Chemistry of Life Matter Matter Makes up everything




- Slides: 19

Chemistry of Life Matter

Matter Makes up everything • Matter is anything that has mass and takes up space (volume) • Basic unit of matter is the atom.

Atoms • Atoms are the smallest unit of matter made of protons, neutrons and electrons. • Atoms have very specific properties that make them the building blocks of everything we know.

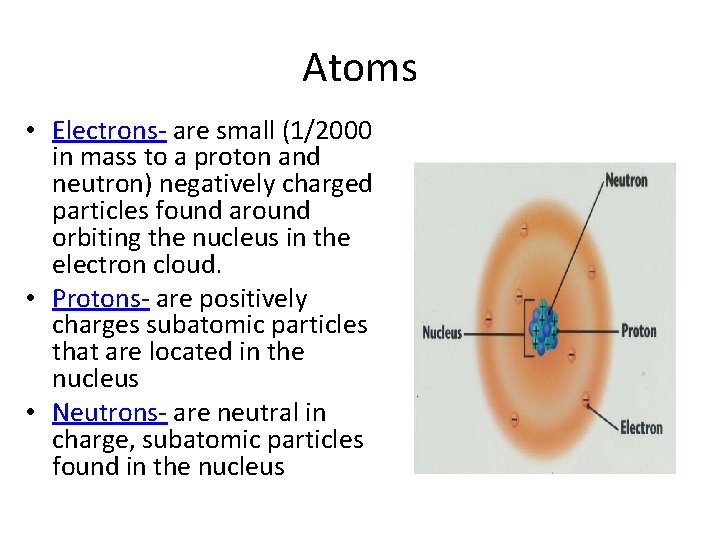
Atoms • Electrons- are small (1/2000 in mass to a proton and neutron) negatively charged particles found around orbiting the nucleus in the electron cloud. • Protons- are positively charges subatomic particles that are located in the nucleus • Neutrons- are neutral in charge, subatomic particles found in the nucleus

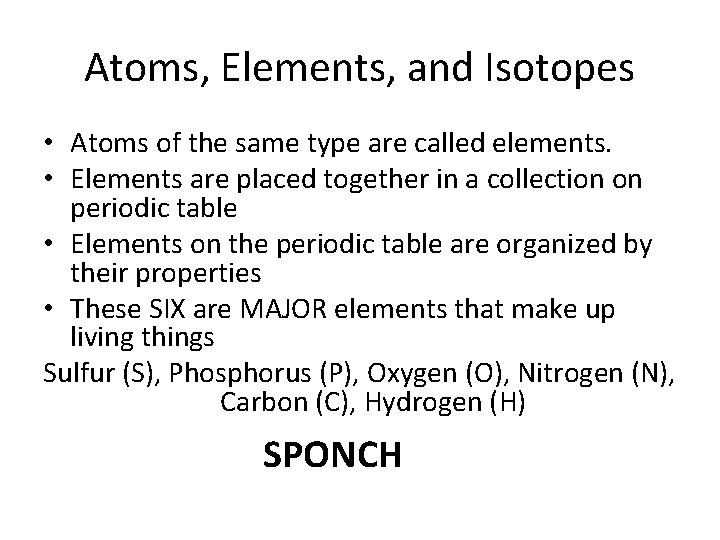
Atoms, Elements, and Isotopes • Atoms of the same type are called elements. • Elements are placed together in a collection on periodic table • Elements on the periodic table are organized by their properties • These SIX are MAJOR elements that make up living things Sulfur (S), Phosphorus (P), Oxygen (O), Nitrogen (N), Carbon (C), Hydrogen (H) SPONCH

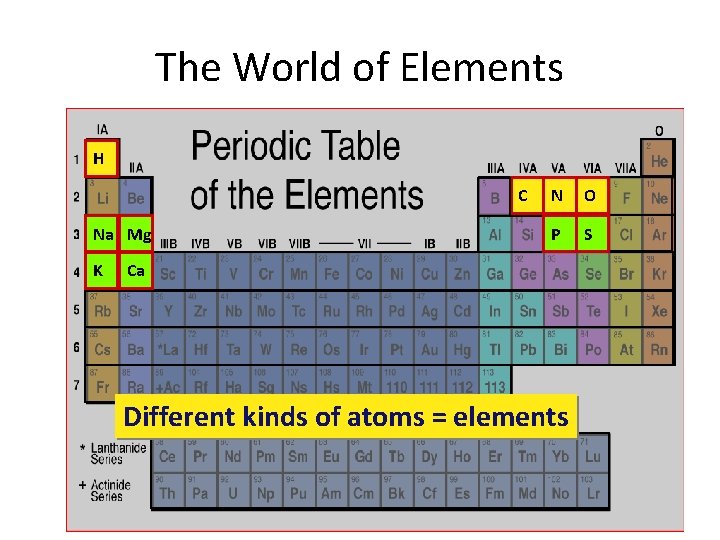
The World of Elements H C Na Mg K N O P S Ca Different kinds of atoms = elements

Atoms, Elements, and Isotopes • Other elements that make up living systems are Calcium (Ca), Sodium (Na), Magnesium (Mg) and Potassium (K) • All elements are given a name and a symbol. The symbol may appear to not match up with its name but that is because the symbol is from its Latin name.

