Gases Dr Ron Rusay Copyright 2007 2010 R






























































- Slides: 92

Gases Dr. Ron Rusay © Copyright 2007 -2010 R. J. Rusay

Gases � Uniformly fill any container. � Exert pressure on its surroundings. � Mix completely with other gases

Gases: Pressure, Mass, Volume, Temperature

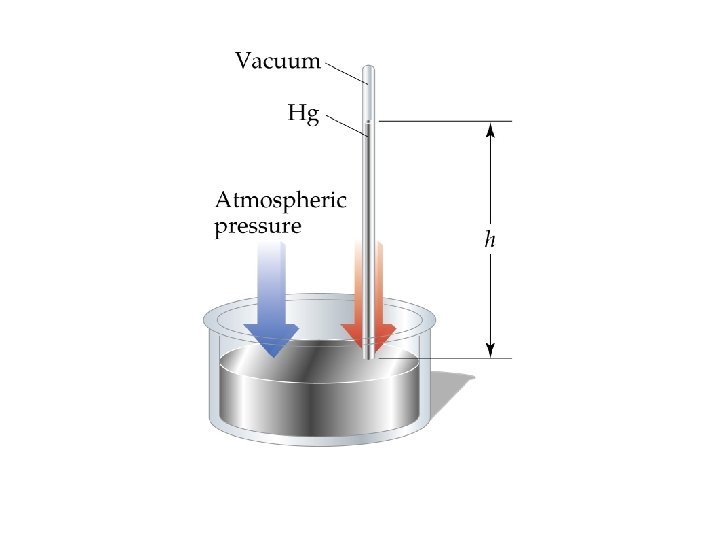
Pressure � � is equal to force/unit area SI units = Newton/meter 2 = 1 Pascal (Pa) 1 standard atmosphere = 101, 325 Pa 1 standard atmosphere = 1 atm = 760 mm Hg = 760 torr

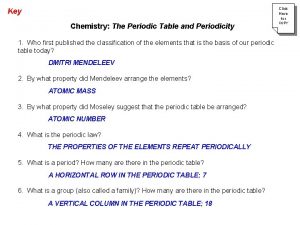
QUESTION Four bicycle tires are inflated to the following pressures. Which one has the highest pressure? Tire A 3. 42 atm; Tire B 48 lbs/sq in; Tire C 305 k. Pa; Tire D 1520 mm. Hg. (Recall; 1. 00 atm = 760 mm. Hg = 14. 7 lb/sq in = 101. 3 k. Pa) A. B. C. D. Tire A Tire B Tire C Tire D

ANSWER A) Even though it has the smallest number, it represents the highest pressure of the four. When all four are changed to a common label (use conversion factors and dimensional analysis) 3. 42 atm is a higher pressure than the others.


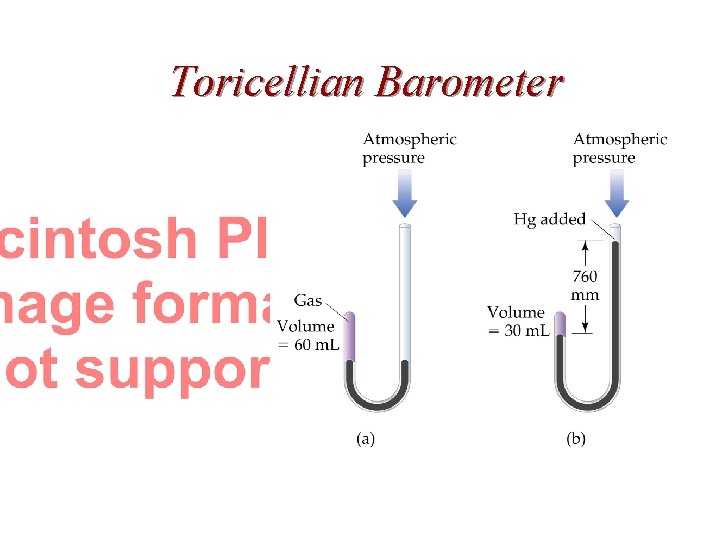
Toricellian Barometer


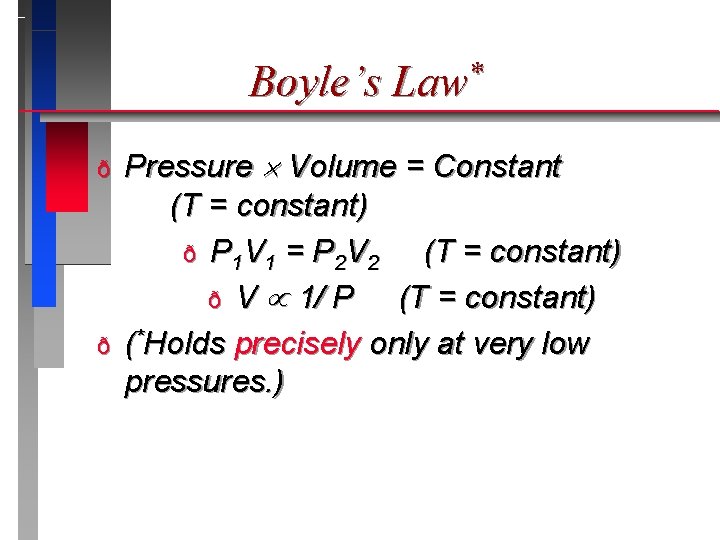
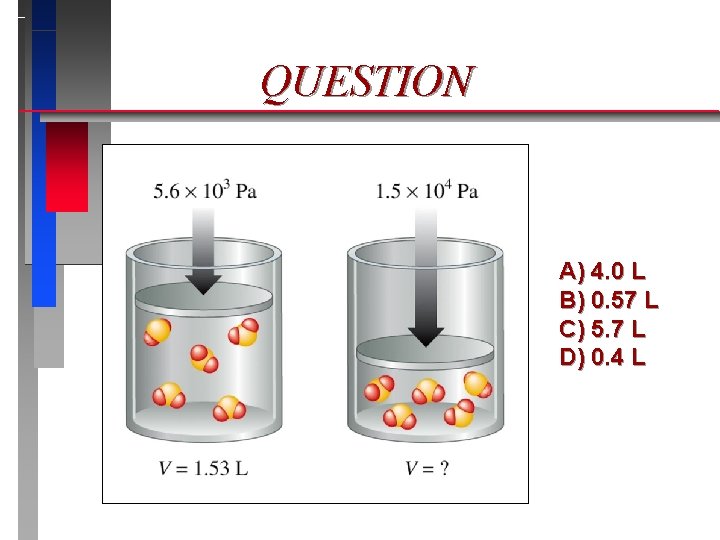
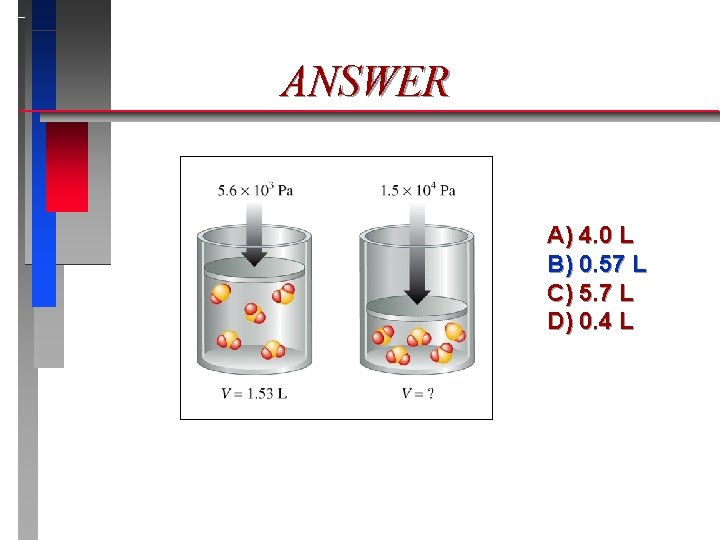
Pressure & Volume Boyle’s Law*

