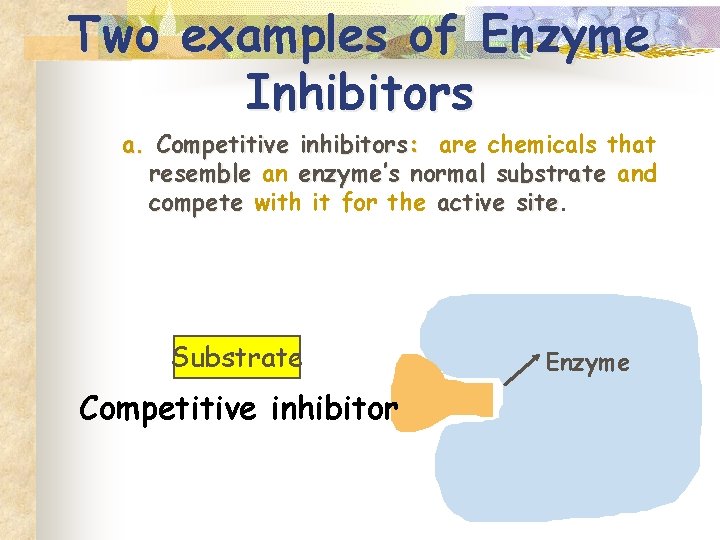
Two examples of Enzyme Inhibitors a. Competitive inhibitors: are chemicals that resemble an enzyme’s normal substrate and compete with it for the active site Substrate Competitive inhibitor Enzyme

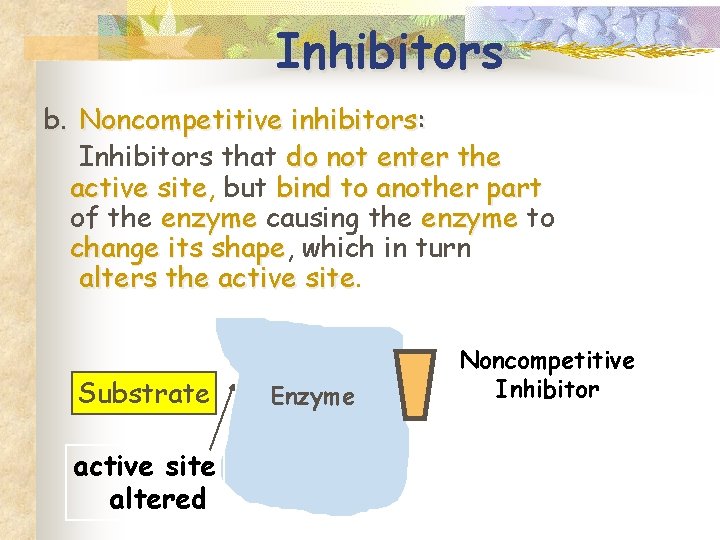
Inhibitors b. Noncompetitive inhibitors: Inhibitors that do not enter the active site, site but bind to another part of the enzyme causing the enzyme to change its shape, shape which in turn alters the active site Substrate active site altered Enzyme Noncompetitive Inhibitor
 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Composition of matter section 1
Composition of matter section 1 Everything around you is made up of
Everything around you is made up of Percent composition examples
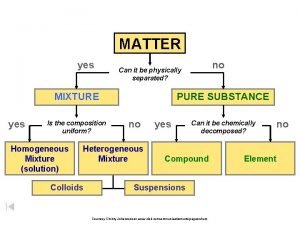
Percent composition examples Is the composition uniform
Is the composition uniform Composition of matter flow chart
Composition of matter flow chart Study of the composition structure and properties
Study of the composition structure and properties Section 1 composition of matter
Section 1 composition of matter Is the composition uniform?
