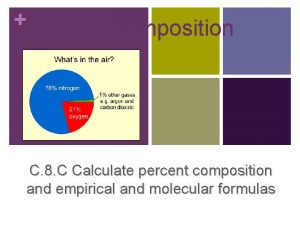
CHEMISTRY Composition of Matter Everything in universe is






















- Slides: 56

CHEMISTRY

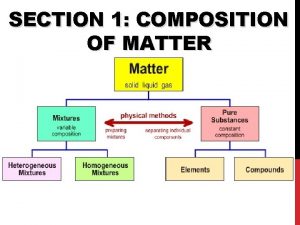
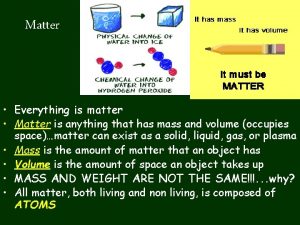
Composition of Matter - Everything in universe is composed of matter Matter is anything that occupies space & has mass Mass – quantity of matter an object has Weight – pull of gravity on an object

Elements Pure substances that cannot be broken down chemically into simpler kinds of matter More than 100 elements (92 naturally occurring)

90% of the mass of an organism is composed of 4 elements (oxygen, carbon, hydrogen and nitrogen) Each element unique chemical symbol Consists of 1 -2 letters First letter is always capitalized

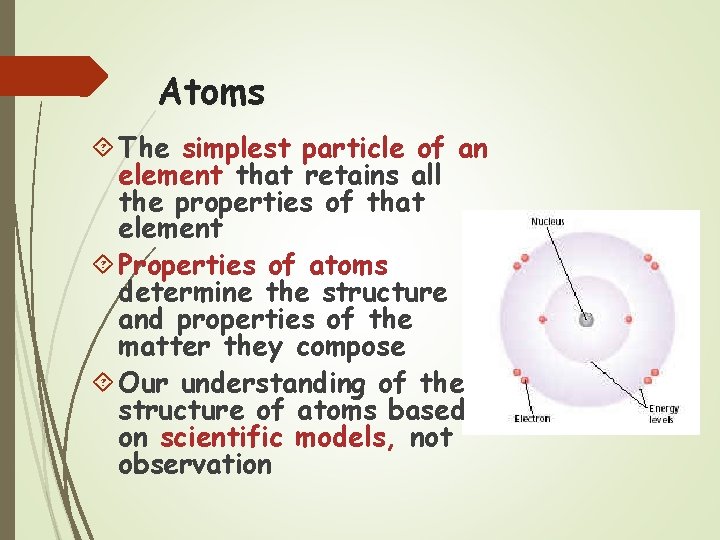
Atoms The simplest particle of an element that retains all the properties of that element Properties of atoms determine the structure and properties of the matter they compose Our understanding of the structure of atoms based on scientific models, not observation

Main Regions of an Atom Nucleus Electron Cloud

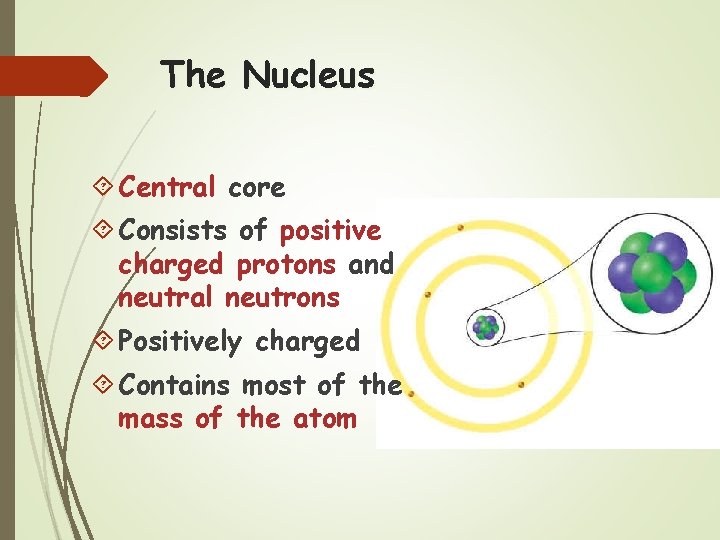
The Nucleus Central core Consists of positive charged protons and neutral neutrons Positively charged Contains most of the mass of the atom

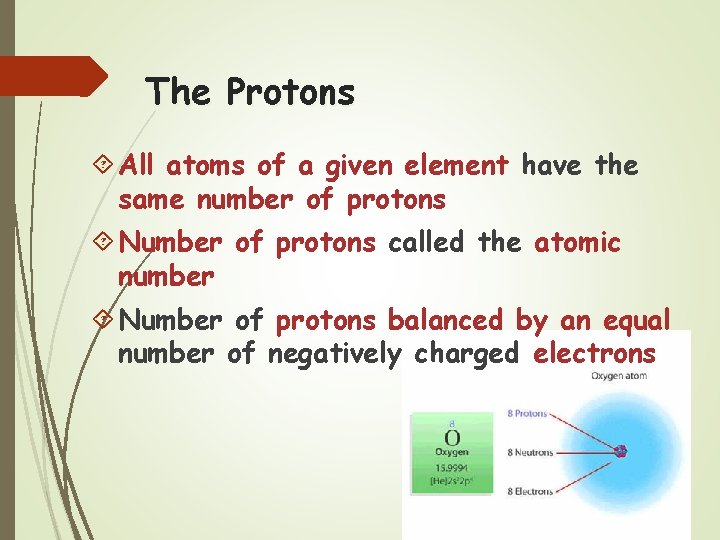
The Protons All atoms of a given element have the same number of protons Number of protons called the atomic number Number of protons balanced by an equal number of negatively charged electrons