Boyle’s Law* ð ð Pressure Volume = Constant (T = constant) ð P 1 V 1 = P 2 V 2 (T = constant) ð V 1/ P (T = constant) (*Holds precisely only at very low pressures. )

QUESTION A) 4. 0 L B) 0. 57 L C) 5. 7 L D) 0. 4 L

ANSWER A) 4. 0 L B) 0. 57 L C) 5. 7 L D) 0. 4 L

An empty one gallon can is hooked to a vacuum pump. What do you expect to happen?

Explain why the can collapsed.

Pressure vs. Volume ð

Ideal Gases Real vs. “Ideal” Definition: A gas that strictly obeys Boyle’s Law is called an ideal gas.

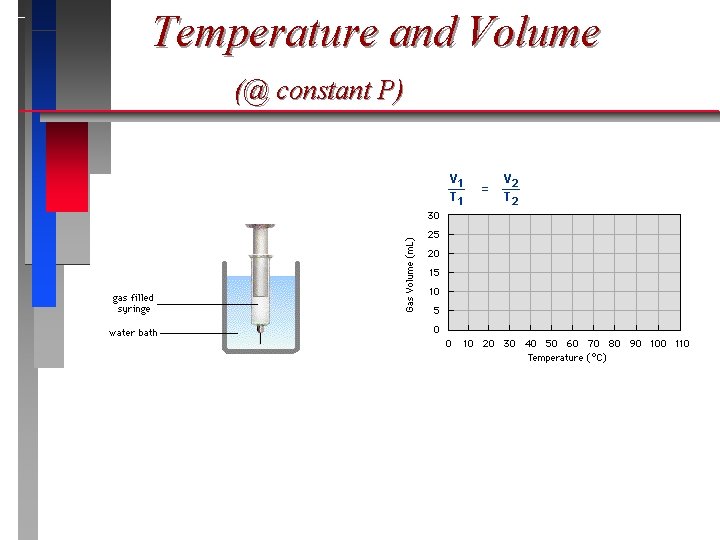
Temperature & Volume

Temperature & Volume

Charles’s Law ð The volume of a gas is directly proportional to temperature, and extrapolates to zero at zero Kelvin. ð V = T (P = constant) = a proportionality constant

Temperature and Volume (@ constant P)

Charles’s Law

Pressure vs. Temperature (@ constant V) Changing Volume

The Meaning of Temperature Kelvin temperature is an index of the random motions of gas particles (higher T means greater motion. ) ð

QUESTION Kinetic molecular theory helps explain the definition of temperature based on molecular motion. Which statement describes an important aspect of this connection? A) Temperature is inversely related to the kinetic energy of the gas particles. B) At the same temperature, more massive gas particles will be moving faster than less massive gas particles. C) As the temperature of a gas sample increases, the average velocity of the gas particles increases. D) Kinetic energy is directly related to temperature. This is valid for any units of temperature.

ANSWER C) states a correct connection between temperature and velocity. The important relationship is summarized in the following equation: ½ mass velocity 2 = 3/2 RT (T must be in K).

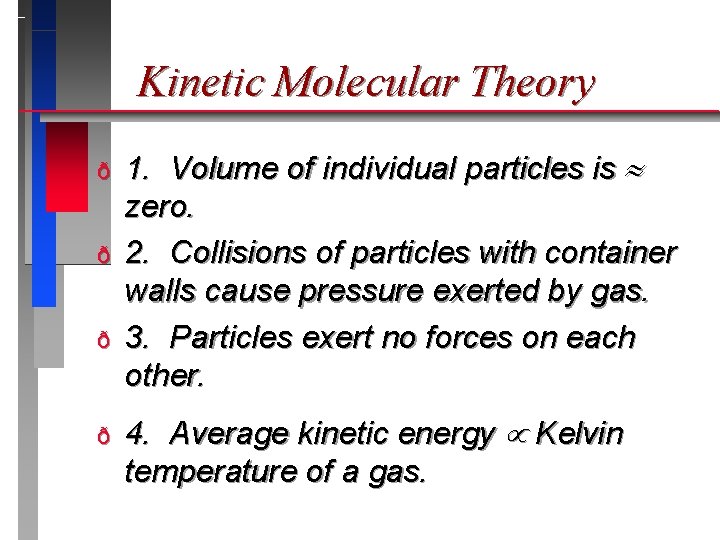
Kinetic Molecular Theory ð ð 1. Volume of individual particles is zero. 2. Collisions of particles with container walls cause pressure exerted by gas. 3. Particles exert no forces on each other. 4. Average kinetic energy Kelvin temperature of a gas.

Molecular Motion / Theory The Meaning of Temperature (Kelvin) is an index of the random motions of gas particles (higher T means greater motion. )

Velocity & Temperature

QUESTION Why is it critical that the temperature be held constant when applying Boyle’s law to changing the pressure of a trapped gas? A) Gas molecules may expand at higher temperatures; this would change the volume. B) Changing the temperature causes the gas to behave in non-ideal fashion. C) Changing the temperature affects the average particle speed, which could affect the pressure. D) Allowing the temperature to drop below 0°C would cause the trapped gas to no longer follow Boyle’s Law.

