Matter and Composition What is matter MATTER is











- Slides: 20

Matter and Composition

What is matter? Ø MATTER is anything which has mass and occupies space. Ø Matter is all things that we can see, feel, and smell in our daily living.

Kinetic Theory of Matter: Molecules are always moving. This is known as the kinetic theory of matter. Ø Molecules move due to a temperature increase or decrease Ø We measure this kinetic energy with a thermometer as temperature. Ø The greater the material‘s kinetic internal energy, the higher the temperature of that material. Ø http: //www. youtube. com/watch? feature= endscreen&NR=1&v=s-Kvo. Vzuk. Ho

Temperature vs. Heat Ø Heat is the energy flow between objects of different temperature. Ø Heat and temperature are NOT the same. Ø Temperature is a number that is related to the average kinetic energy of the molecules of a substance.

Temperature vs. Heat (continued) Ø Heat is a measurement of the total energy in a substance. That total energy is made up of not only of the kinetic energies of the molecules of the substance, but total energy is also made up of the potential energies of the molecules.

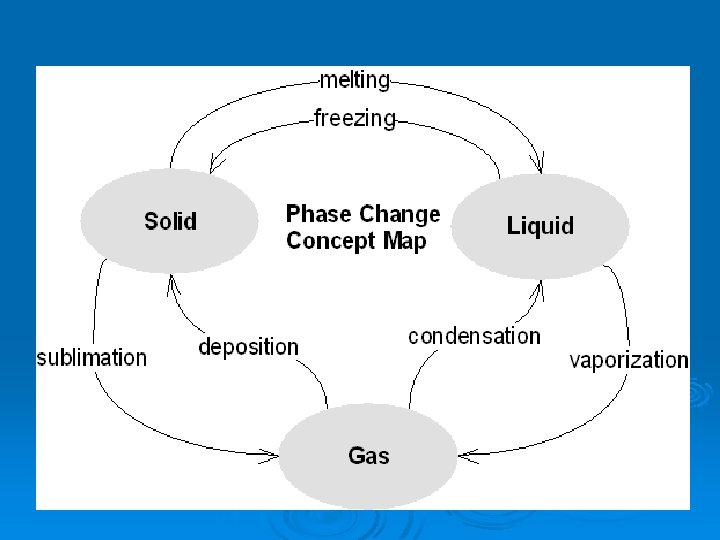
Solids Liquids THREE (3) Gases STATES OF MATTER

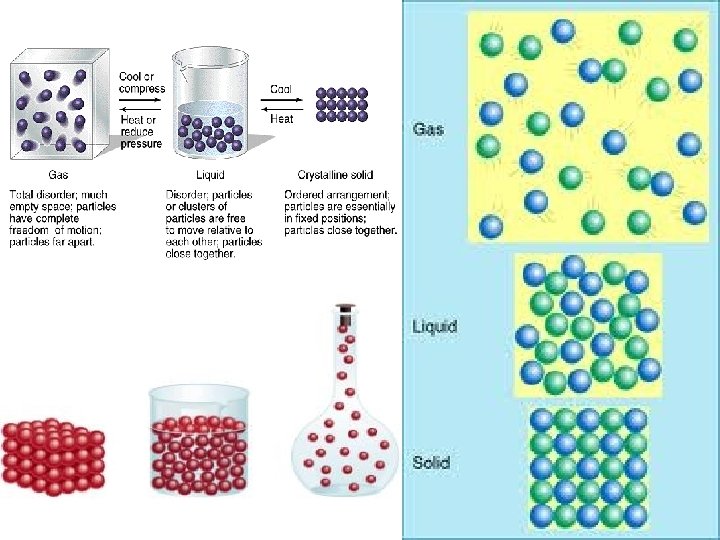
Phases of Matter: Solid l matter that has definite volume and shape. l molecules are packed together tightly and move slowly. Ø Liquid l matter that has definite volume but not shape. l molecules of a liquid are loosely packed and move with greater speed, l a liquid can flow and spread. Ø Gas l matter that has no definite volume or shape. Molecules of a gas are so loosely arranged and move so rapidly that they will fill their container. Plasma (similar to a gas) No definite volume or shape. Very loosely arranged, reacts to electromagnetism, usually has higher temperatures than other gases, most abundant phase of matter in the universe. Ø


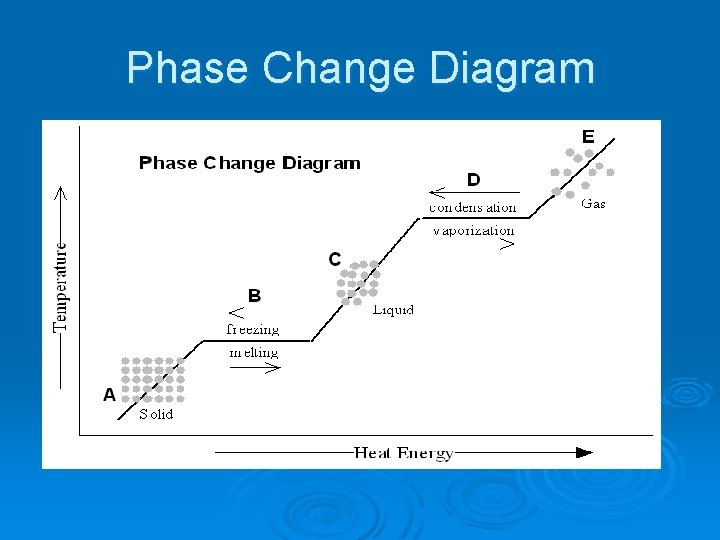
Phase Change Descriptions: Ø Melting l Ø Freezing l Ø the change from gas to liquid. Sublimation l Ø vaporization from within as well as from the surface of a liquid. Condensation l Ø vaporization from the surface of a liquid. Boiling l Ø the change from liquid to gas. Evaporation l Ø the change from liquid to solid. Vaporization l Ø the change from solid to liquid. the change from solid to gas. Deposition l the change from gas to solid.