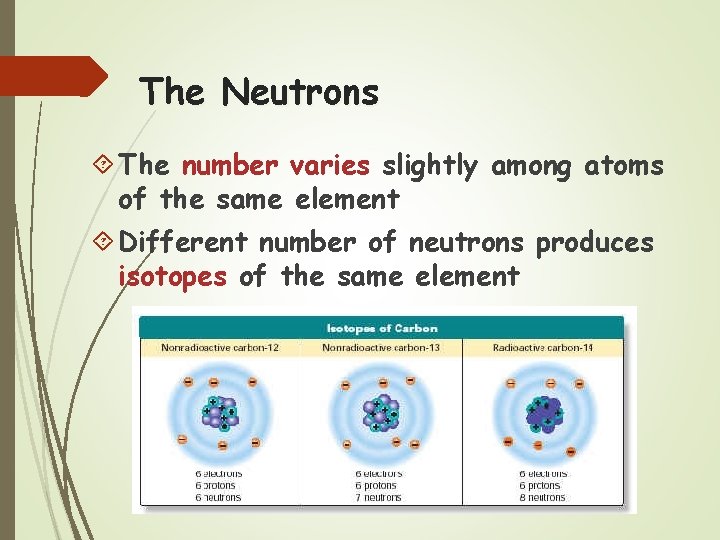
The Neutrons The number varies slightly among atoms of the same element Different number of neutrons produces isotopes of the same element

Atomic Mass Protons & neutrons are found in the nucleus of an atom Protons and neutrons each have a mass of 1 amu (atomic mass unit) The atomic mass of an atom is found by adding the number of protons & neutrons in an atom

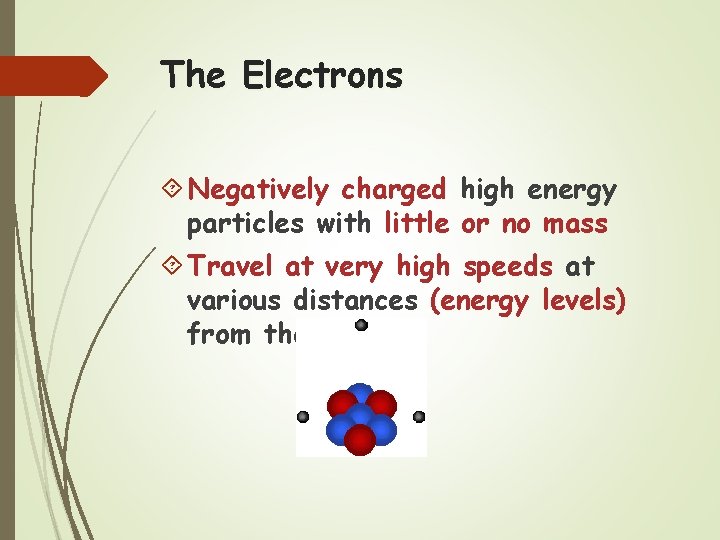
The Electrons Negatively charged high energy particles with little or no mass Travel at very high speeds at various distances (energy levels) from the nucleus

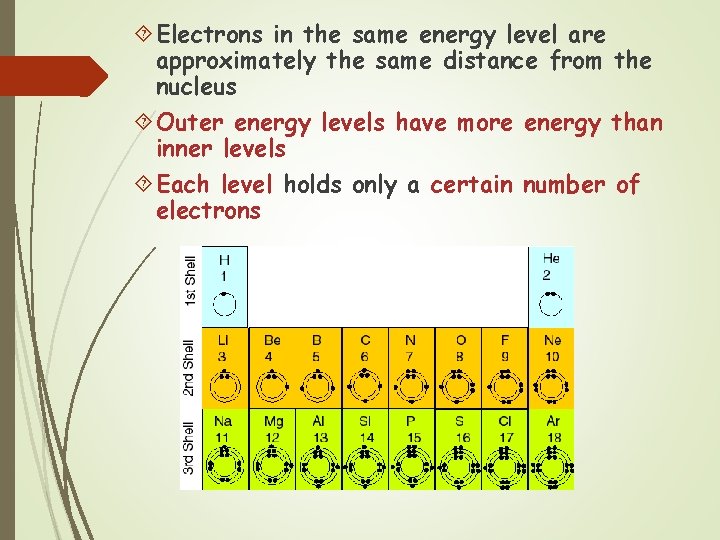
Electrons in the same energy level are approximately the same distance from the nucleus Outer energy levels have more energy than inner levels Each level holds only a certain number of electrons

Energy Levels Atoms have 7 energy levels The levels are K (closest to the nucleus), L, M, N, O, P, Q (furthest from the nucleus) The K level can only hold 2 electrons Levels L – Q can hold 8 electrons (octet rule)

Periodic Table Elements are arranged by their atomic number on the Periodic Table The horizontal rows are called Periods & tell the number of energy levels Vertical groups are called Families & tell the outermost number of electrons


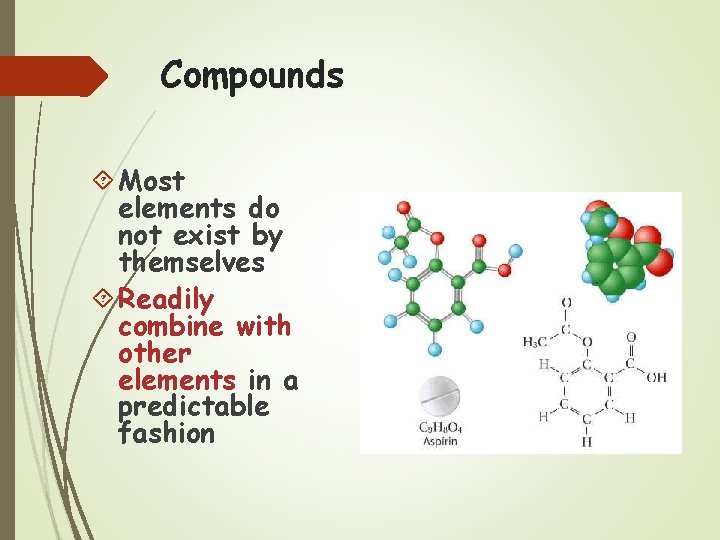
Compounds Most elements do not exist by themselves Readily combine with other elements in a predictable fashion