ANSWER C) provides the connection between temperature and pressure that would introduce another variable when studying the pressurevolume relationship. Boyle’s law shows that if the molecules of a trapped gas maintain the same average velocity when their volume is changed, the pressure will be inversely related to the volume change.


QUESTION As the temperature of a gas increases, which statement best correlates to information about molecular velocity? A) The average molecular velocity will increase, but the distribution of molecular velocities will stay the same. B) The average molecular velocity will stay the same, but the molecular velocity distribution will spread. C) The average molecular velocity will increase, and the distribution of the molecular velocities will spread. D) The average molecular velocity will stay the same, and the distribution of the molecular velocities will stay the same.

ANSWER C) accurately reflects the connection between molecular velocity and an increase in temperature. Kelvin temperature is directly related to molecular velocity - with greater temperature the distribution of available velocities also increases.

View Gas Molecules Applet http: //chemconnections. org/Java/molecules/index. html View Molecular Vibrations: IRGas. Tutor http: //chemistry. beloit. edu/Warming/pages/infrared. html

Changes in Temperature ( PV&T)

Pressure, Volume & Temperature

Applying a Gas Law Provide 3 different sets of conditions (Pressure and Temperature) which can account for the volume of the gas decreasing by 1/2. Cases 1 -3 in the handout.

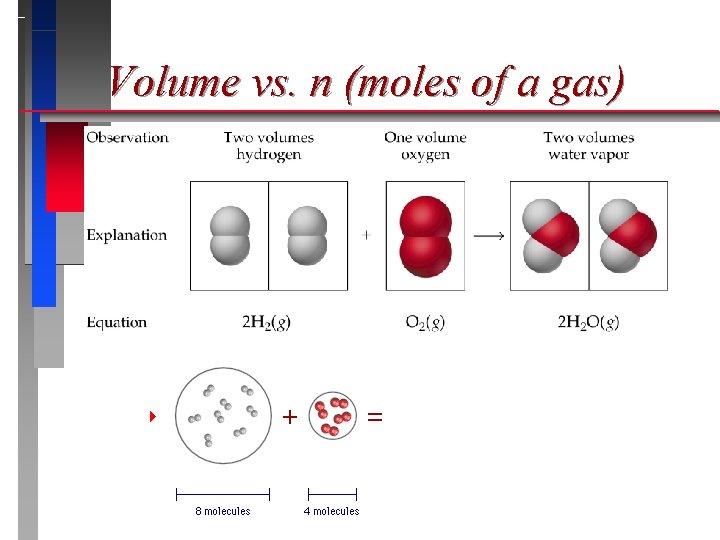
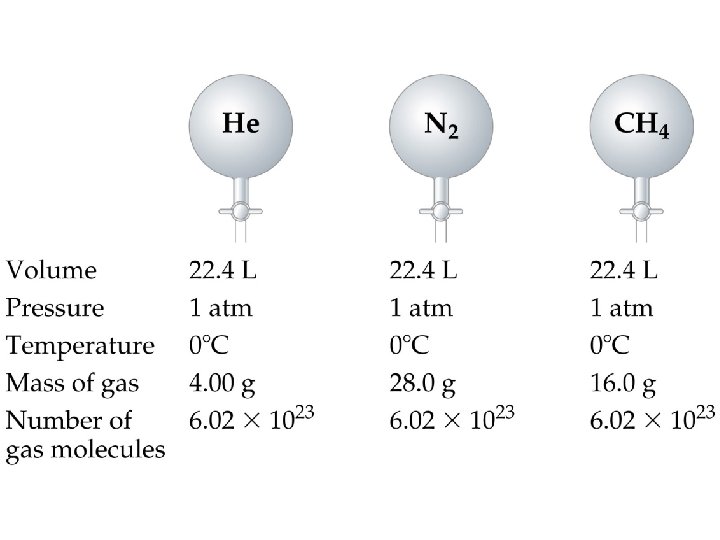
Avogadro’s Law ð ð For a gas at constant temperature and pressure, the volume is directly proportional to the number of moles of gas (at low pressures). V = n ð = proportionality constant ð V = volume of the gas n = number of moles of gas ð

Volume vs. n (moles of a gas)


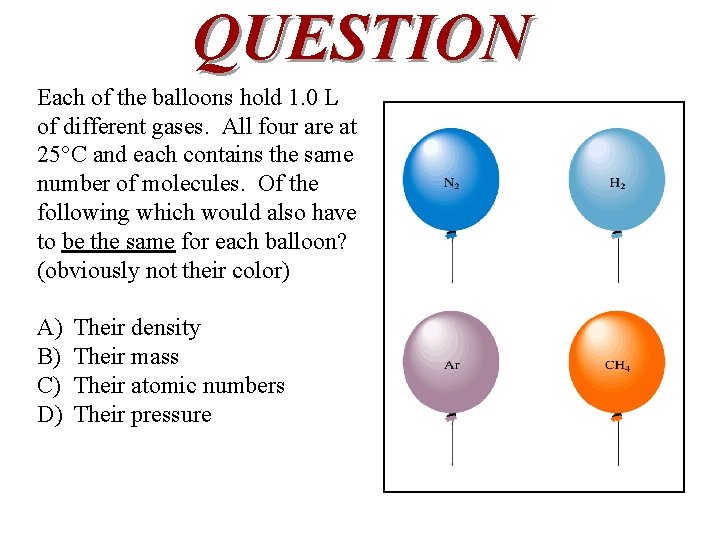
QUESTION Each of the balloons hold 1. 0 L of different gases. All four are at 25°C and each contains the same number of molecules. Of the following which would also have to be the same for each balloon? (obviously not their color) A) B) C) D) Their density Their mass Their atomic numbers Their pressure

ANSWER D) is consistent with Avogadro’s Law. The temperature, pressure, number of moles, and volume are related for a sample of trapped gas. If two samples have three variables out of the four the same, the fourth variable must be the same as well.

