What Is Everything Made of If it has



























- Slides: 66

What Is Everything Made of? If it has mass and volume it’s…

Examples of Matter.

What Is Not Matter. • Energy (heat, light, electricity, etc). • Forces (gravity magnetism and the nuclear forces) • Space itself is a 3 D “fabric” but not matter.

Law of Conservation of Mass. • Matter cannot be created nor destroyed, except in a nuclear reaction when it becomes energy E=mc 2. • A burning log does not disappear, collect all the smoke and ashes and the mass is the same.

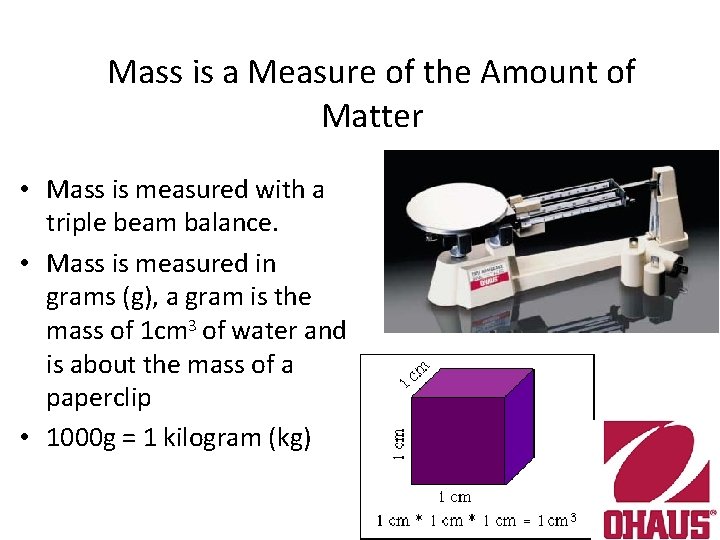
Mass is a Measure of the Amount of Matter • Mass is measured with a triple beam balance. • Mass is measured in grams (g), a gram is the mass of 1 cm 3 of water and is about the mass of a paperclip • 1000 g = 1 kilogram (kg)

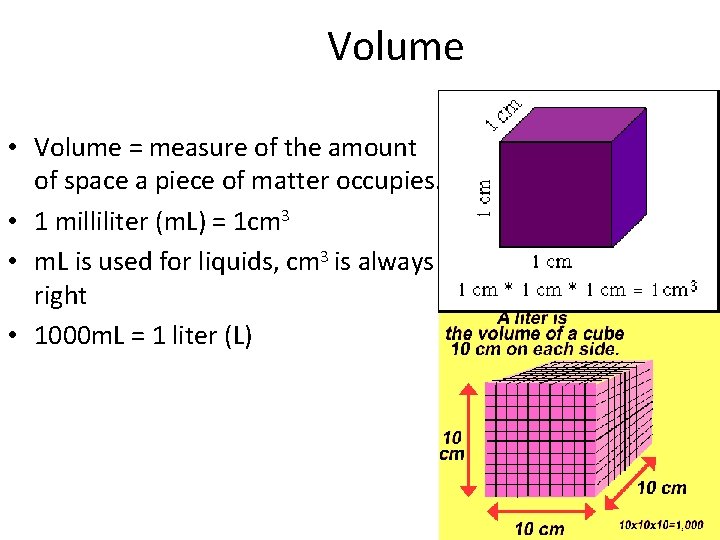
Volume • Volume = measure of the amount of space a piece of matter occupies. • 1 milliliter (m. L) = 1 cm 3 • m. L is used for liquids, cm 3 is always right • 1000 m. L = 1 liter (L)

Measuring Volume • Use a graduated cylinder for liquids. • Measure at the bottom of the meniscus • Use an overflow can for oddly shaped objects

Measuring • Measure to 2 decimal places with a ruler or triple beam balance • Measure to 1 decimal place with a graduated cylinder. • Last number is always a guess. • When calculating, your answer can never have more decimals than your data.

Estimate the volume of each example • 82. 7 m. L • 58. 0 m. L • 52. 2 m. L

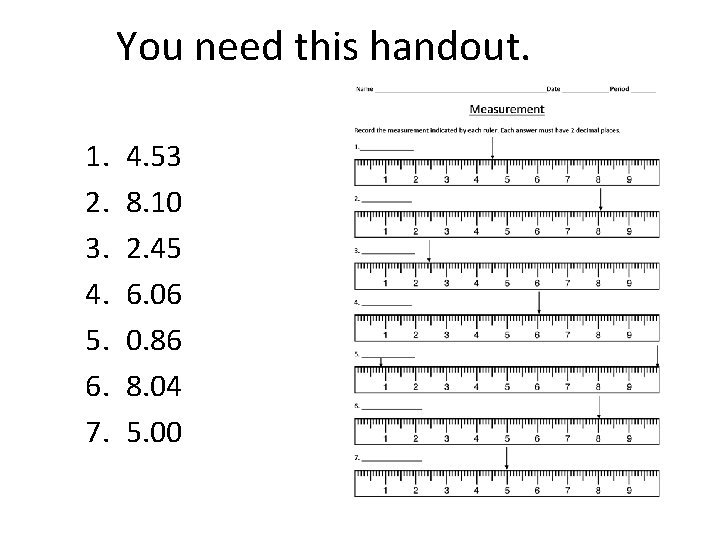
You need this handout. 1. 2. 3. 4. 5. 6. 7. 4. 53 8. 10 2. 45 6. 06 0. 86 8. 04 5. 00

Dihydrogen Monoxide • Tare a container then measure mass every 10 m. L up to 60 m. L. Graph your data • If you are accurate it will form a line, the more accurate you are, the closer your data will be to a line. • When real scientists do an experiment, their hope is their data will form a line or curve. • Draw the line of best fit. Be sure to go through (x=0, y=0)

Mass vs Weight

All Matter has Gravity • The elephant has more matter so it has more mass and more gravity. • Even dust is matter so it has gravity.

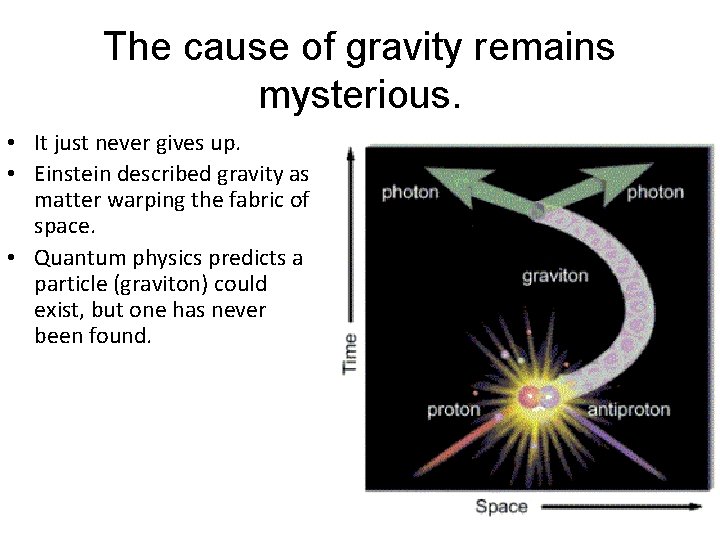
The cause of gravity remains mysterious. • It just never gives up. • Einstein described gravity as matter warping the fabric of space. • Quantum physics predicts a particle (graviton) could exist, but one has never been found.