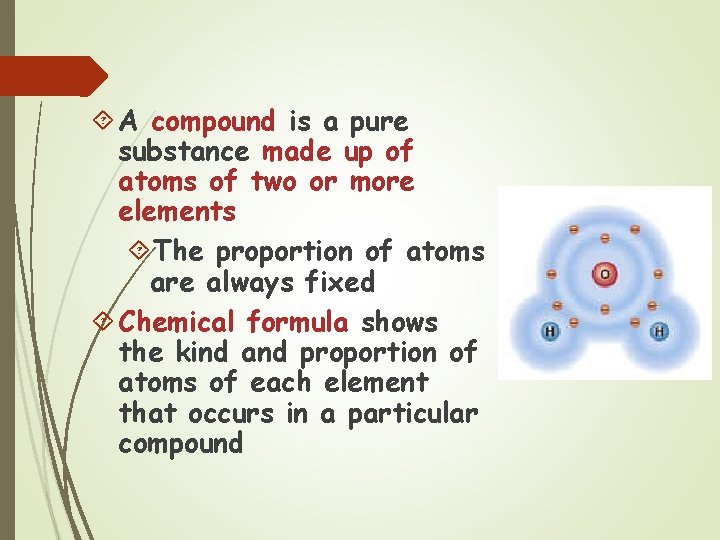
A compound is a pure substance made up of atoms of two or more elements The proportion of atoms are always fixed Chemical formula shows the kind and proportion of atoms of each element that occurs in a particular compound

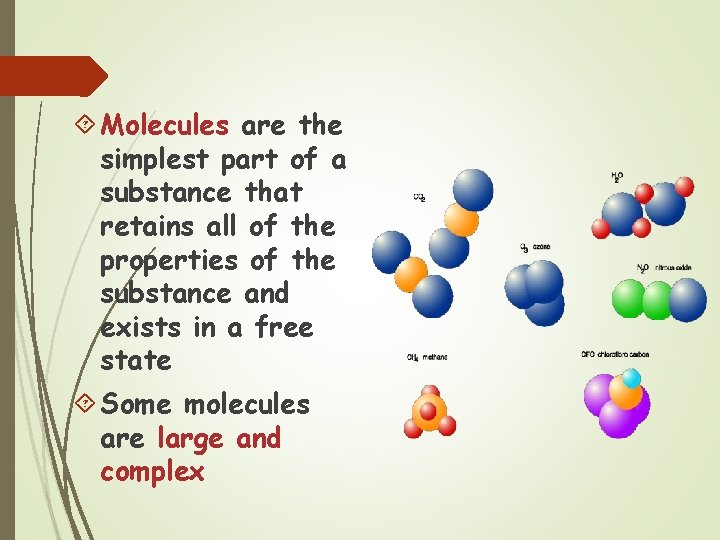
Molecules are the simplest part of a substance that retains all of the properties of the substance and exists in a free state Some molecules are large and complex

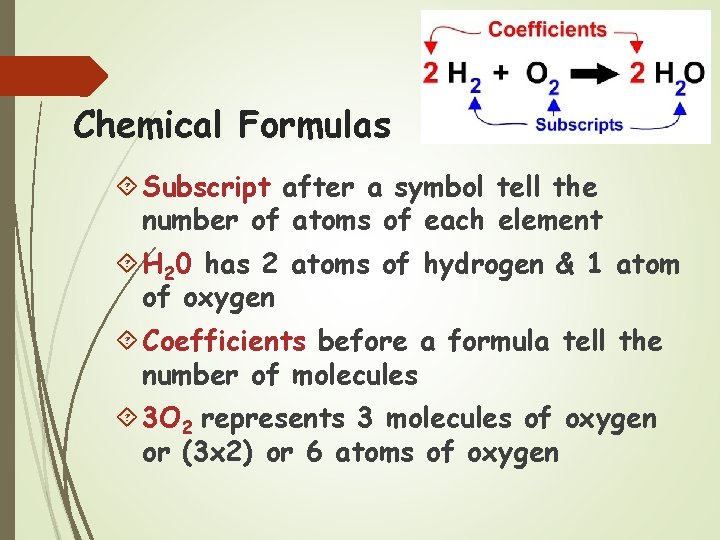
Chemical Formulas Subscript after a symbol tell the number of atoms of each element H 20 has 2 atoms of hydrogen & 1 atom of oxygen Coefficients before a formula tell the number of molecules 3 O 2 represents 3 molecules of oxygen or (3 x 2) or 6 atoms of oxygen

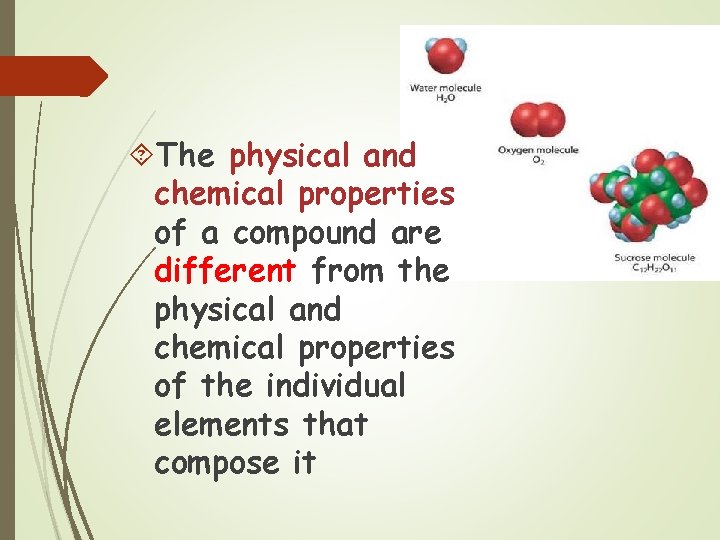
The physical and chemical properties of a compound are different from the physical and chemical properties of the individual elements that compose it