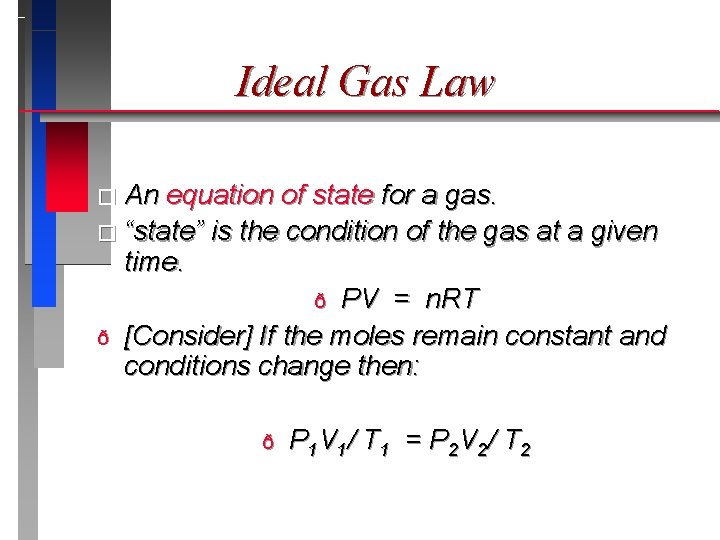
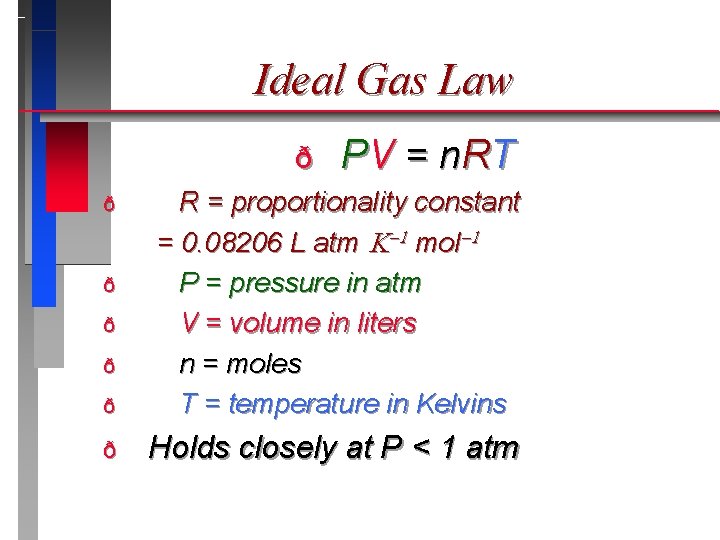
Ideal Gas Law � An equation of state for a gas. � “state” is the condition of the gas at a given time. ð PV = n. RT ð [Consider] If the moles remain constant and conditions change then: ð P 1 V 1/ T 1 = P 2 V 2/ T 2

QUESTION If a person exhaled 125 m. L of CO 2 gas at 37. 0°C and 0. 950 atm of pressure, what would this volume be at a colder temperature of 10. 0°C and 0. 900 atm of pressure? A) B) C) D) 3. 12 m. L 0. 130 L 0. 120 L 22. 4 L

ANSWER C) correctly accounts for the changing conditions. The combination of Charles and Boyle’s laws provides the mathematical basis for the calculation. After converting the temperature to K units, the equation P 1 V 1/T 1 = P 2 V 2/T 2 may be used by solving for V 2.

Ideal Gas Law ð P V = n RT ð R = proportionality constant = 0. 08206 L atm mol P = pressure in atm V = volume in liters n = moles T = temperature in Kelvins ð Holds closely at P < 1 atm ð ð

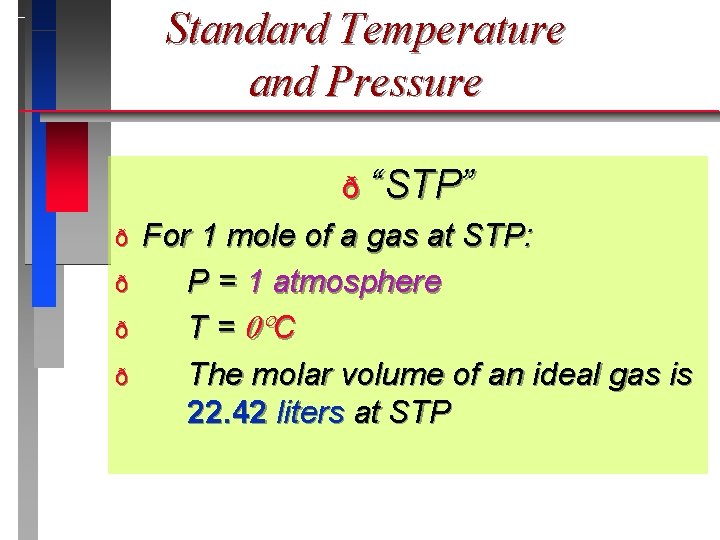
Standard Temperature and Pressure ð “STP” ð ð For 1 mole of a gas at STP: P = 1 atmosphere T = C The molar volume of an ideal gas is 22. 42 liters at STP

QUESTION If a 10. 0 L sample of a gas at 25°C suddenly had its volume doubled, without changing its temperature what would happen to its pressure? What could be done to keep the pressure constant without changing the temperature? A) The pressure would double; nothing else could be done to prevent this. B) The pressure would double; the moles of gas could be doubled. C) The pressure would decrease by a factor of two; the moles of gas could be halved. D) The pressure would decrease by a factor of two; the moles could be doubled.

ANSWER D) describes two opposing changes. When the volume increases, the pressure of a trapped gas will decrease (at constant temperature and constant moles of gas). However, if the pressure drops, more collisions could be restored by adding more particles of gas in the same ratio as the pressure decline.


QUESTION A typical total capacity for human lungs is approximately 5, 800 m. L. At a temperature of 37°C (average body temperature) and pressure of 0. 98 atm, how many moles of air do we carry inside our lungs when inflated? (R = 0. 08206 L atm/ K mol) A) B) C) D) E) 1. 9 mol 0. 22 mol 230 mol 2. 20 mol: Moles can harm a person’s lungs.