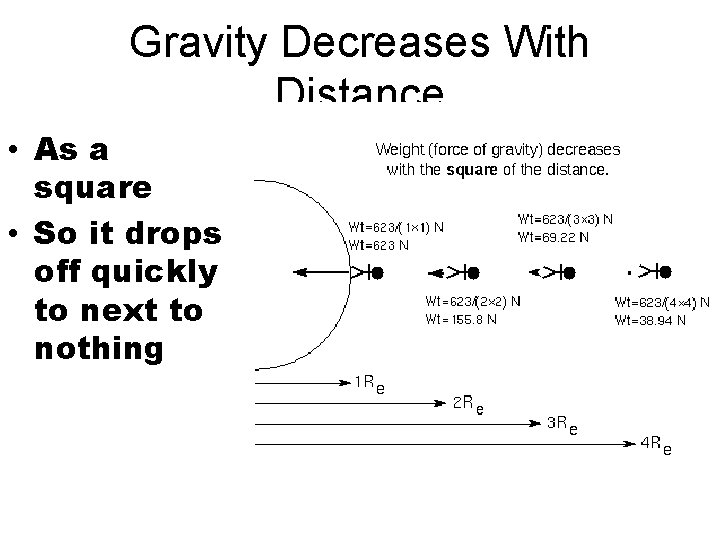
Gravity Decreases With Distance • As a square • So it drops off quickly to next to nothing

Gravity isn’t the same everywhere on the Earth • Gravity at any location depends upon many factors such as the makeup and thickness of the crust • Imagine two gravity meters, the on the ground is closer to the center of the Earth so it reads higher.

Weight is the Pull of Gravity • Weight is a measure of the pull of gravity. • Weight is measured with a spring that is compressed or stretched.

How can something be nothing? • Far enough from the pull of a planet, an astronaut could become weightless. • The astronaut and the space suit are made of something… • but, according to weight they are nothing, which is impossible!

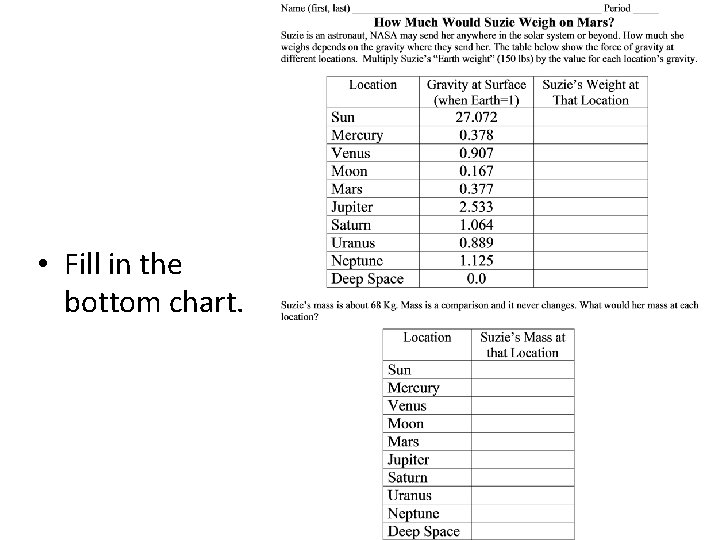
Another Problem • Weight changes when you’re standing on a different planet or on the moon • The Earth’s mass is six times the moons. • On Earth the astronaut weighs 185 lbs. • Moon has 1/6 th the gravity so the astronaut weighs only 31 lbs. • Weight is not about the astronaut, it’s about what object they’re standing on.

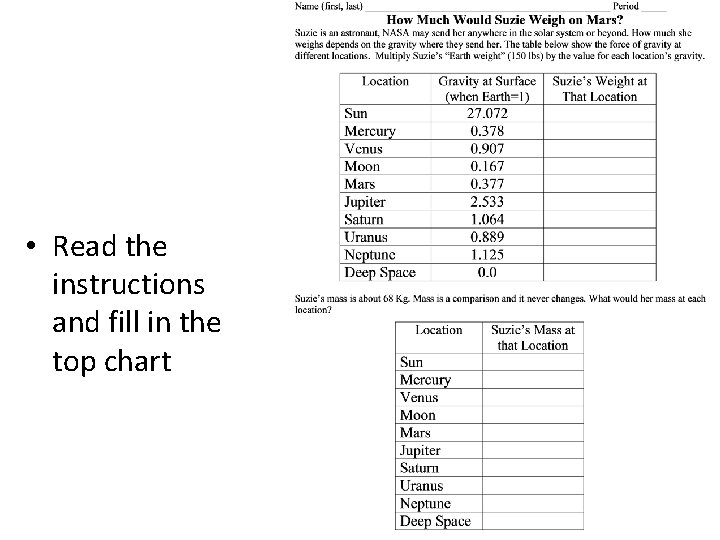
• Read the instructions and fill in the top chart

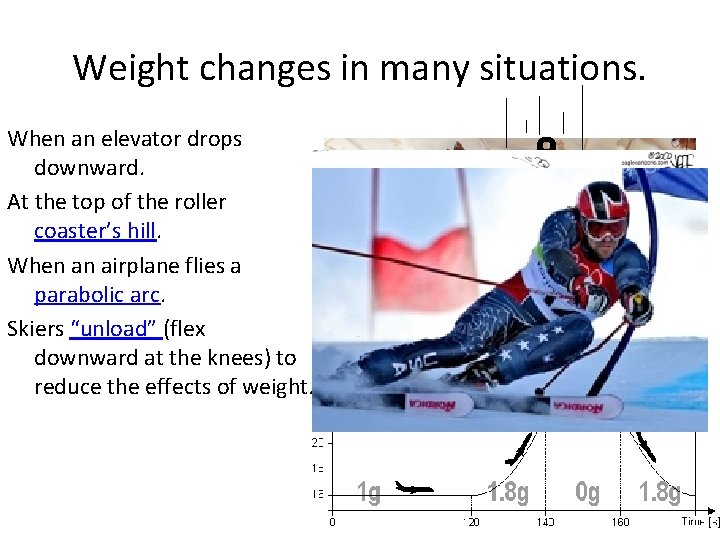
Weight changes in many situations. When an elevator drops downward. At the top of the roller coaster’s hill. When an airplane flies a parabolic arc. Skiers “unload” (flex downward at the knees) to reduce the effects of weight.

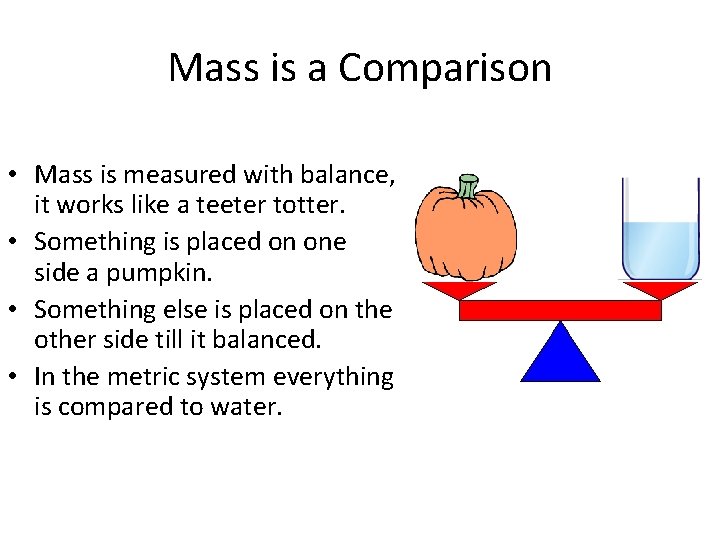
Mass is a Comparison • Mass is measured with balance, it works like a teeter totter. • Something is placed on one side a pumpkin. • Something else is placed on the other side till it balanced. • In the metric system everything is compared to water.

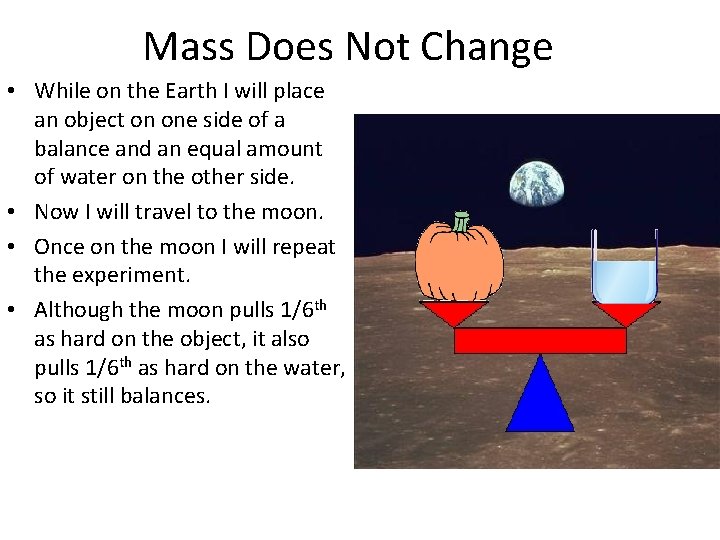
Mass Does Not Change • While on the Earth I will place an object on one side of a balance and an equal amount of water on the other side. • Now I will travel to the moon. • Once on the moon I will repeat the experiment. • Although the moon pulls 1/6 th as hard on the object, it also pulls 1/6 th as hard on the water, so it still balances.

• Fill in the bottom chart.

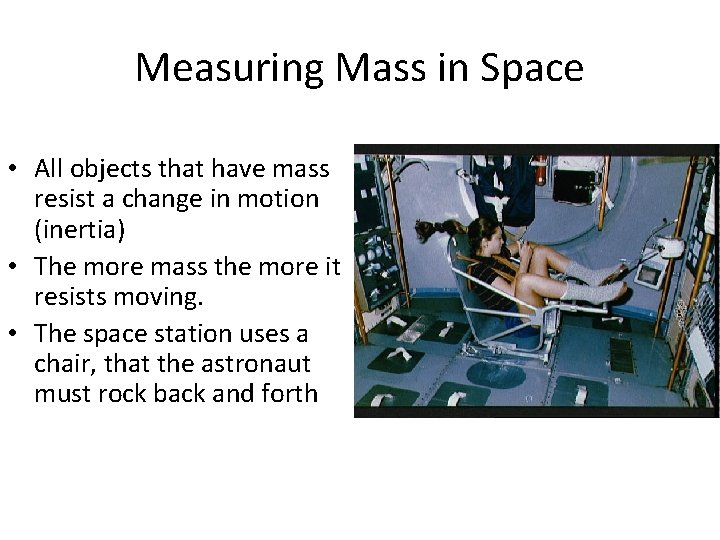
Measuring Mass in Space • All objects that have mass resist a change in motion (inertia) • The more mass the more it resists moving. • The space station uses a chair, that the astronaut must rock back and forth

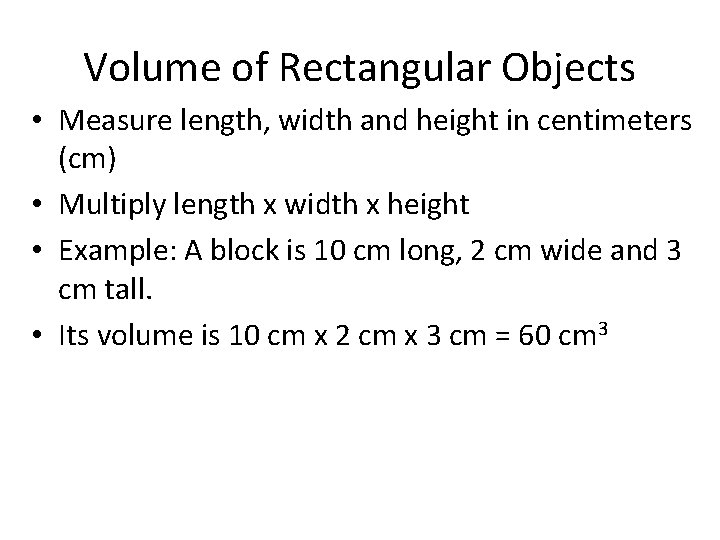
Volume of Rectangular Objects • Measure length, width and height in centimeters (cm) • Multiply length x width x height • Example: A block is 10 cm long, 2 cm wide and 3 cm tall. • Its volume is 10 cm x 2 cm x 3 cm = 60 cm 3

D = m/v • Denser things sink. • The density of pure water is 1. 0 g/cm 3 by definition. • Anything denser sinks; anything less dense floats. • Saltwater is denser than fresh. • Density can be used to identify substances.

Example: An object has a mass of 200. 00 g and a volume of 340. 00 cm 3. What is its density and will it sink or will it float? • • D=m/v D= 200. 00 g/340. 0 cm 3 D=0. 6 g/cm 3 It will float because it’s density is less than 1. 0 g/cm 3

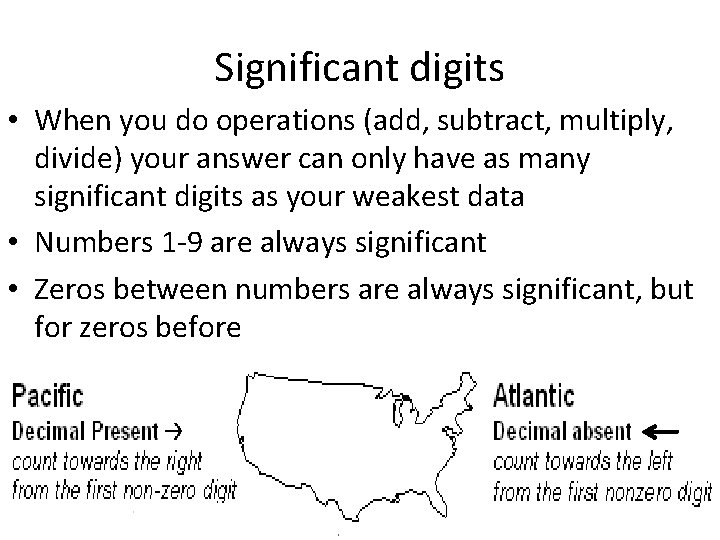
Significant digits • When you do operations (add, subtract, multiply, divide) your answer can only have as many significant digits as your weakest data • Numbers 1 -9 are always significant • Zeros between numbers are always significant, but for zeros before