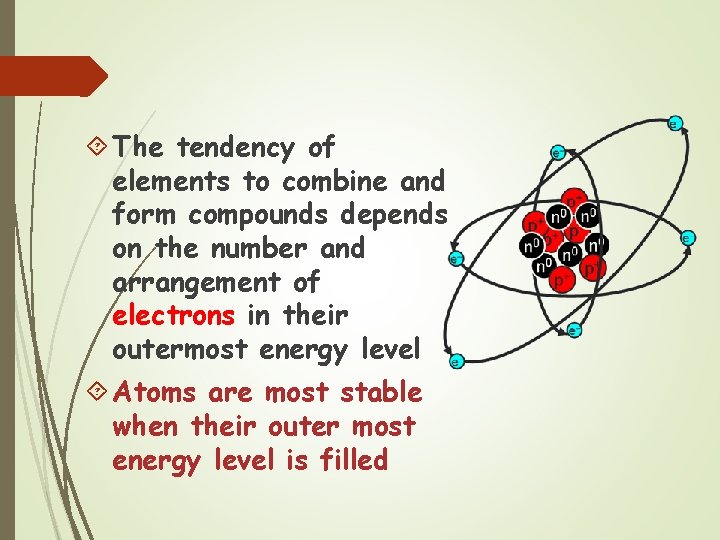
The tendency of elements to combine and form compounds depends on the number and arrangement of electrons in their outermost energy level Atoms are most stable when their outer most energy level is filled

Most atoms are not stable in their natural state Tend to react (combine) with other atoms in order to become more stable (undergo chemical reactions) Chemical equations represent chemical reactions

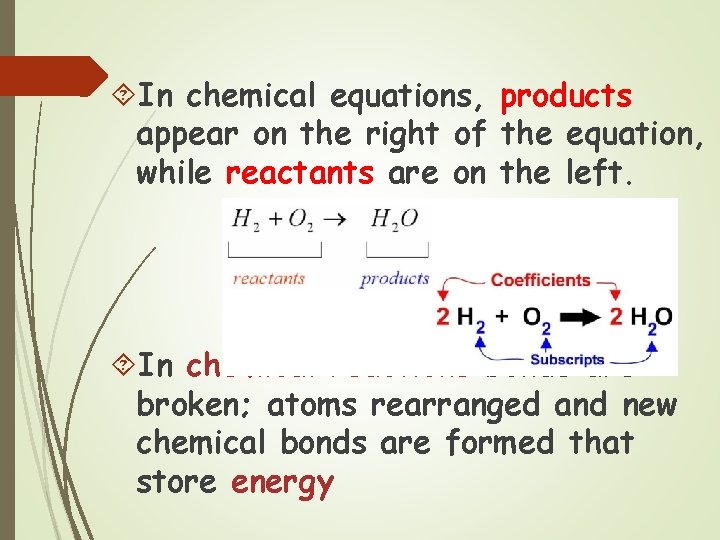
In chemical equations, products appear on the right of the equation, while reactants are on the left. In chemical reactions bonds are broken; atoms rearranged and new chemical bonds are formed that store energy

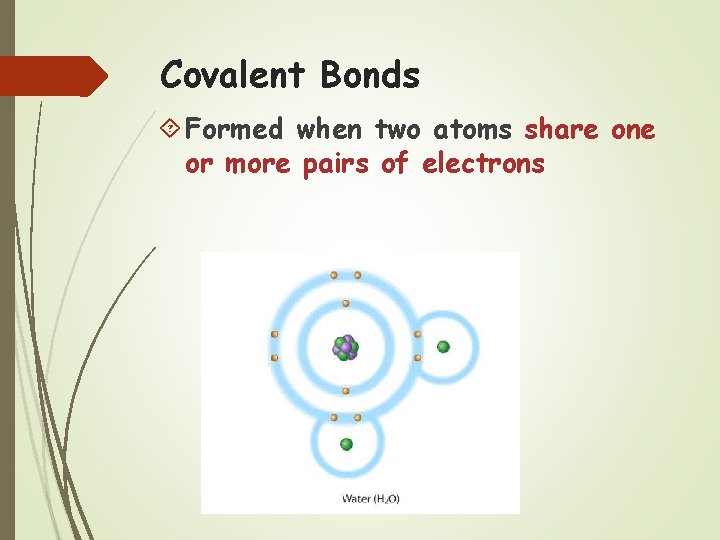
Covalent Bonds Formed when two atoms share one or more pairs of electrons

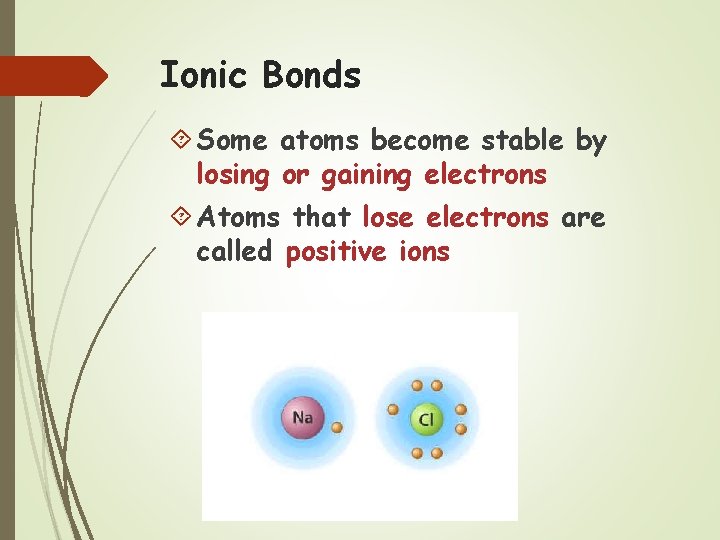
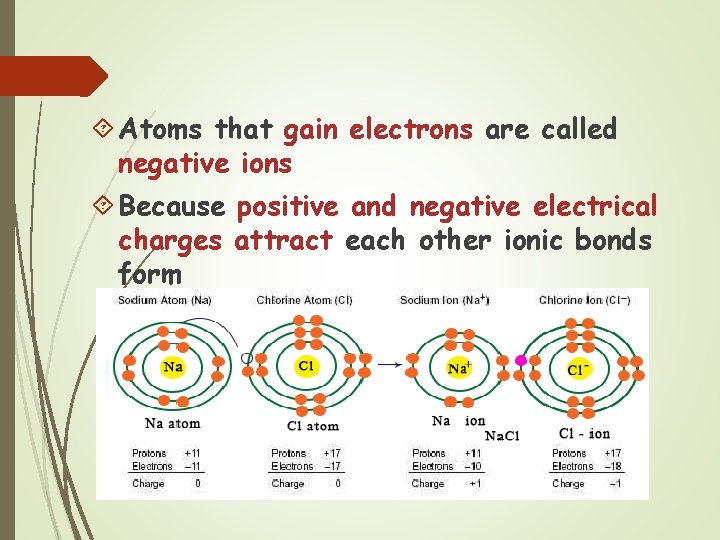
Ionic Bonds Some atoms become stable by losing or gaining electrons Atoms that lose electrons are called positive ions

Atoms that gain electrons are called negative ions Because positive and negative electrical charges attract each other ionic bonds form

Energy and Matter Energy The ability to do work or cause change Occurs in various forms Can be converted to another form Forms important to biological systems are chemical, thermal, electrical and mechanical energy Free energy is the energy in a system that is available for work

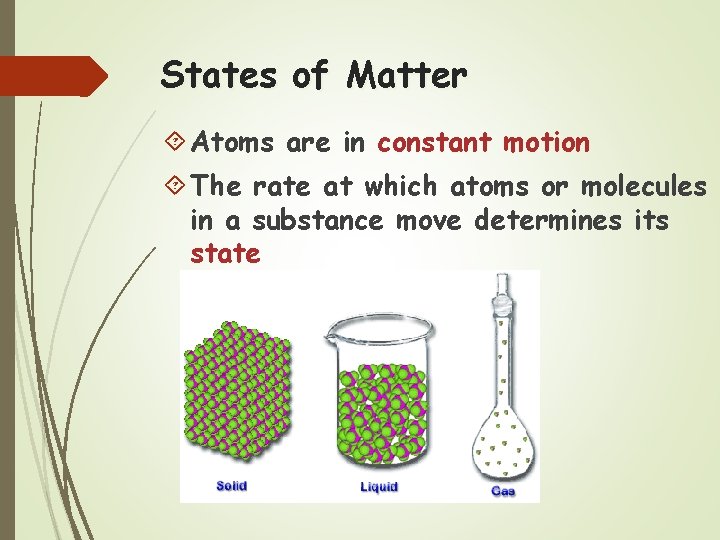
States of Matter Atoms are in constant motion The rate at which atoms or molecules in a substance move determines its state

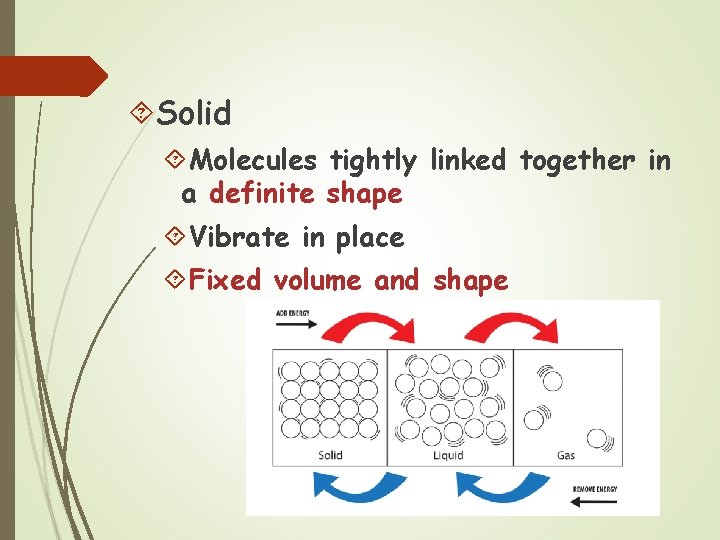
Solid Molecules tightly linked together in a definite shape Vibrate in place Fixed volume and shape

Liquids Molecules not as tightly linked as a solid Maintain fixed volume Able to flow and conform to shape of container

Gas Molecules have little or no attraction to each other Fill the volume of the occupied container Move most rapidly To cause a substance to change state, thermal energy (heat) must be added to or removed from a substance

Energy and Chemical Reactions Living things undergo thousands of chemical reactions as part of the life process

Many are very complex involving multistep sequences called biochemical pathways Chemical equations represent chemical reactions Reactants are shown on the left side of the equation Products are shown on the right side

The number of each kind of atom must be the same on either side of the arrow (equation must be balanced) So, in reactions the amount of product must equal the amount of reactants. Bonds may be broken or made forming new compounds

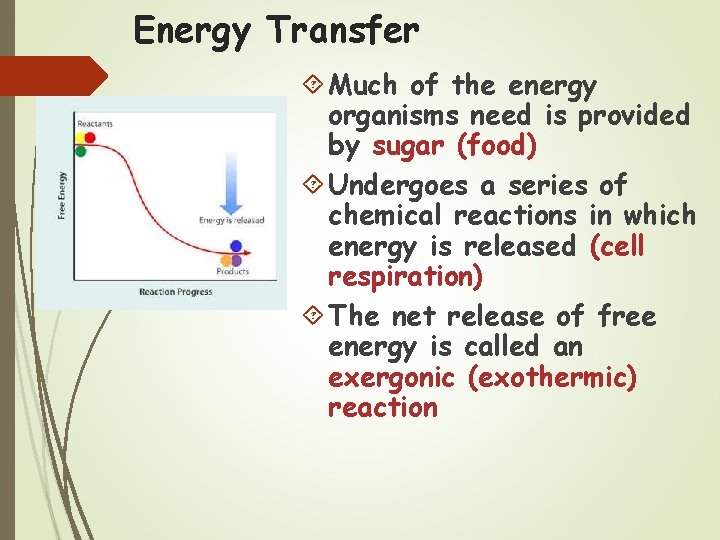
Energy Transfer Much of the energy organisms need is provided by sugar (food) Undergoes a series of chemical reactions in which energy is released (cell respiration) The net release of free energy is called an exergonic (exothermic) reaction

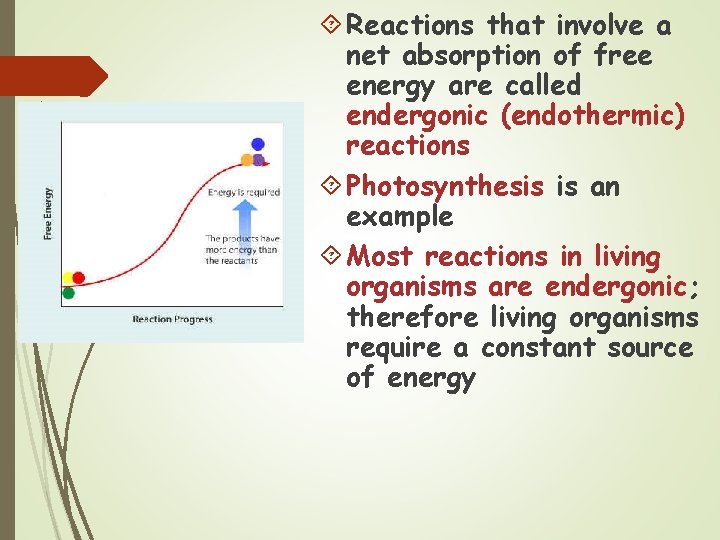
Reactions that involve a net absorption of free energy are called endergonic (endothermic) reactions Photosynthesis is an example Most reactions in living organisms are endergonic; therefore living organisms require a constant source of energy

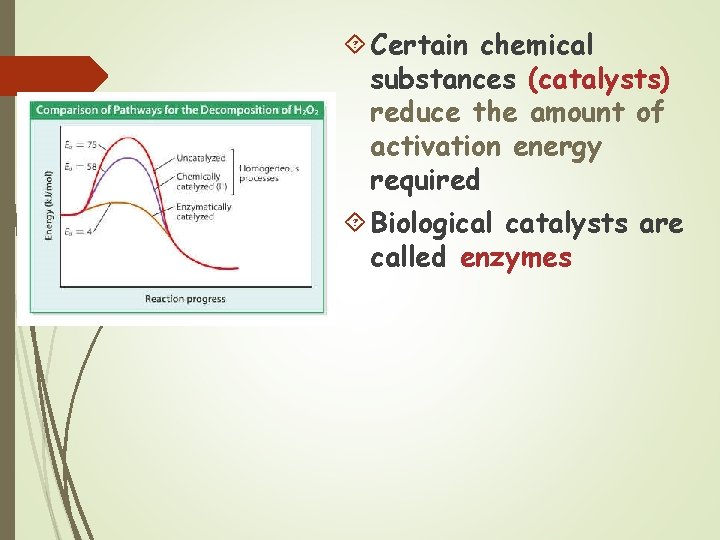
Most chemical reactions require energy to begin The amount of energy needed to start the reaction is called activation energy

Certain chemical substances (catalysts) reduce the amount of activation energy required Biological catalysts are called enzymes

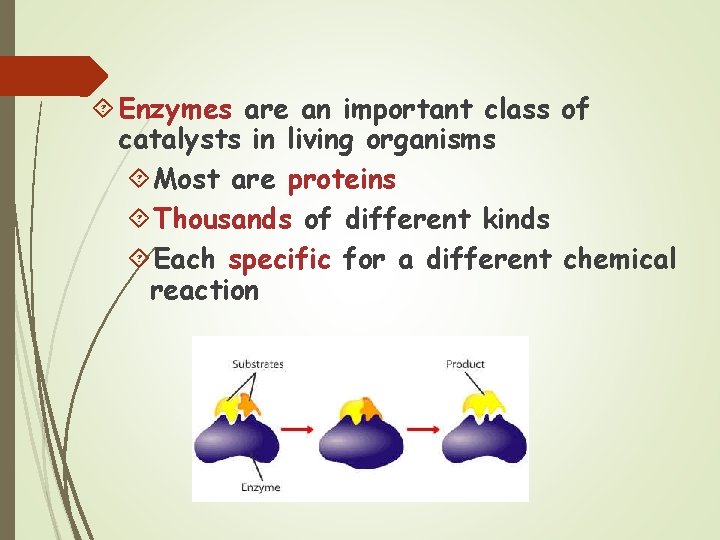
Enzymes are an important class of catalysts in living organisms Most are proteins Thousands of different kinds Each specific for a different chemical reaction

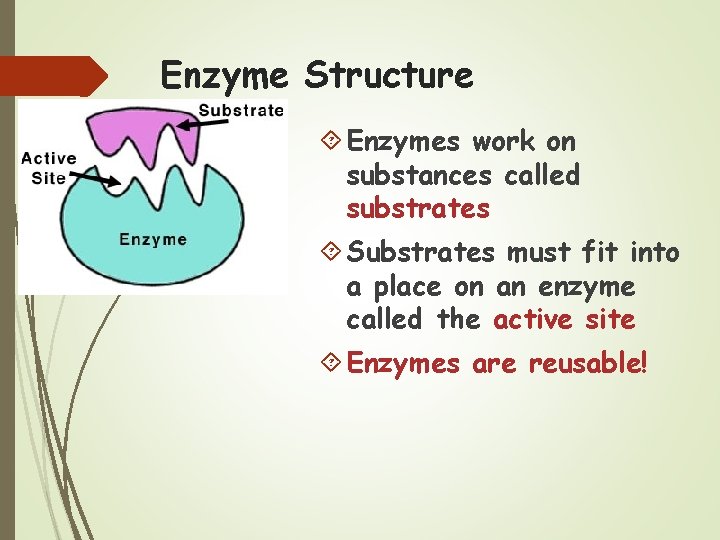
Enzyme Structure Enzymes work on substances called substrates Substrates must fit into a place on an enzyme called the active site Enzymes are reusable!

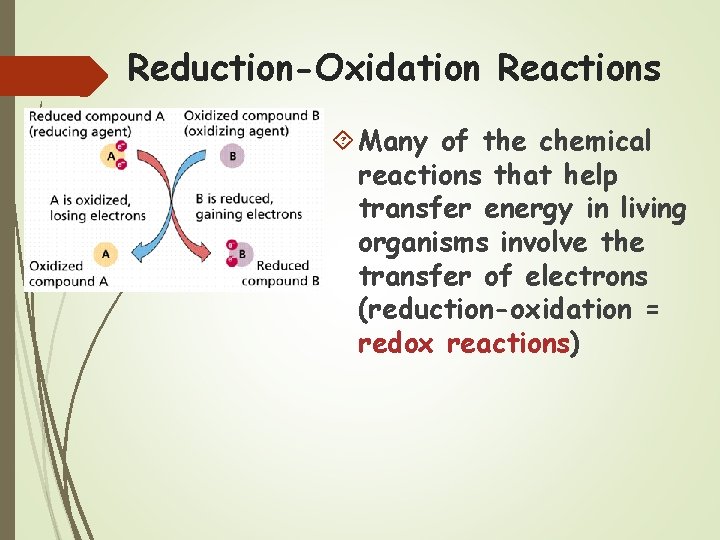
Reduction-Oxidation Reactions Many of the chemical reactions that help transfer energy in living organisms involve the transfer of electrons (reduction-oxidation = redox reactions)

Oxidation reaction – reactant loses electron(s) becoming more positive

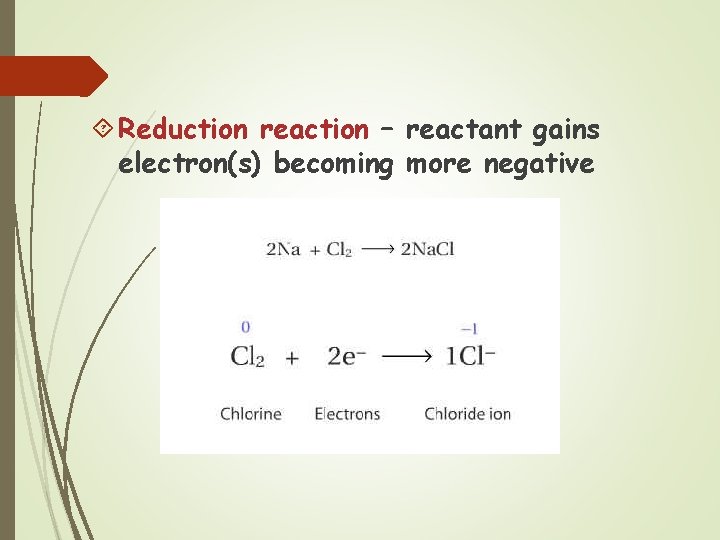
Reduction reaction – reactant gains electron(s) becoming more negative

Solutions

Solutions A solution is a mixture in which 2 or more substances are uniformly distributed in another substance

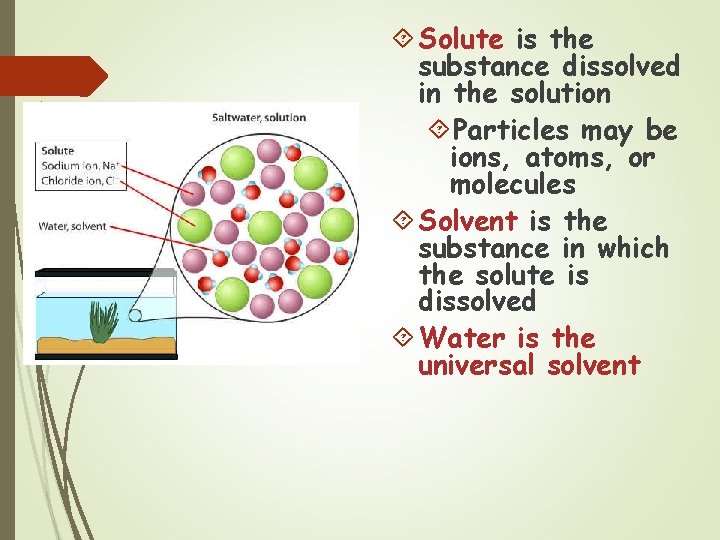
Solute is the substance dissolved in the solution Particles may be ions, atoms, or molecules Solvent is the substance in which the solute is dissolved Water is the universal solvent

Solutions can be composed of varying proportions of a given solute in a given solvent --vary in concentration (measurement of the amount of solute) A saturated solution is one in which no more solute can be dissolved Aqueous solution (water) are universally important to living things

Dissociation of water Breaking apart of the water molecule into two ions of opposite charge (due to strong attraction of oxygen atom of one molecule for H atom of another water molecule) H 2 O H+ + water hydrogen ion OH- hydroxide ion

Acids and Bases One of the most important aspects of a living system is the degree of acidity or alkalinity

Acids Number of hydronium ions in solutions is greater than the number of hydroxide ions HCl H+ + Cl-

Bases Number of hydroxide ions in solution is greater than the number of hydronium ions Na. OH Na+ + OH-

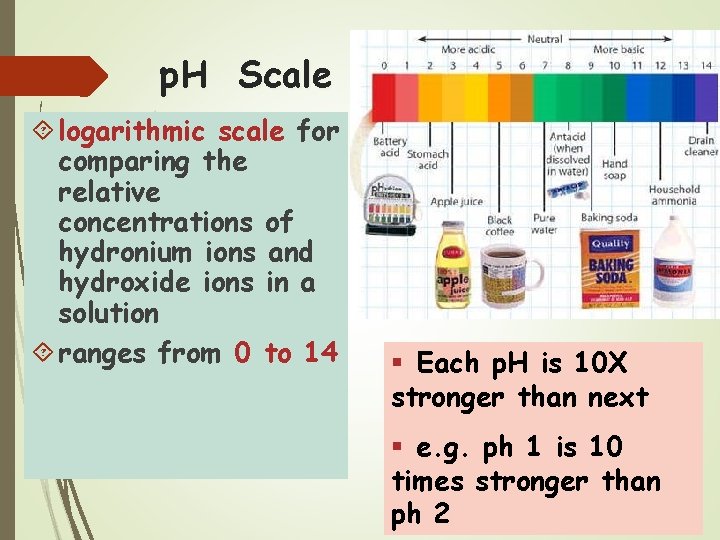
p. H Scale logarithmic scale for comparing the relative concentrations of hydronium ions and hydroxide ions in a solution ranges from 0 to 14 § Each p. H is 10 X stronger than next § e. g. ph 1 is 10 times stronger than ph 2

the lower the p. H the stronger the acid the higher the p. H the stronger the base p. H 7. 0 is neutral

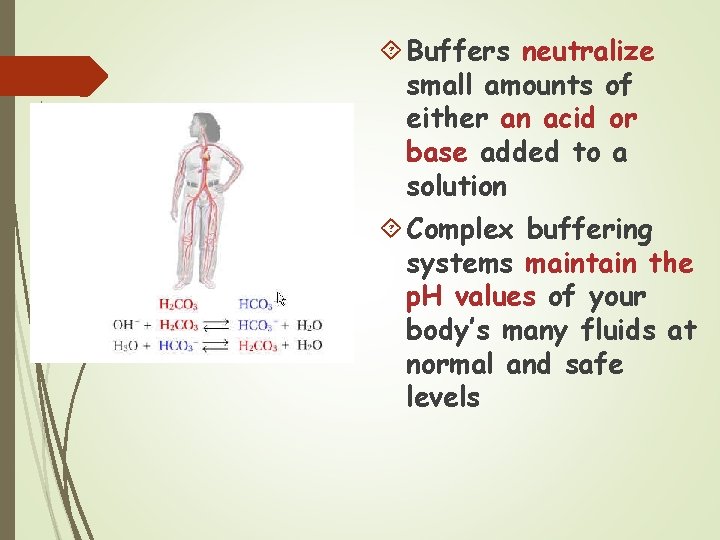
Buffers Control of p. H is very important Most enzymes function only within a very narrow p. H Control is accomplished with buffers made by the body Buffers keep a neutral p. H (p. H 7)

Buffers neutralize small amounts of either an acid or base added to a solution Complex buffering systems maintain the p. H values of your body’s many fluids at normal and safe levels

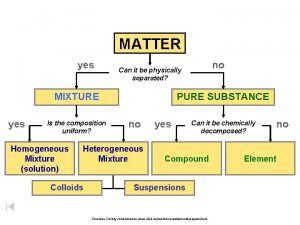
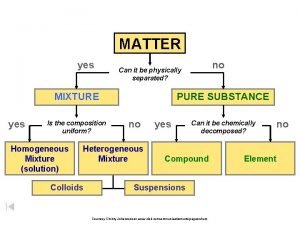
 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What is the answer to life the universe and everything
What is the answer to life the universe and everything What is everything around us made of
What is everything around us made of Is the composition uniform yes or no
Is the composition uniform yes or no Composition of matter flow chart
Composition of matter flow chart The study of composition structure and properties of matter
The study of composition structure and properties of matter What is dissolution
What is dissolution Composition uniform
Composition uniform Matter flow chart
Matter flow chart Composition of matter chapter 9
Composition of matter chapter 9 Composition of matter section 1
Composition of matter section 1 Classifying matter concept map
Classifying matter concept map Composition of matter flow chart
Composition of matter flow chart Number of proton
Number of proton Properties of solids liquids and gases
Properties of solids liquids and gases A type of matter with a fixed composition
A type of matter with a fixed composition Percent composition examples
Percent composition examples White matter
White matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Arbor vitae
Arbor vitae Gray matter and white matter
Gray matter and white matter Pallium telencephalon
Pallium telencephalon Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Chemistry matter and its changes
Chemistry matter and its changes Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 assessment the mole answer key
Chapter 10 assessment the mole answer key Examples of matter in chemistry
Examples of matter in chemistry Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 2 matter and change answer key
2 matter and change answer key Flowchart undissolved solids
Flowchart undissolved solids Graphic organizer about matter
Graphic organizer about matter Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Duproc
Duproc Ordonnanceur dollar universe
Ordonnanceur dollar universe Orsyp
Orsyp Orbit venus
Orbit venus There is no neutral ground in the universe
There is no neutral ground in the universe The last book in the universe
The last book in the universe ❄️ heat death of the universe
❄️ heat death of the universe Parall universe
Parall universe Degenerate era of the universe
Degenerate era of the universe Portland state scholarships
Portland state scholarships Dollar universe tutorial
Dollar universe tutorial Dollar universe scheduler tool tutorial
Dollar universe scheduler tool tutorial Open univers
Open univers Henry disapproves of stealing jelly beans
Henry disapproves of stealing jelly beans Richard pogge
Richard pogge Entropy system and surroundings
Entropy system and surroundings Chapter 26 exploring the universe answers
Chapter 26 exploring the universe answers