ANSWER B) is correct. The units for temperature must be in K, pressure in atm, and volume in L. Then using the universal constant 0. 08206 L atm/ K mol in the PV = n. RT equation moles may be solved. n O (g) = PV / RT 2

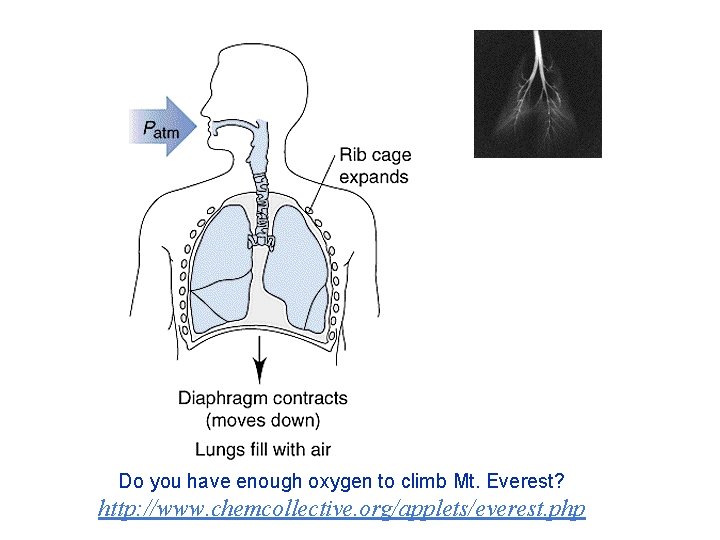
Do you have enough oxygen to climb Mt. Everest? http: //www. chemcollective. org/applets/everest. php

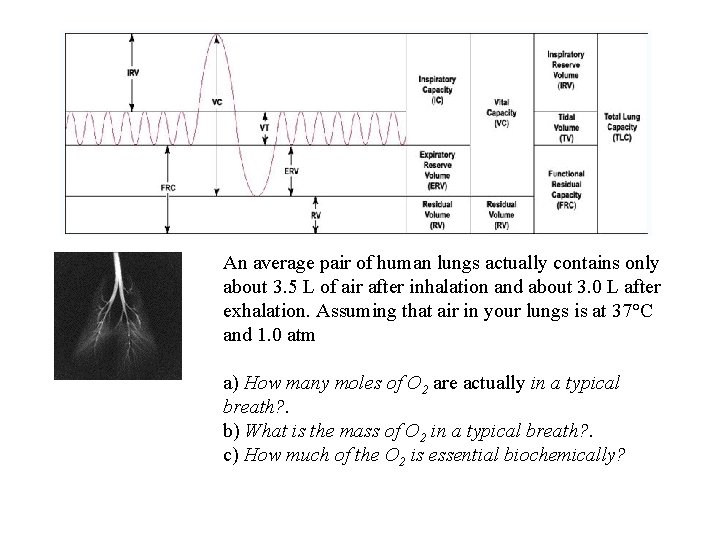
An average pair of human lungs actually contains only about 3. 5 L of air after inhalation and about 3. 0 L after exhalation. Assuming that air in your lungs is at 37°C and 1. 0 atm a) How many moles of O 2 are actually in a typical breath? . b) What is the mass of O 2 in a typical breath? . c) How much of the O 2 is essential biochemically?

QUESTION The primary source of exhaled CO 2 is from the combustion of glucose, C 6 H 12 O 6 (molar mass = 180. g/mol. ). The balanced equation is shown here: C 6 H 12 O 6 (aq) + 6 O 2 (g) 6 CO 2 (g) + 6 H 2 O (l) If you oxidized 5. 42 grams of C 6 H 12 O 6 while tying your boots to climb Mt. Everest, how many liters of O 2 @ STP conditions did you use? A) B) C) D) 0. 737 L 0. 672 L 4. 05 L 22. 4 L

ANSWER C), assuming you were not holding your breath, is correct. The number of moles of glucose must first be determined (5. 42/180. = 0. 0301 moles), then this is multiplied by 6 to account for the stoichiometric ratio between glucose and oxygen. From this, PV = n. RT may be used with the appropriate substitutions.

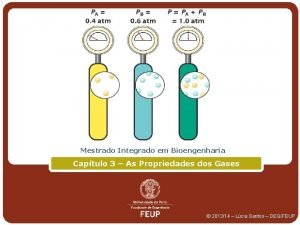
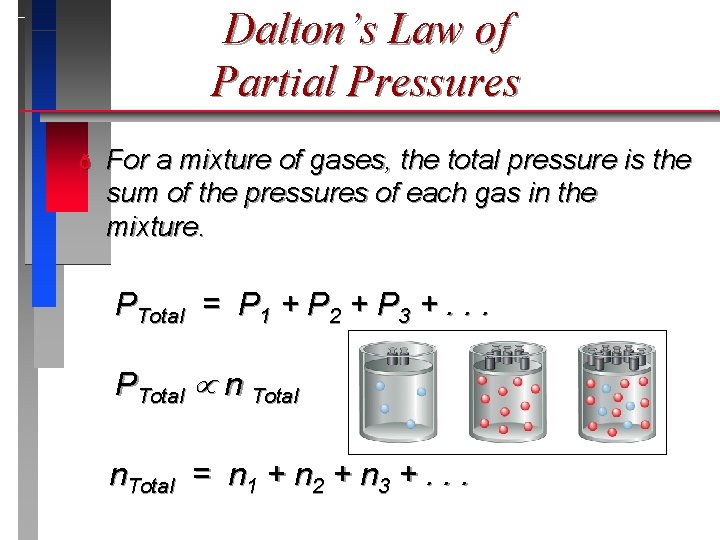
Dalton’s Law of Partial Pressures ð For a mixture of gases, the total pressure is the sum of the pressures of each gas in the mixture. PTotal = P 1 + P 2 + P 3 +. . . PTotal n Total n. Total = n 1 + n 2 + n 3 +. . .

Dalton’s Law of Partial Pressures ð For a mixture of gases, the partial gas pressure and total pressure equal the mole fraction of each gas in the mixture. P 1 / PTotal = n 1 / n. Total

QUESTION If the mole fraction of O 2 in our atmosphere at standard conditions is approximately 0. 209, what is the partial pressure of the oxygen in every breath you take? A) B) C) D) 1. 00 atm 4. 78 atm 159 torr 3640 mm. Hg

ANSWER C) shows the correct pressure. This calculation is based on the application of Dalton’s law of partial pressures. For one atmosphere of pressure 0. 209 is caused by oxygen so 760 . 209 = 159 torr.

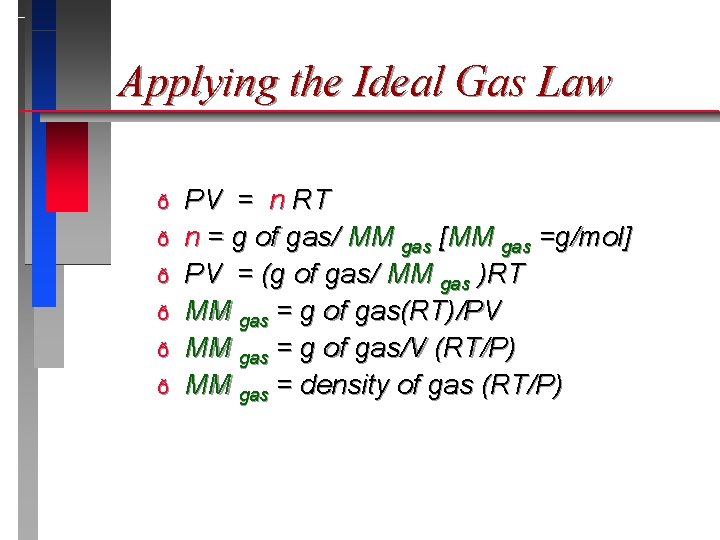
Applying the Ideal Gas Law ð ð ð PV = n RT n = g of gas/ MM gas [MM gas =g/mol] PV = (g of gas/ MM gas )RT MM gas = g of gas(RT)/PV MM gas = g of gas/V (RT/P) MM gas = density of gas (RT/P)

QUESTION Under STP conditions what is the density of O 2 gas? A) B) C) D) Not enough information is given to solve this. 1. 31 g/L 1. 43 g/L 0. 999 g/L

ANSWER C) is the correct density for O 2 at STP. At STP conditions the volume of one mole of a gas is approximately 22. 4 L. One mole of oxygen = 32. 0 grams. The density is calculated by dividing mass by volume. Or ðdensity of gas (g/L) = MM gas P/ RT

QUESTION Which sequence represents the gases in order of increasing density at STP? A) Fluorine < Carbon monoxide < Chlorine < Argon B) Carbon monoxide < Fluorine < Argon < Chlorine C) Argon < Carbon monoxide < Chlorine < Fluorine D) Fluorine < Chlorine < Carbon monoxide < Argon

ANSWER Which sequence represents the gases in order of increasing density at STP? A) Fluorine < Carbon monoxide < Chlorine < Argon B) Carbon monoxide < Fluorine < Argon < Chlorine C) Argon < Carbon monoxide < Chlorine < Fluorine D) Fluorine < Chlorine < Carbon monoxide < Argon

QUESTION The density of an unknown atmospheric gas pollutant was experimentally determined to be 1. 964 g/ L @ 0 o. C and 760 torr. • What is the molar mass of the gas? • What might the gas be? A) CO B) SO 2 C) H 2 O D) CO 2

ANSWER 1. 964 g/ L @ 0 o. C and 760 torr. R = 0. 08206 L atm mol o. C K torr atm MM gas = density of gas (RT/P) MM gas = 1. 964 g/ L x 0. 08206 L atm mol x 273 K/ 760 torr x 760 torr/ 1 atm MM MMgas gas==44. 0 g/mol D) CO 2

QUESTION Freon-12 had been widely used as a refrigerant in air conditioning systems. However, it has been shown to be a greenhouse gas and destroy the ozone layer. What is the molar mass of Freon-12 if 9. 27 grams was collected by water displacement, in a 2. 00 liter volume at 30. 0°C and 764 mm. Hg. Water’s vapor pressure at this temperature is approximately 31. 8 mm. Hg. A) B) C) D) 120. g/mol 12. 0 g/mol 115 g/mol 92. 7 g/mol

ANSWER A) is the molar mass of Freon-12. The pressure must be corrected for the presence of water by subtracting 31. 8 mm. Hg from the total pressure. This should also be converted to atm. The temperature must be converted to K. Then PV = n. RT can be used if n is written as g/mm and solved for mm.

QUESTION The aroma of fresh raspberries can be attributed, at least in part, to 3(para-hydroxyphenyl)-2 -butanone. What is the molar mass of this pleasant smelling compound if at 1. 00 atmosphere of pressure and 25. 0°C, 0. 0820 grams has a volume of 12. 2 m. L? A) B) C) D) 13. 8 g/mol 164 g/mol 40. 9 g/mol 224 g/mol

ANSWER B) is correct. Using PV = n. RT and properly substituting 0. 0122 L for V, 298 K for T, using 0. 08206 for R and solving for n the number of moles represented by 0. 0820 grams can be obtained. Then the grams in one mole can be found.

Diffusion and Effusion Diffusion: describes the mixing of gases. The rate of diffusion is the rate of gas mixing. Effusion: describes the passage of gas into an evacuated chamber.

Effusion

Effusion and Diffusion Effusion: Diffusion:

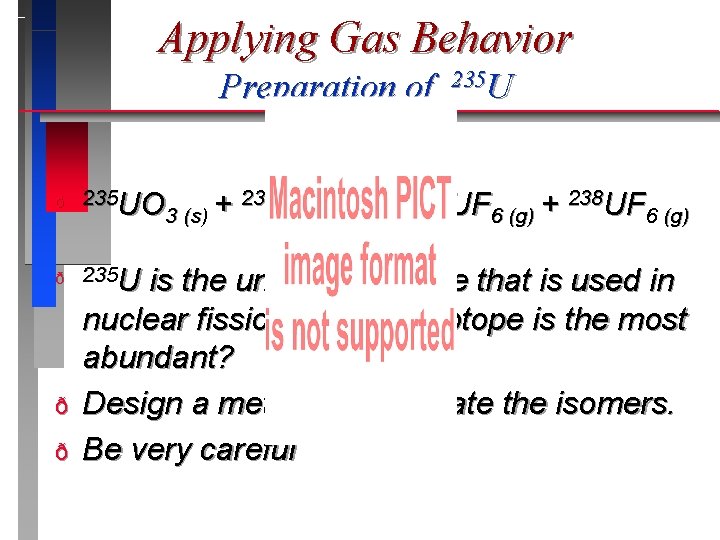
Applying Gas Behavior Preparation of ð 235 UO 238 UO + 3 (s) ð 235 U ð ð 235 UF 6 (g) + 238 UF 6 (g) is the unstable isotope that is used in nuclear fission. Which isoptope is the most abundant? Design a method to separate the isomers. Be very careful

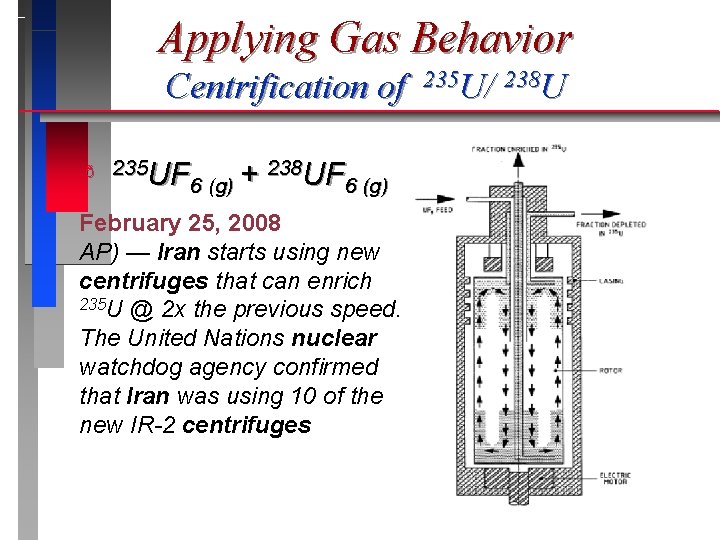
Applying Gas Behavior Centrification of ð 235 UF 238 UF + 6 (g) U-238, moves toward the outside of the cylinder and U 235, collects closer to the center. The stream that is slightly enriched in U-235 is withdrawn and fed into the next higher stage, while the slightly depleted stream is recycled back into the next lower stage. 235 U/ 238 U

Applying Gas Behavior Centrification of ð 235 U/ 238 U 235 UF 238 UF + 6 (g)

Applying Gas Behavior Centrification of ð 235 UF 238 UF + 6 (g) February 25, 2008 AP) — Iran starts using new centrifuges that can enrich 235 U @ 2 x the previous speed. The United Nations nuclear watchdog agency confirmed that Iran was using 10 of the new IR-2 centrifuges 235 U/ 238 U

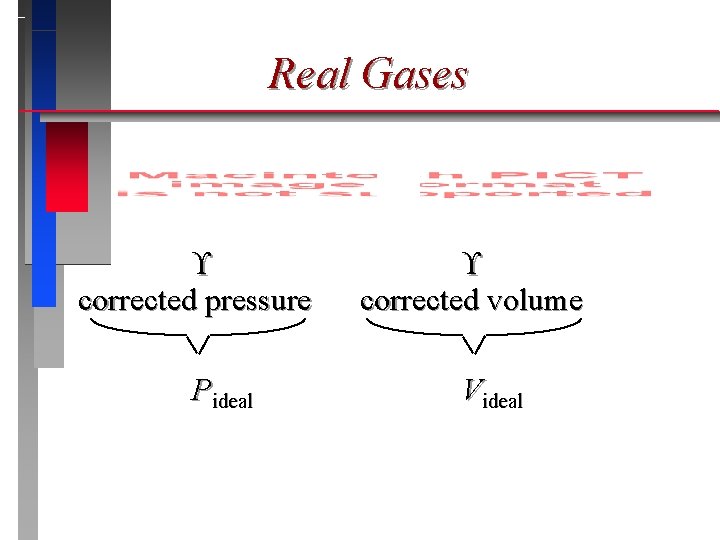
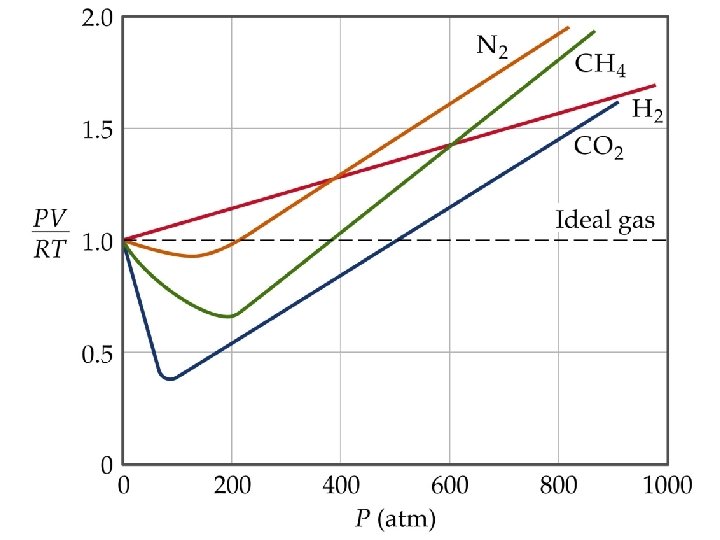
Real Gases Must correct ideal gas behavior when at high pressure (smaller volume) and low temperature (attractive forces become important).

Real Gases corrected pressure Pideal corrected volume Videal


Real Gases Volume vs. Temperature @ constant P

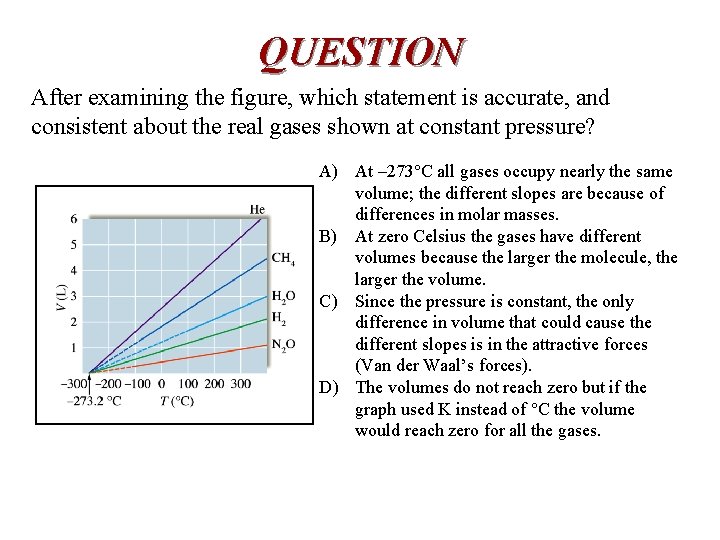
QUESTION After examining the figure, which statement is accurate, and consistent about the real gases shown at constant pressure? A) At – 273°C all gases occupy nearly the same volume; the different slopes are because of differences in molar masses. B) At zero Celsius the gases have different volumes because the larger the molecule, the larger the volume. C) Since the pressure is constant, the only difference in volume that could cause the different slopes is in the attractive forces (Van der Waal’s forces). D) The volumes do not reach zero but if the graph used K instead of °C the volume would reach zero for all the gases.

ANSWER C) is the correct conclusion. If all the gases had the same attractive forces (with constant pressure) the volumes would be proportional to their speeds and inversely proportional to their molecular weights.


QUESTION Real gases exhibit their most “ideal” behavior at which relative conditions? A) B) C) D) Low temperatures and low pressures High temperatures and high pressures High temperatures and low pressures Low temperatures and high pressures

ANSWER C) provides the correct choice. At those conditions gas molecules are farthest apart and exert their least influence on each othereby permitting their behavior to follow mathematical predictions.

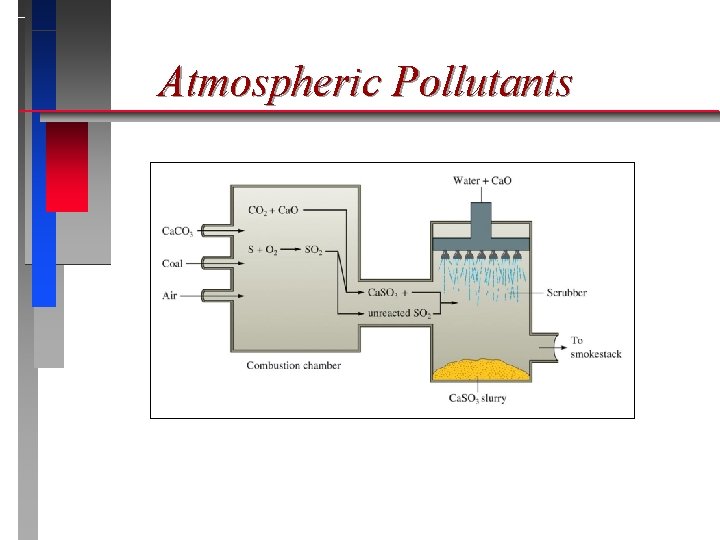
Atmospheric Pollutants

Atmospheric Pollutants


Gases & Airbags Use of Chemical Reactions and Physical Properties Workshop: Gases II
 Rusay
Rusay Copyright 2007
Copyright 2007 Copyright 2007
Copyright 2007 Copyright 2010 pearson education inc
Copyright 2010 pearson education inc Copyright 2010 pearson education inc
Copyright 2010 pearson education inc Copyright 2010 pearson education inc
Copyright 2010 pearson education inc Composition copyright example
Composition copyright example Copyright 2010 pearson education inc
Copyright 2010 pearson education inc Copyright 2010 pearson education inc
Copyright 2010 pearson education inc Copyright 2010 pearson education inc
Copyright 2010 pearson education inc C-929-a
C-929-a Pearson education inc all rights reserved
Pearson education inc all rights reserved Copyright 2010
Copyright 2010 Copyright 2010 pearson education inc
Copyright 2010 pearson education inc Variazioni finanziarie attive
Variazioni finanziarie attive Nwoz
Nwoz Copyright 2010 pearson education inc
Copyright 2010 pearson education inc Inorganic gases
Inorganic gases Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Teoria cinetica de los gases
Teoria cinetica de los gases Boyle's law statement
Boyle's law statement Lightest members of noble gases
Lightest members of noble gases Stp for gases
Stp for gases Properties of gas
Properties of gas Propiedades del argon
Propiedades del argon Atomic mass number of boron
Atomic mass number of boron Los gases nobles se caracterizan por
Los gases nobles se caracterizan por Characteristics of ideal gases
Characteristics of ideal gases What are the greenhouse gases
What are the greenhouse gases Air mixture of gases
Air mixture of gases Greenhouse gases composition
Greenhouse gases composition Molecular theory of gases and liquids
Molecular theory of gases and liquids Aterial blood gases
Aterial blood gases Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Chapter 11 review gases section 1
Chapter 11 review gases section 1 Why was the term inert gases dropped?
Why was the term inert gases dropped? Kinetic theory of gases
Kinetic theory of gases Process of liquid to gas
Process of liquid to gas Specific heat of monatomic gas
Specific heat of monatomic gas Which of the following gases will effuse the most rapidly
Which of the following gases will effuse the most rapidly Gases have low densities
Gases have low densities Plant tissue and organs
Plant tissue and organs Cuales son los gases nobles
Cuales son los gases nobles Degree of freedom of an ideal gas
Degree of freedom of an ideal gas Teoria cinetica de los gases
Teoria cinetica de los gases Gases dosage form
Gases dosage form Group in periodic table
Group in periodic table Pgas=patm+ph
Pgas=patm+ph Manejo de cilindros de gases comprimidos
Manejo de cilindros de gases comprimidos Is the greenhouse effect good or bad
Is the greenhouse effect good or bad Grahams law
Grahams law Ec of xenon
Ec of xenon Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Aterial blood gases
Aterial blood gases Are noble gases representative elements
Are noble gases representative elements Physical characteristics of gases
Physical characteristics of gases Ley de avogadro
Ley de avogadro How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases What human activity produces the most greenhouse gases
What human activity produces the most greenhouse gases Globo aerostatico ley de los gases
Globo aerostatico ley de los gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Kinetic theory of gases postulates
Kinetic theory of gases postulates Physical properties of gases
Physical properties of gases Periodic table noble gases
Periodic table noble gases Fraccion molar gases
Fraccion molar gases Why are gases eaiser to compress than solids or liquids?
Why are gases eaiser to compress than solids or liquids? Stoichiometry of gases
Stoichiometry of gases Partial pressures of gases pogil answers
Partial pressures of gases pogil answers Gases characteristics
Gases characteristics The thin envelope of gases
The thin envelope of gases Ideal gas law equation example
Ideal gas law equation example Properties of gas
Properties of gas Ear words
Ear words Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Elementos gases
Elementos gases General gas equation is
General gas equation is Lei de charles
Lei de charles What are the general properties of gases
What are the general properties of gases Presiones parciales
Presiones parciales Fugacity of real gases
Fugacity of real gases Physical characteristics of gases
Physical characteristics of gases Características de los gases nobles
Características de los gases nobles Equação geral dos gases
Equação geral dos gases Kinetic molecular theory of solids
Kinetic molecular theory of solids Constante dos gases ideais
Constante dos gases ideais Difusibilidad de los gases
Difusibilidad de los gases Teoría cinético molecular de los gases
Teoría cinético molecular de los gases Exchange of gases in earthworm
Exchange of gases in earthworm Isomásicos
Isomásicos Pv=cte
Pv=cte Diffusion of gases across respiratory membranes:
Diffusion of gases across respiratory membranes: Factores que afectan a los gases
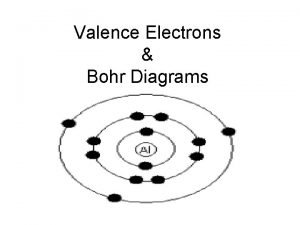
Factores que afectan a los gases Valence electrons bohr diagram
Valence electrons bohr diagram