Phase Change Diagram

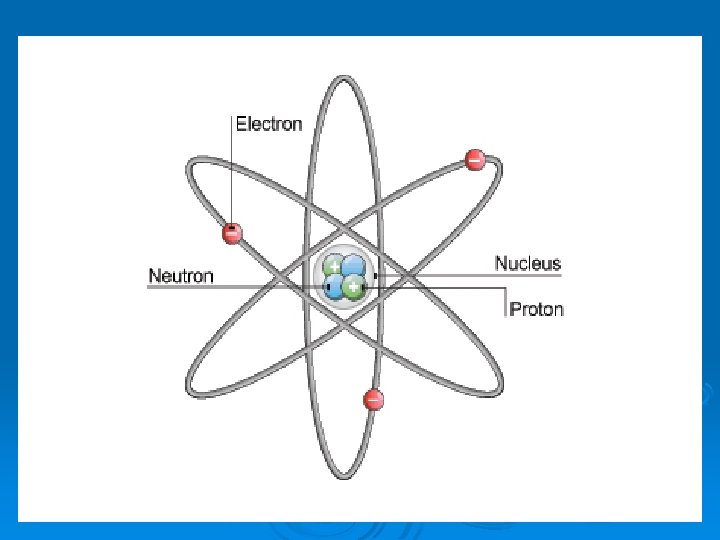
What is an atom? Ø Atoms are composed of particles called protons, electrons and neutrons. Ø Protons carry a positive electrical charge Ø Electrons carry a negative electrical charge Ø Neutrons carry no electrical charge at all


What is an element? Ø An element is a substance that is made entirely from one type of atom. Ø An ELEMENT is the simplest form of matter which can not be changed further by chemical or physical methods

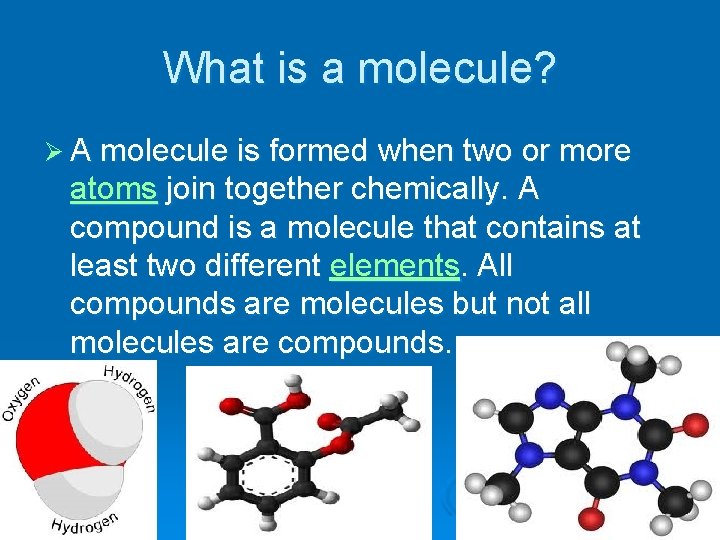
What is a molecule? Ø A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds.

What is a compound? Ø two or more different elements in a chemically combined form Ø has a fixed ratio determining the composition Example, a molecule of water contains two hydrogen atoms and one oxygen atom, (written as H 2 O ). Water is always H 2 O and not HO or H 3 O.

What is a mixture? Ø A mixture is a substance made by combining two or more different materials in such a way that no chemical reaction occurs. Ø A mixture can usually be separated back into its original components. Ø Some examples of mixtures are a tossed salad, salt water and a mixed bag of M&M's candy.

Types of Mixtures Ø A homogeneous mixture Ø A heterogeneous mixture has the same uniform consists of visibly appearance and different substances or composition throughout. phases. The three Many homogeneous phases or states of mixtures are commonly matter are gas, liquid, referred to as solutions. and solid.

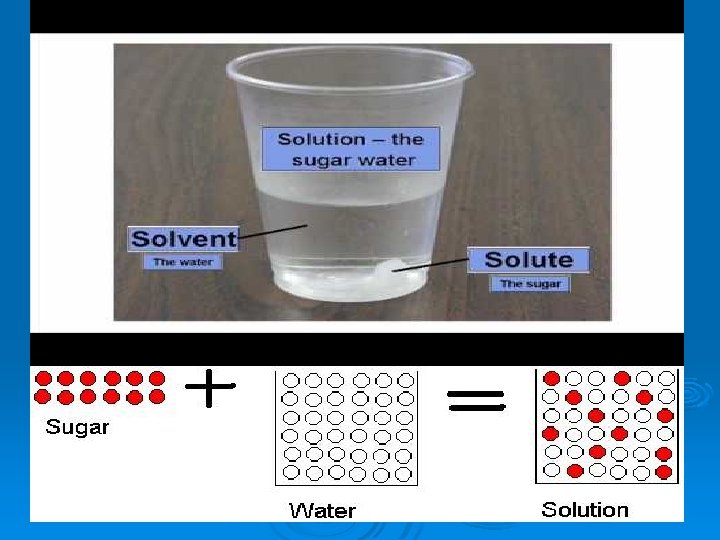
What is a Solution? Ø A solution is a mixture of two or more substances in a single phase. Ø At least two substances must be mixed in order to have a solution. Ø The substance in the smallest amount and the one that dissolves or disperses is called the SOLUTE. Ø The substance in the larger amount is called the SOLVENT.