Examples

Example 2: A graduated cylinder starts with 45. 12 m. L of water. An object with a mass of 12. 30 g is lowered into the water. The water in the cylinder goes up to 56. 78 m. L. What is the density of the object? • • • Volume = 56. 78 m. L - 45. 12 m. L Volume = 11. 66 ml D= m/v D= 12. 30 g/11. 65 cm 3 D=1. 16 g/cm 3 (we only get to keep 1 decimal)

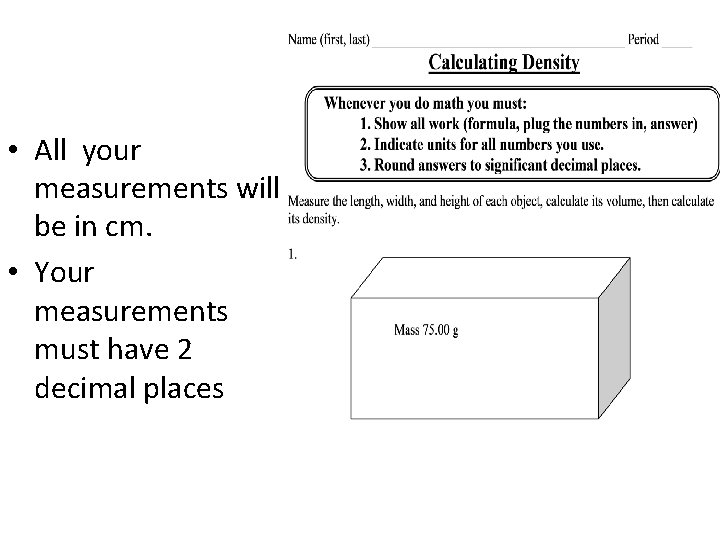
• All your measurements will be in cm. • Your measurements must have 2 decimal places

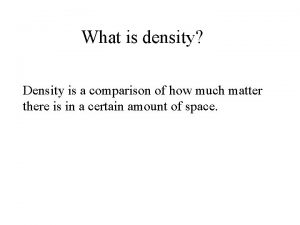
What is density? • Density is a comparison of how much matter there is in a certain amount of space. • How big something is compared to how heavy it is.

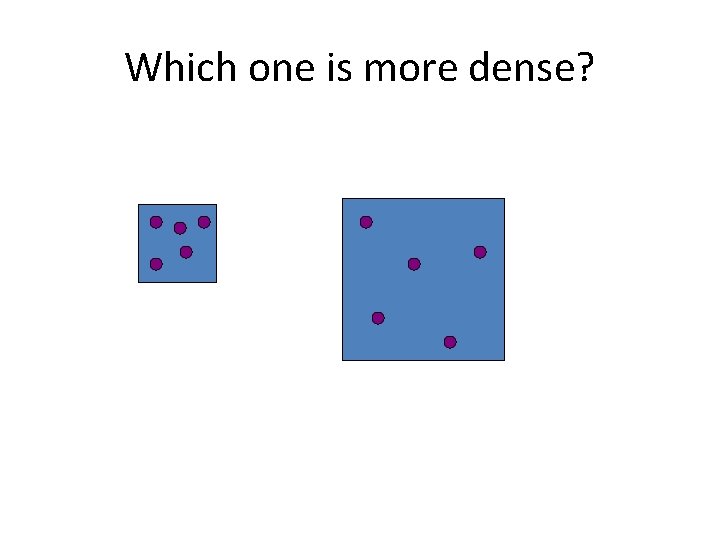
Which one is more dense?

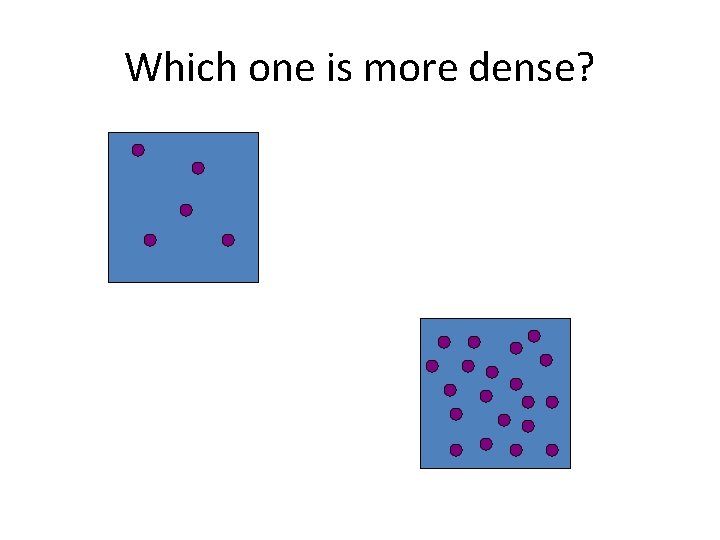
Which one is more dense?

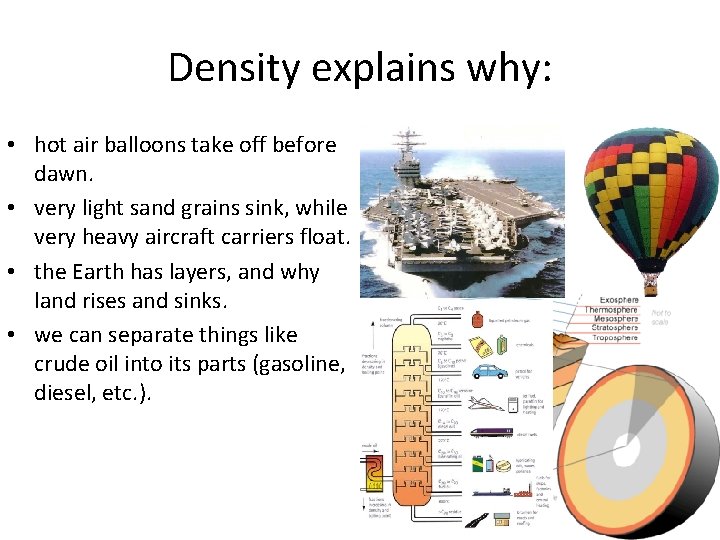
Density explains why: • hot air balloons take off before dawn. • very light sand grains sink, while very heavy aircraft carriers float. • the Earth has layers, and why land rises and sinks. • we can separate things like crude oil into its parts (gasoline, diesel, etc. ).

Liquid Layers • If you pour together liquids that don’t mix and have different densities, they will form liquid layers. • The liquid with the highest density will be on the bottom. • The liquid with the lowest density will be on the top.

Liquid Layers • Imagine that the liquids have the following densities: – 10 g/cm 3. 3 g/cm 3. – 6 g/cm 3. 5 g/cm 3. • Which number would go with which layer?

Liquid Layers • Imagine that the liquids on the right have the following densities: – 15 g/cm 3 – 3 g/cm 3 – 7 g/cm 3 10 g/cm 3 9 g/cm 3 12 g/cm 3 • Match the colors to the correct densities. 3 g/cm 3 7 g/cm 3 9 g/cm 3 10 g/cm 3 12 g/cm 3 15 g/cm 3

Review • What is the formula for density? • What happens if you pour together liquids that have different densities? • Will the liquid on the top have the highest or lowest density? • Will the liquid on the bottom have the highest or lowest density?

Air Pressure/Density

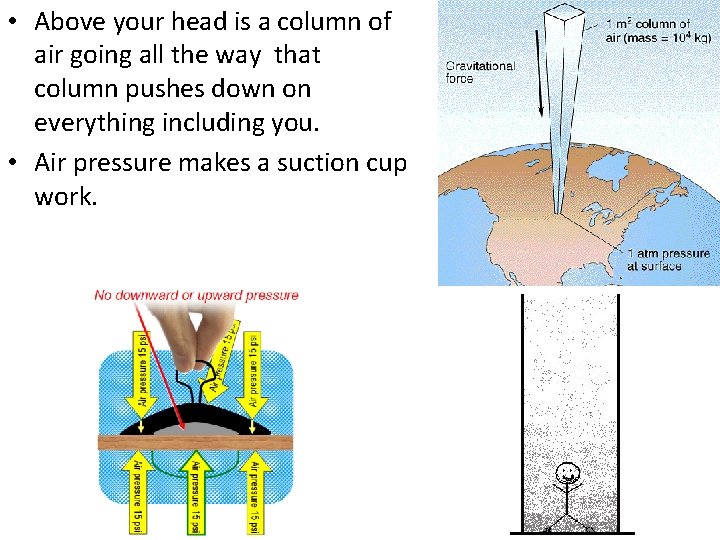
• Above your head is a column of air going all the way that column pushes down on everything including you. • Air pressure makes a suction cup work.

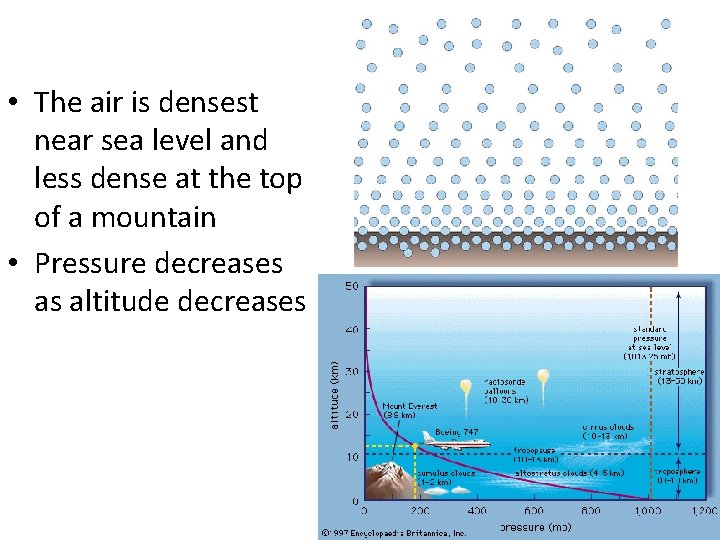
• The air is densest near sea level and less dense at the top of a mountain • Pressure decreases as altitude decreases

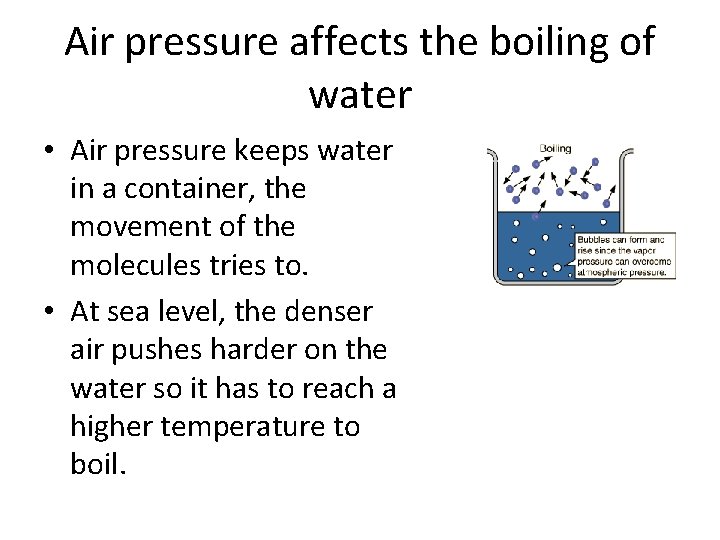
Air pressure affects the boiling of water • Air pressure keeps water in a container, the movement of the molecules tries to. • At sea level, the denser air pushes harder on the water so it has to reach a higher temperature to boil.

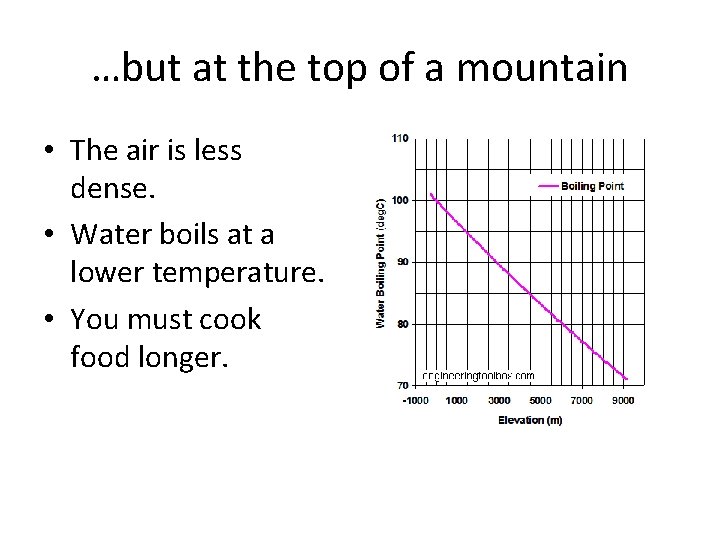
…but at the top of a mountain • The air is less dense. • Water boils at a lower temperature. • You must cook food longer.

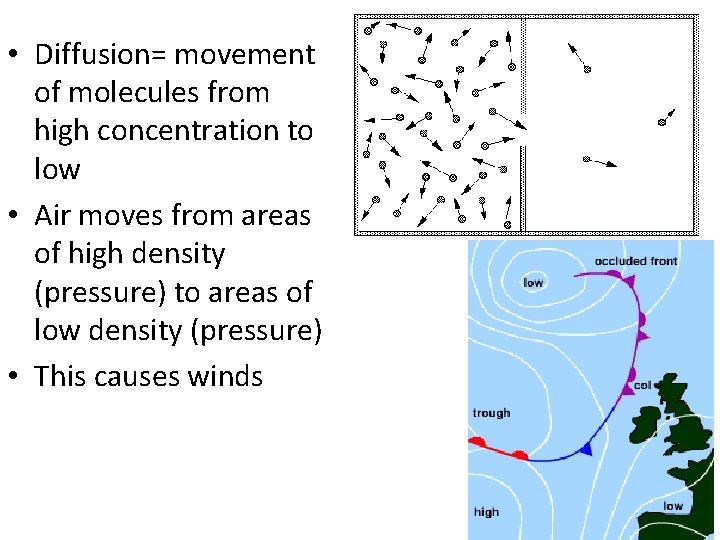
• Diffusion= movement of molecules from high concentration to low • Air moves from areas of high density (pressure) to areas of low density (pressure) • This causes winds