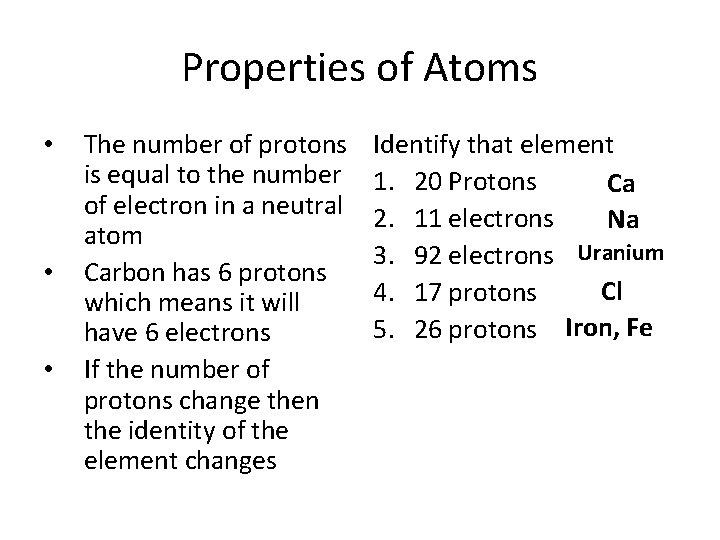
Properties of Atoms • • • The number of protons is equal to the number of electron in a neutral atom Carbon has 6 protons which means it will have 6 electrons If the number of protons change then the identity of the element changes Identify that element 1. 20 Protons Ca 2. 11 electrons Na 3. 92 electrons Uranium Cl 4. 17 protons 5. 26 protons Iron, Fe

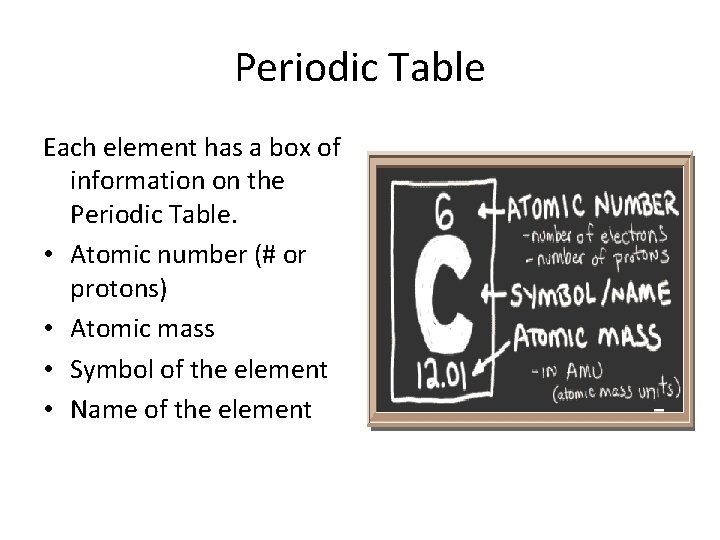
Periodic Table Each element has a box of information on the Periodic Table. • Atomic number (# or protons) • Atomic mass • Symbol of the element • Name of the element

Elements form Compounds are substances formed by chemical combination of 2 or more elements in definite proportions. Compounds are represented by chemical formulas CO CO 2

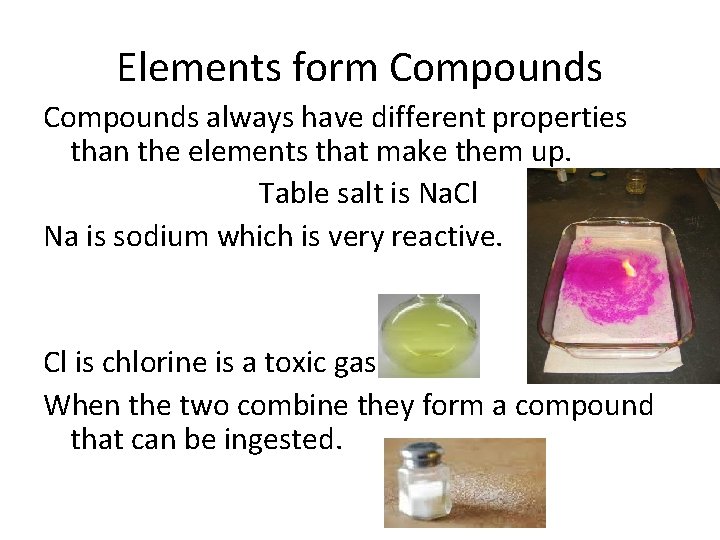
Elements form Compounds always have different properties than the elements that make them up. Table salt is Na. Cl Na is sodium which is very reactive. Cl is chlorine is a toxic gas. When the two combine they form a compound that can be ingested.