Is the composition uniform? Composition of matter flow chart
Composition of matter flow chart What is composition in matter
What is composition in matter Composition of matter section 1
Composition of matter section 1 Classifying matter concept map
Classifying matter concept map Composition of matter flow chart
Composition of matter flow chart Number of proton
Number of proton Properties of solids liquids and gases
Properties of solids liquids and gases A type of matter with a fixed composition
A type of matter with a fixed composition Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Definition of substance
Definition of substance Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry: matter and change chapter 10 the mole answer key
Chemistry: matter and change chapter 10 the mole answer key Examples of matter in chemistry
Examples of matter in chemistry 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change answer key chapter 2
Chemistry matter and change answer key chapter 2 Matter flowchart chemistry
Matter flowchart chemistry Graphic organizer for classification
Graphic organizer for classification Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter Gray matter vs white matter
Gray matter vs white matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Gray matter and white matter
Gray matter and white matter Frontal and parietal lobes
Frontal and parietal lobes Flow energy review
Flow energy review Everything around us is called
Everything around us is called Lord i lift everything to you
Lord i lift everything to you Resurrection changes everything
Resurrection changes everything What is the answer to life the universe and everything
What is the answer to life the universe and everything Survive asl
Survive asl Air resistance surface area
Air resistance surface area The avalanche devoured everything in its path
The avalanche devoured everything in its path Is anything that occupies space and has mass.
Is anything that occupies space and has mass. Lesson 3 : everything begins with cells
Lesson 3 : everything begins with cells Is everything relative
Is everything relative Attitude is everything quotes
Attitude is everything quotes The forklift hand signal for dog everything means
The forklift hand signal for dog everything means God see everything
God see everything Does everything fall at the same speed
Does everything fall at the same speed Everything's an argument chapter 1
Everything's an argument chapter 1 Everything that can be invented has been invented
Everything that can be invented has been invented 31536000 in scientific notation
31536000 in scientific notation Everything that sorrounds us
Everything that sorrounds us Fnet formula
Fnet formula Behold i make everything new
Behold i make everything new How to put god first in everything you do
How to put god first in everything you do Schema on the fly splunk
Schema on the fly splunk Everything that surrounds an organism
Everything that surrounds an organism Genesee park to othello
Genesee park to othello Everything you need to know about the odyssey
Everything you need to know about the odyssey R alliteration
R alliteration Jesus you are lord
Jesus you are lord Immutability changes everything
Immutability changes everything Hand signals for cranes
Hand signals for cranes Everything that lives and moves about will be food for you
Everything that lives and moves about will be food for you Steven johnson everything bad is good for you
Steven johnson everything bad is good for you Computer science is changing everything
Computer science is changing everything Arnold wesker chips with everything
Arnold wesker chips with everything Section 1: ecosystems: everything is connected
Section 1: ecosystems: everything is connected Grieve everything we covid
Grieve everything we covid Today is a beautiful day tag questions
Today is a beautiful day tag questions Good knowing
Good knowing Technique is everything
Technique is everything Testing single phase motor
Testing single phase motor Whats the basic unit for volume
Whats the basic unit for volume Happy no fear
Happy no fear God saw everything he made and it was good
God saw everything he made and it was good God looked at everything he had made
God looked at everything he had made Everything around you is made up of
Everything around you is made up of Everything psychological is biological
Everything psychological is biological Attitude changes everything
Attitude changes everything How everything
How everything How everything
How everything What is everything made of
What is everything made of Excellence in everything we do
Excellence in everything we do Marketing is everything
Marketing is everything Material mean
Material mean There is a crack in everything
There is a crack in everything Tag question everything
Tag question everything Put everything away
Put everything away What happened is everything ok
What happened is everything ok In everything give thanks to the lord
In everything give thanks to the lord He who defends everything defends nothing
He who defends everything defends nothing God does everything for our good story
God does everything for our good story Be happy not because everything is good
Be happy not because everything is good Pick up everything
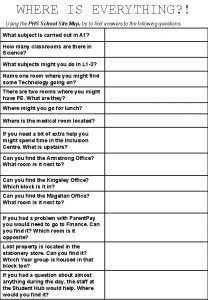
Pick up everything Where is everything
Where is everything Where is everything
Where is everything Where is everything
Where is everything Hot air balloon
Hot air balloon Hindu beliefs about god
Hindu beliefs about god Everything toolbar
Everything toolbar Kellogg's pep vitamins advertisement analysis
Kellogg's pep vitamins advertisement analysis Everything finder
Everything finder Tag question everything
Tag question everything Public sentiment is everything
Public sentiment is everything Everything is poison
Everything is poison We will not be shaken we will not be moved
We will not be shaken we will not be moved Everything hinges on
Everything hinges on Everything that is alive needs energy
Everything that is alive needs energy 100 similes
100 similes Everything has been invented
Everything has been invented How neurons communicate
How neurons communicate Everything is nothing
Everything is nothing Branch of chemical engineering
Branch of chemical engineering Put everything together
Put everything together