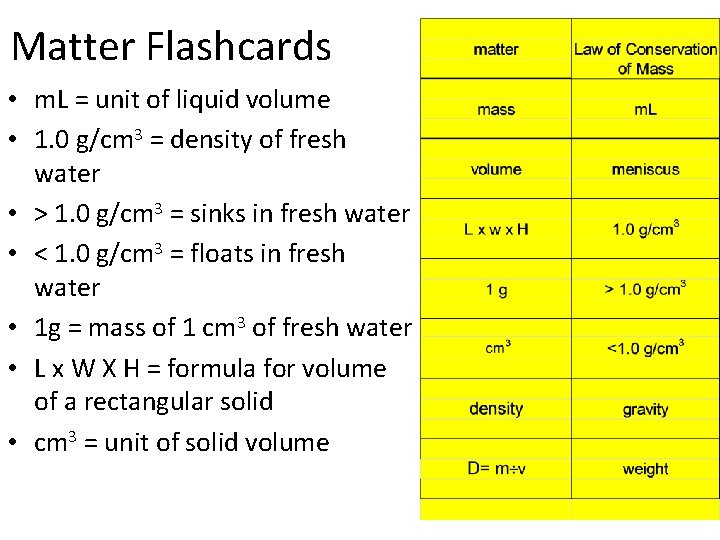
Matter Flashcards • m. L = unit of liquid volume • 1. 0 g/cm 3 = density of fresh water • > 1. 0 g/cm 3 = sinks in fresh water • < 1. 0 g/cm 3 = floats in fresh water • 1 g = mass of 1 cm 3 of fresh water • L x W X H = formula for volume of a rectangular solid • cm 3 = unit of solid volume

Solid, Liquid, Gas (a) Particles in solid (b) Particles in liquid (c) Particles in gas

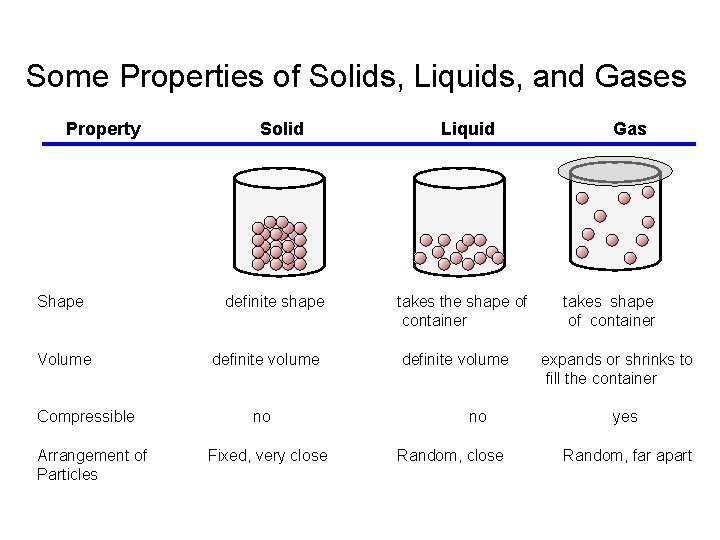
Some Properties of Solids, Liquids, and Gases Property Shape Volume Compressible Arrangement of Particles Solid definite shape definite volume no Fixed, very close Liquid takes the shape of container definite volume no Random, close Gas takes shape of container expands or shrinks to fill the container yes Random, far apart

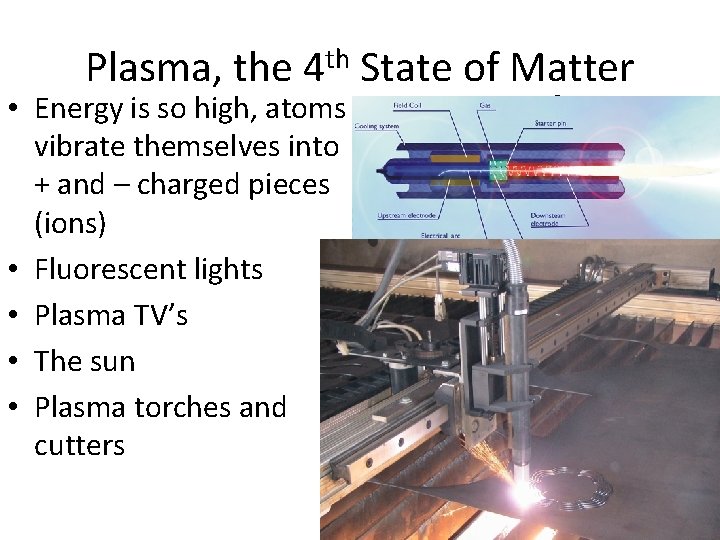
Plasma, the 4 th State of Matter • Energy is so high, atoms vibrate themselves into + and – charged pieces (ions) • Fluorescent lights • Plasma TV’s • The sun • Plasma torches and cutters

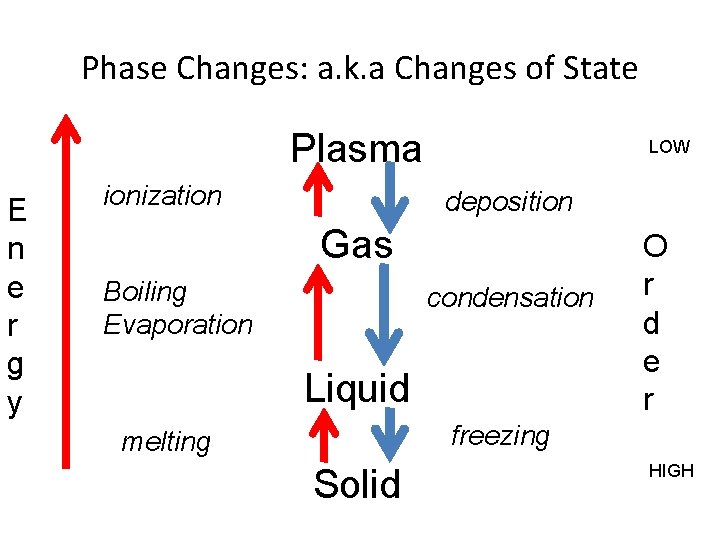
Phase Changes: a. k. a Changes of State Plasma E n e r g y ionization LOW deposition Gas Boiling Evaporation condensation Liquid O r d e r freezing melting Solid HIGH

Sublimation • When a solid goes directly to a gas without ever becoming a liquid. • Dry ice (solid CO 2) • Food in the freezer (freezer burn)

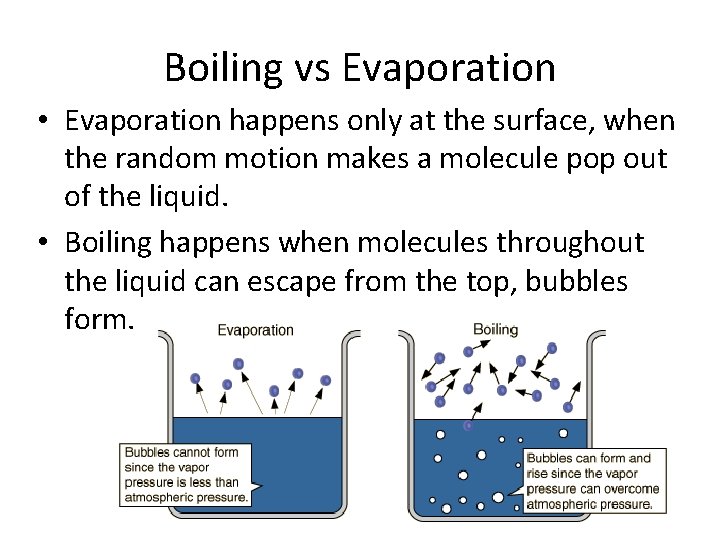
Boiling vs Evaporation • Evaporation happens only at the surface, when the random motion makes a molecule pop out of the liquid. • Boiling happens when molecules throughout the liquid can escape from the top, bubbles form.

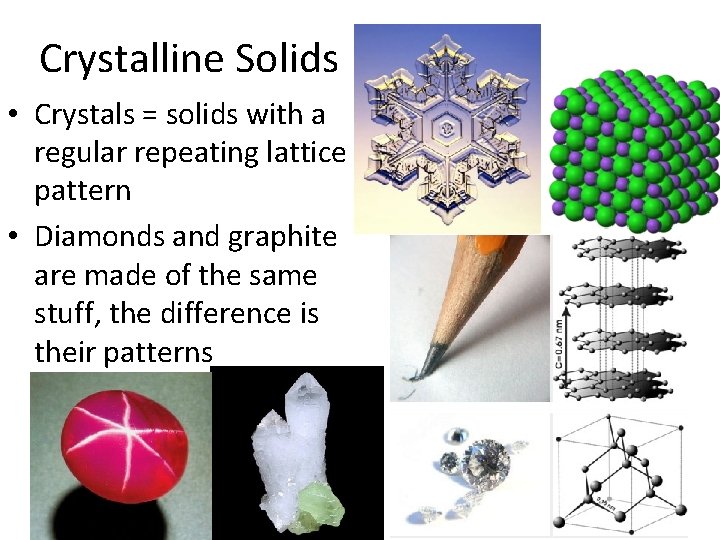
Crystalline Solids • Crystals = solids with a regular repeating lattice pattern • Diamonds and graphite are made of the same stuff, the difference is their patterns

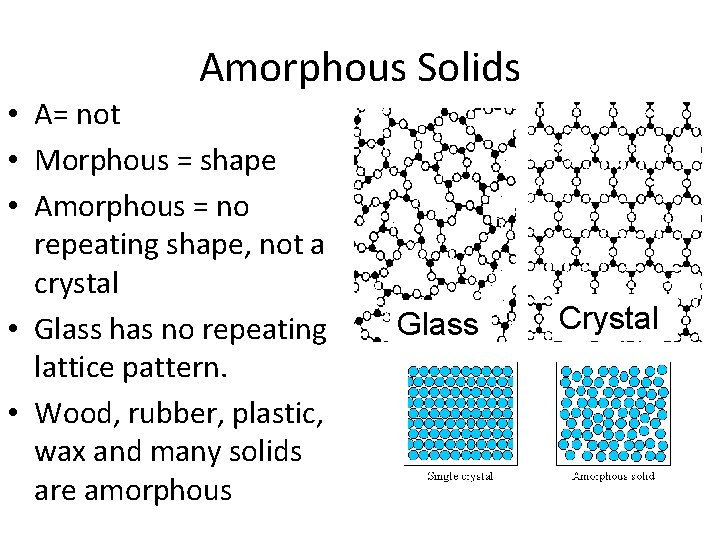
Amorphous Solids • A= not • Morphous = shape • Amorphous = no repeating shape, not a crystal • Glass has no repeating lattice pattern. • Wood, rubber, plastic, wax and many solids are amorphous Glass Crystal

Melting and Boiling Points • Every substance has a melting and boiling point that can be used to identify it. • Being frozen does not mean cold, tungsten stays frozen until it 5, 555 o. C • Boiling does not mean hot, helium boils at -268 o. C.

Example: Nitrogen • Element # 7 on the Periodic Chart • Pure nitrogen atoms travel as twins that are almost impossible to separate. • Only microbes (bacteria) can eat nitrogen from the air • Your body needs nitrogen to make DNA, and muscles (among other things)

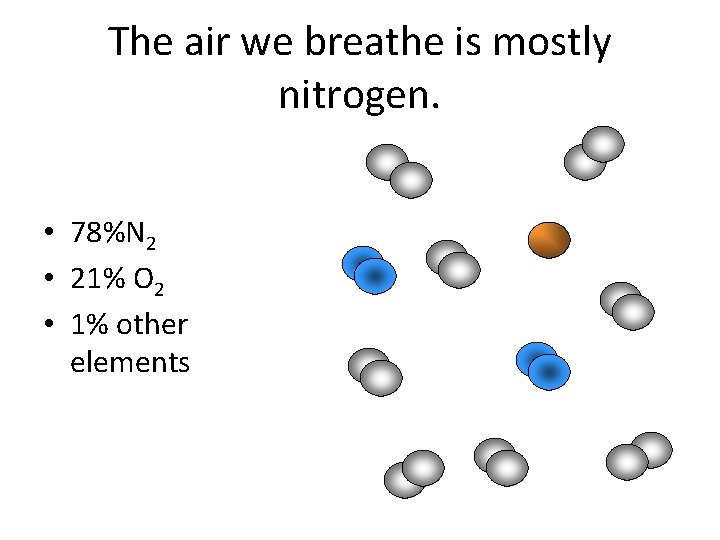
The air we breathe is mostly nitrogen. • 78%N 2 • 21% O 2 • 1% other elements

Uses for Nitrogen • • Fertilizer Explosives Car tires, nitrous Freezing things

Properties of Nitrogen • • Colorless Odorless Will not burn MELTING POINT: − 210°C BOILING POINT: − 196°C DENSITY : 0. 0012506 g/cm 3

Lauric Acid • Main acid in coconut oil and palm kernel oil. • Antimicrobial • White, powdery solid with faint odor of bay oil or soap. • Used in many soaps and shampoos.

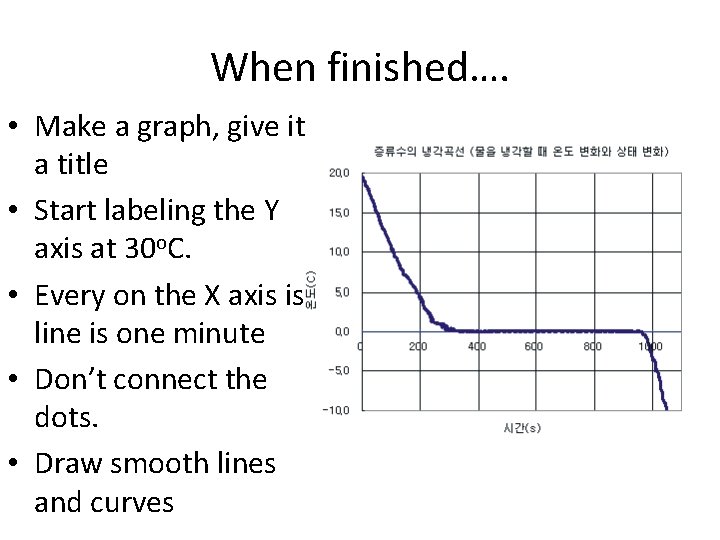
You will need this handout. • Record the temperature every 30 seconds. • Write an observation every thirty seconds

When finished…. • Make a graph, give it a title • Start labeling the Y axis at 30 o. C. • Every on the X axis is line is one minute • Don’t connect the dots. • Draw smooth lines and curves