Elements form Compounds by chemical bonds • Chemical bonds are interactions between two elements electrons • The electrons involved in bonding are called valence electrons • TWO TYPES of BONDS: IONIC BONDS COVALENT BONDS

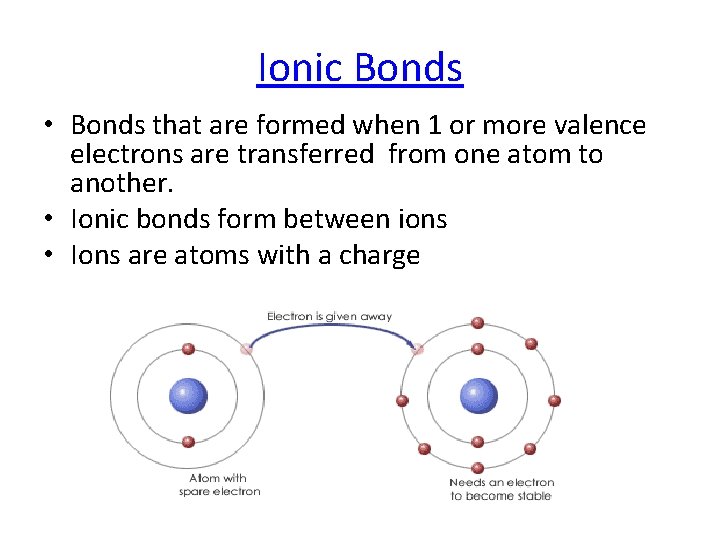
Ionic Bonds • Bonds that are formed when 1 or more valence electrons are transferred from one atom to another. • Ionic bonds form between ions • Ions are atoms with a charge

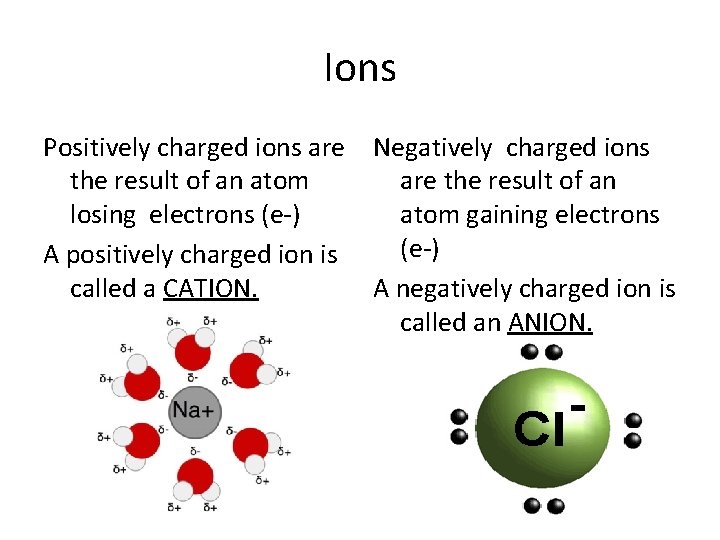
Ions Positively charged ions are Negatively charged ions the result of an atom are the result of an losing electrons (e-) atom gaining electrons (e-) A positively charged ion is called a CATION. A negatively charged ion is called an ANION.