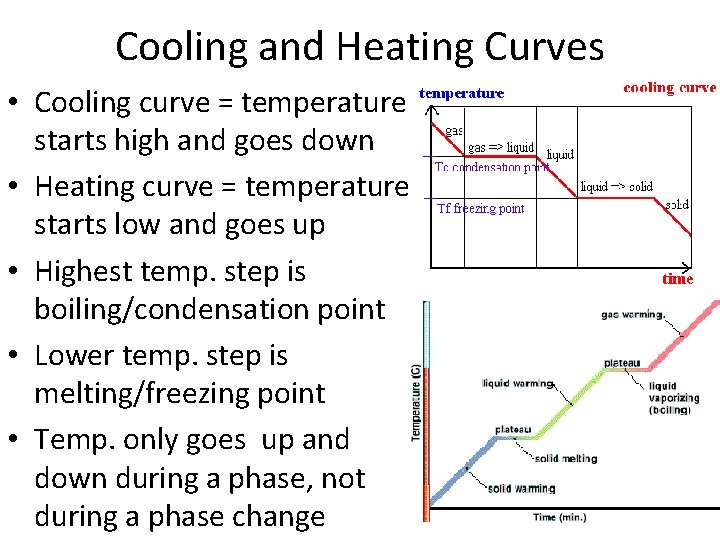
Cooling and Heating Curves • Cooling curve = temperature starts high and goes down • Heating curve = temperature starts low and goes up • Highest temp. step is boiling/condensation point • Lower temp. step is melting/freezing point • Temp. only goes up and down during a phase, not during a phase change

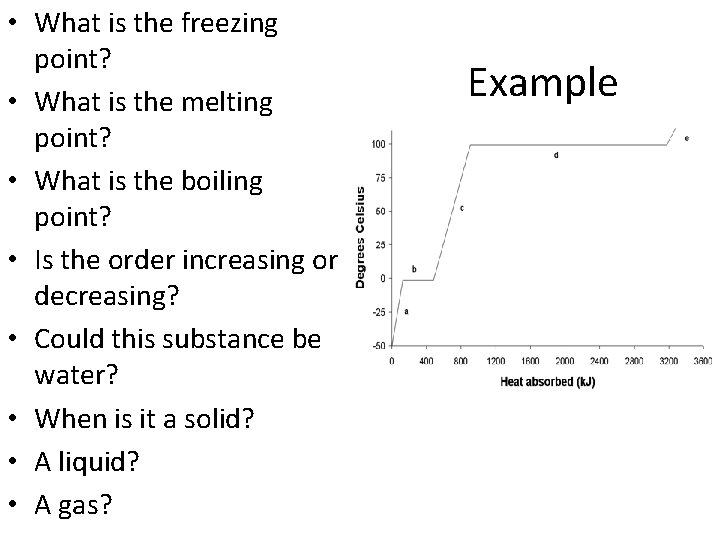
• What is the freezing point? • What is the melting point? • What is the boiling point? • Is the order increasing or decreasing? • Could this substance be water? • When is it a solid? • A liquid? • A gas? Example

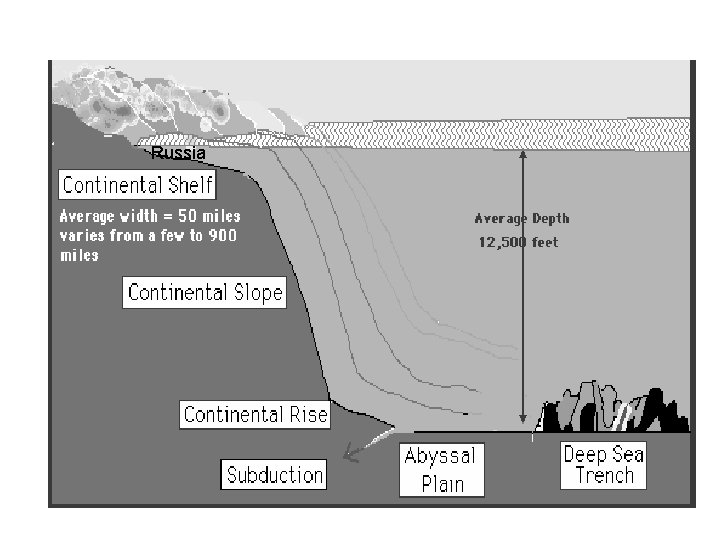
Russia
 God looked at everything he had made
God looked at everything he had made God saw everything
God saw everything Everything that sorrounds us
Everything that sorrounds us What is it
What is it Everything around you is made up of
Everything around you is made up of What is everything around us made of
What is everything around us made of What is everything made of
What is everything made of Has everything been invented
Has everything been invented Anything that has mass and volume
Anything that has mass and volume Everything that can be invented has been invented
Everything that can be invented has been invented Anything that has mass and occupies space is called
Anything that has mass and occupies space is called A square plate made of lead has an oval shaped hole
A square plate made of lead has an oval shaped hole He has made us kings and priest
He has made us kings and priest Has any progress been made
Has any progress been made Genesis 41:41
Genesis 41:41 If the law has made you a witness
If the law has made you a witness The bride hath made herself ready
The bride hath made herself ready Spring has sprung the grass has riz
Spring has sprung the grass has riz Density of golden syrup g/cm3
Density of golden syrup g/cm3 Every picture has a story and every story has a moment
Every picture has a story and every story has a moment A problem has been detected and windows shut down
A problem has been detected and windows shut down Mark 4:23-24
Mark 4:23-24 Problem has been detected and windows
Problem has been detected and windows Everything you need to know about the odyssey
Everything you need to know about the odyssey Immutability changes everything
Immutability changes everything Pumpkin spice communion wafers
Pumpkin spice communion wafers Where is everything
Where is everything 31536000 in scientific notation
31536000 in scientific notation Everything begins with cells
Everything begins with cells Tag question everything
Tag question everything Asl everything
Asl everything Test single phase motor
Test single phase motor Attitude changes everything
Attitude changes everything Everything's an argument chapter 13 summary
Everything's an argument chapter 13 summary Computer science is changing everything
Computer science is changing everything The avalanche devoured everything that stood in its way
The avalanche devoured everything that stood in its way Branch of chemical engineering
Branch of chemical engineering Everything finder
Everything finder Splunk schema on the fly
Splunk schema on the fly To defend everything is to defend nothing
To defend everything is to defend nothing Pick up everything
Pick up everything Hebrews 4
Hebrews 4 Marketing is everything
Marketing is everything Lord i lift everything to you
Lord i lift everything to you Not everything that counts can be counted meaning
Not everything that counts can be counted meaning ______ she beautiful?
______ she beautiful? We will not be moved you're standing with us
We will not be moved you're standing with us Travel hand signal
Travel hand signal How can brahman be everywhere and in everything
How can brahman be everywhere and in everything Net force opposite directions
Net force opposite directions Why do objects fall at the same rate
Why do objects fall at the same rate Is everything relative
Is everything relative Newspeak
Newspeak Checklists for everything
Checklists for everything How grieve everything we
How grieve everything we How everything
How everything Put everything together
Put everything together Arnold wesker chips with everything
Arnold wesker chips with everything Shall i compare thee to a summer's day theme
Shall i compare thee to a summer's day theme Tag question everything
Tag question everything Everything that surrounds an organism
Everything that surrounds an organism Why do objects fall at the same rate
Why do objects fall at the same rate Everything works for good
Everything works for good Pershore template
Pershore template Everything is material
Everything is material Resurrection changes everything
Resurrection changes everything Under my wings everything prospers
Under my wings everything prospers