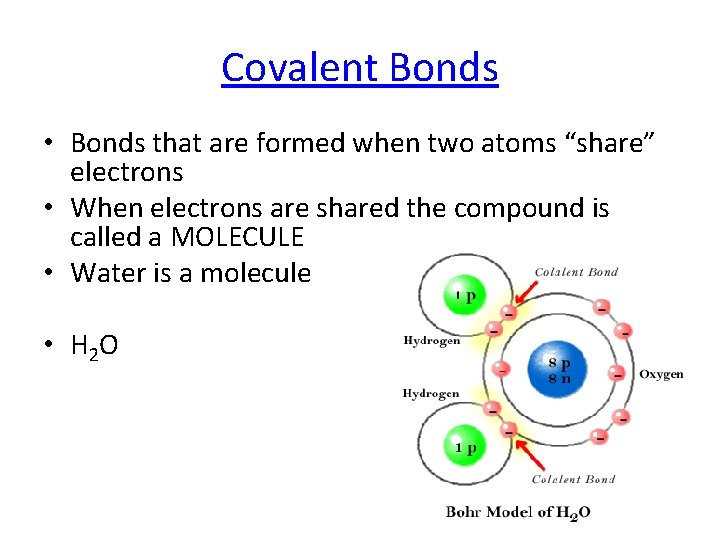
Covalent Bonds • Bonds that are formed when two atoms “share” electrons • When electrons are shared the compound is called a MOLECULE • Water is a molecule • H 2 O

Why do elements bond? • Each element wants 8 electrons in its highest energy level. • This can only happen when elements bond with each other • The 8 electrons are referred to as an OCTET.

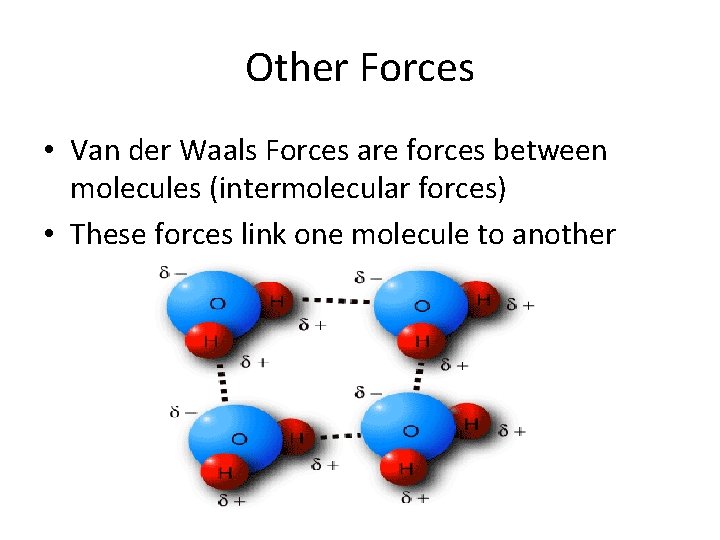
Other Forces • Van der Waals Forces are forces between molecules (intermolecular forces) • These forces link one molecule to another

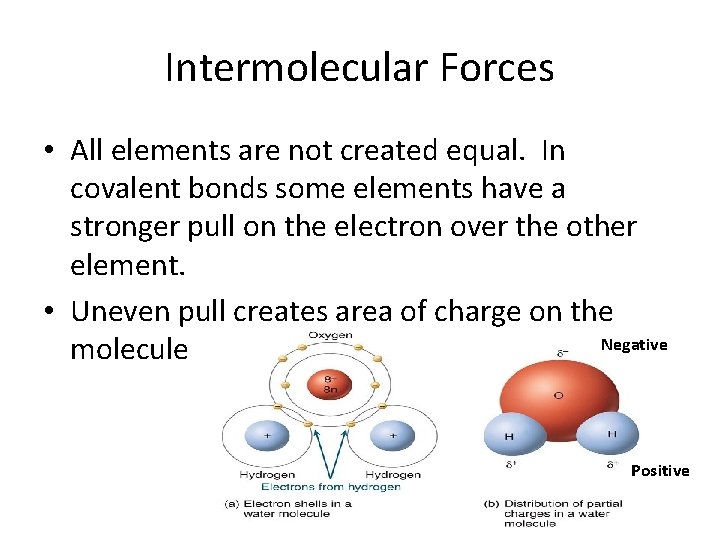
Intermolecular Forces • All elements are not created equal. In covalent bonds some elements have a stronger pull on the electron over the other element. • Uneven pull creates area of charge on the Negative molecule Positive

Van Der Waals Forces • Named after their discovery • Weak attractions between molecules • EXAMPLE: Geckos use Van der Waals forces to climb. • Van der Waals forces create attractions between the gecko’s foot and the surface on which it is climbing.
 Everything around you is made up of
Everything around you is made up of What is the answer to life the universe and everything
What is the answer to life the universe and everything Matter makes it all up
Matter makes it all up Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Threshold 5
Threshold 5 Definition of substance
Definition of substance Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 study guide the mole
Chapter 10 study guide the mole Examples of matter in chemistry
Examples of matter in chemistry Classification of matter flowchart
Classification of matter flowchart Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Chemistry matter and change answer key chapter 2
Chemistry matter and change answer key chapter 2 Matter flowchart chemistry
Matter flowchart chemistry Graphic organizer about matter
Graphic organizer about matter Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter