Nomenclature Compounds Formulas Names Dr Ron Rusay Copyright











![Naming Acids [Compounds with electropositive Hydrogen atom(s)] Naming Acids [Compounds with electropositive Hydrogen atom(s)]](https://slidetodoc.com/presentation_image_h2/57165a66e8a6571b9ac7e51988ba5513/image-23.jpg)



- Slides: 29

Nomenclature (Compounds: Formulas & Names) Dr. Ron Rusay © Copyright 2010 R. J. Rusay

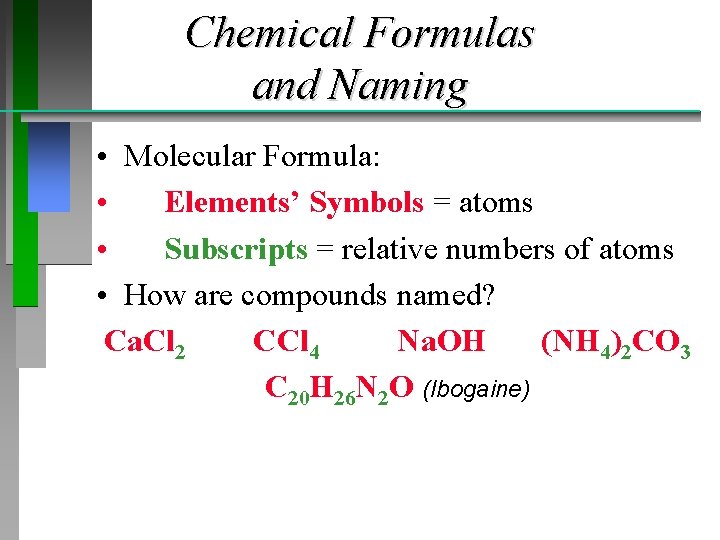
Chemical Formulas and Naming • Molecular Formula: • Elements’ Symbols = atoms • Subscripts = relative numbers of atoms • How are compounds named? Ca. Cl 2 CCl 4 Na. OH (NH 4)2 CO 3 C 20 H 26 N 2 O (Ibogaine)

Nomenclature • Nomenclature: the naming of compounds • Governed by the IUPAC: International Union of Pure and Applied Chemistry • International rules are updated periodically • General schemes and examples follow:


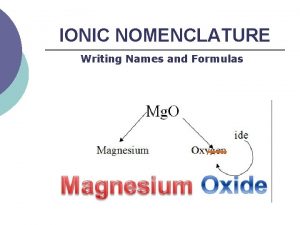
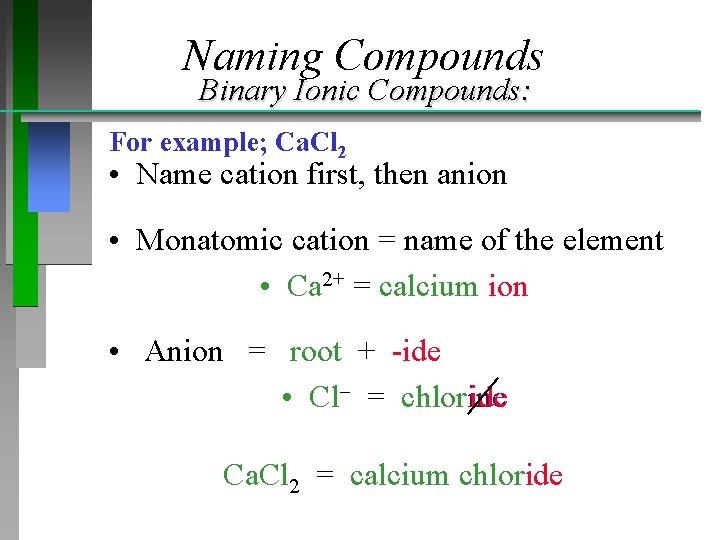
Naming Compounds Binary Ionic Compounds: For example; Ca. Cl 2 • Name cation first, then anion • Monatomic cation = name of the element • Ca 2+ = calcium ion • Anion = root + -ide • Cl = chlorine Ca. Cl 2 = calcium chloride

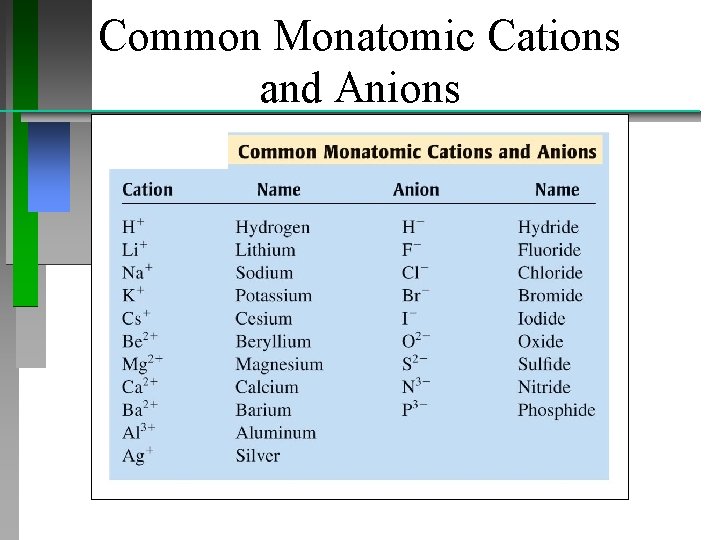
Common Monatomic Cations and Anions

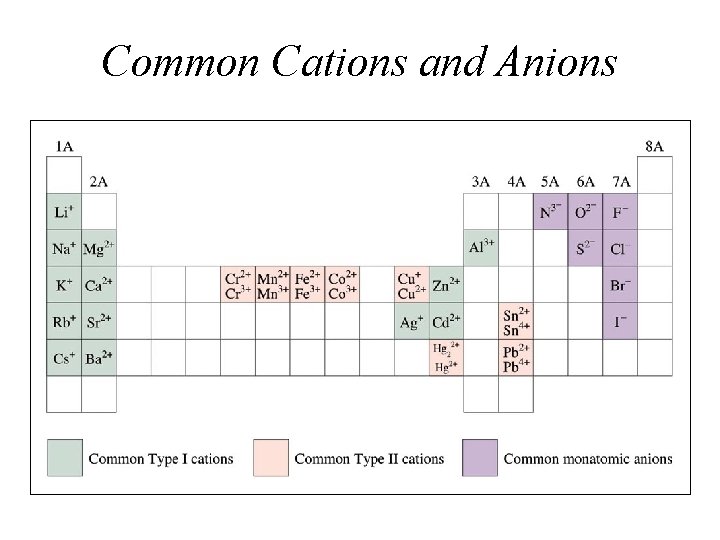
Common Cations and Anions

QUESTION

ANSWER D) lithium chloride. Lithium is a Group IA metal, so it always forms a +1 ion. Therefore, no roman numeral is necessary.

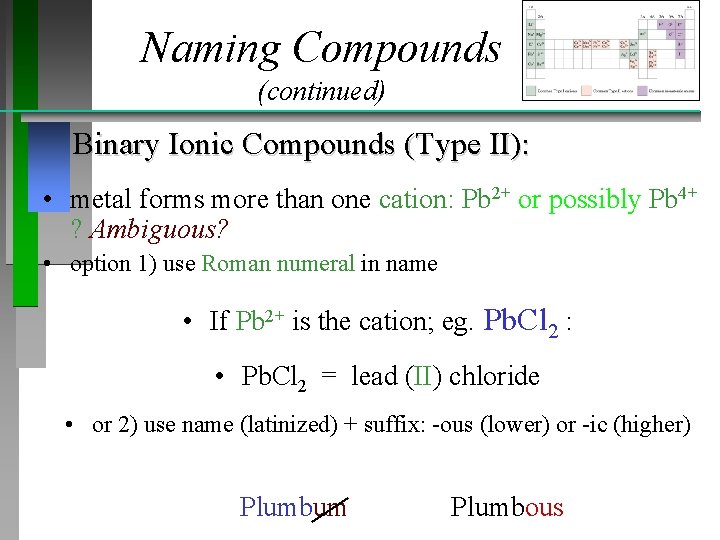
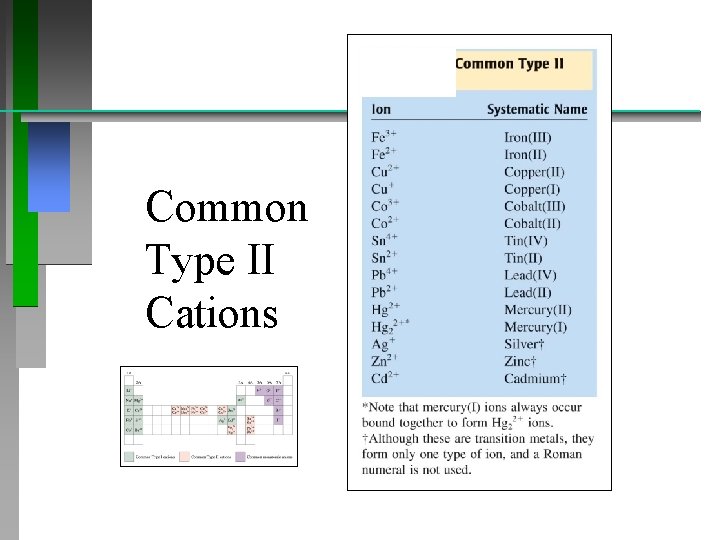
Naming Compounds (continued) Binary Ionic Compounds (Type II): • metal forms more than one cation: Pb 2+ or possibly Pb 4+ ? Ambiguous? • option 1) use Roman numeral in name • If Pb 2+ is the cation; eg. Pb. Cl 2 : • Pb. Cl 2 = lead (II) chloride • or 2) use name (latinized) + suffix: -ous (lower) or -ic (higher) Plumbum Plumbous

Common Type II Cations

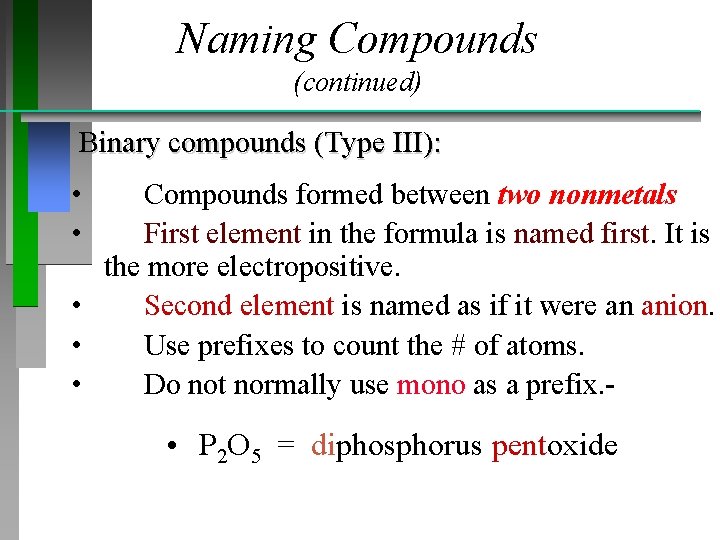
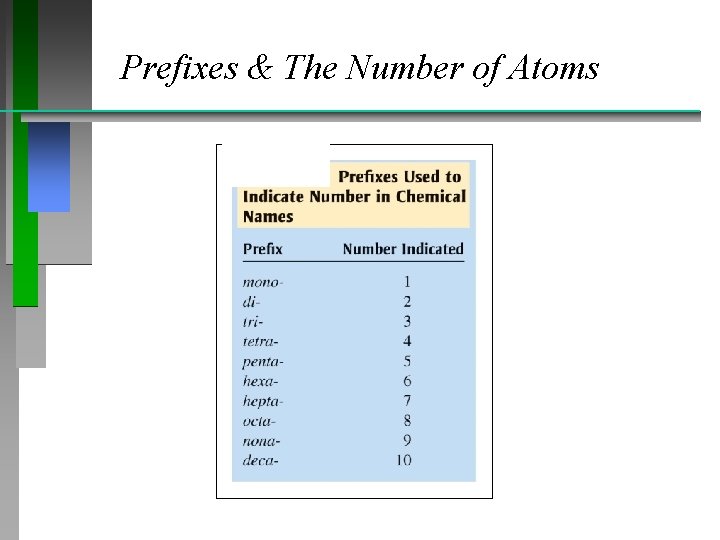
Naming Compounds (continued) Binary compounds (Type III): • • Compounds formed between two nonmetals First element in the formula is named first. It is the more electropositive. • Second element is named as if it were an anion. • Use prefixes to count the # of atoms. • Do not normally use mono as a prefix. - • P 2 O 5 = diphosphorus pentoxide

QUESTION

ANSWER B) iron (II) oxide. Iron is a transition metal that forms more than one type of ion. A roman numeral is needed to indicate which ion is present in the compound.

Prefixes & The Number of Atoms

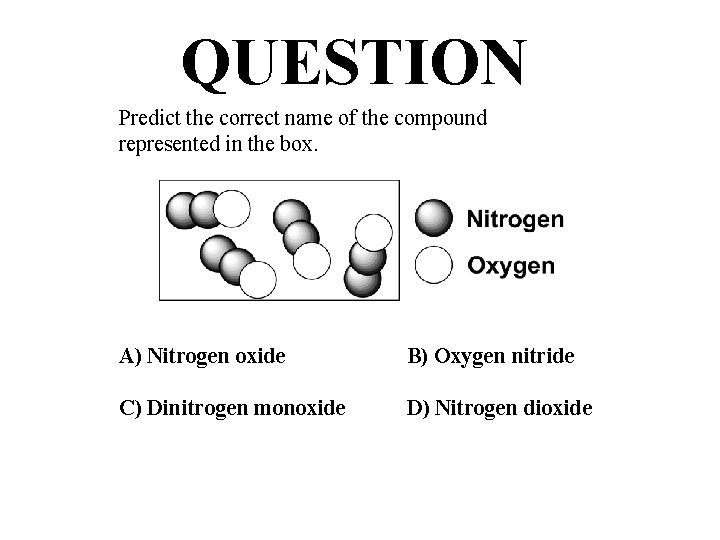
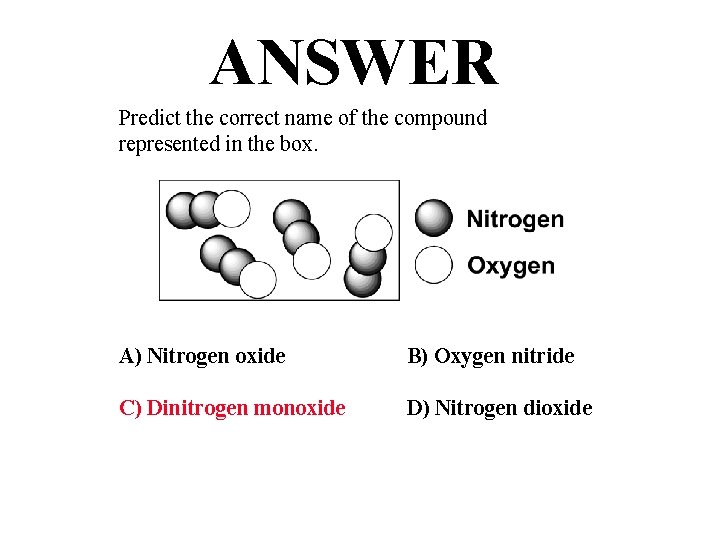
QUESTION Predict the correct name of the compound represented in the box. A) Nitrogen oxide B) Oxygen nitride C) Dinitrogen monoxide D) Nitrogen dioxide

ANSWER Predict the correct name of the compound represented in the box. A) Nitrogen oxide B) Oxygen nitride C) Dinitrogen monoxide D) Nitrogen dioxide

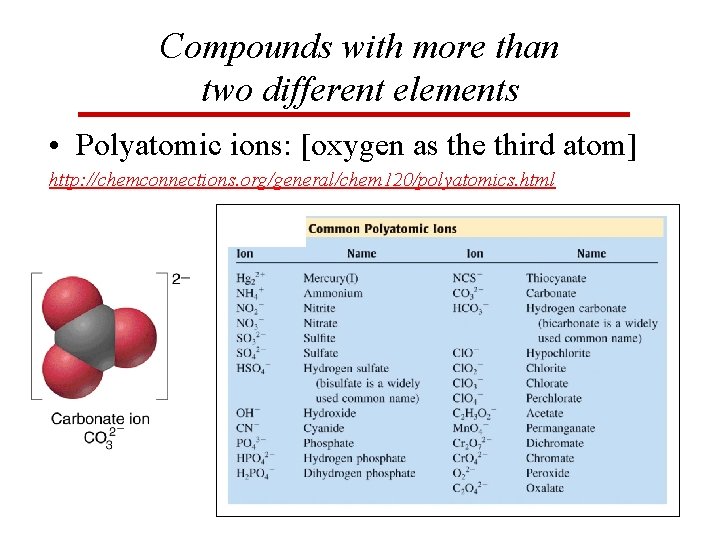
Compounds with more than two different elements • Polyatomic ions: [oxygen as the third atom] http: //chemconnections. org/general/chem 120/polyatomics. html

QUESTION Which of the following provides the correct name for Ca(H 2 PO 4)2? A. B. C. D. Calcium dihydrogen phosphate Calcium (II) hydrogen phosphate Calcium di-dihydrogen phosphate Calcium (II) dihydrogen phosphate

ANSWER Which of the following provides the correct name for Ca(H 2 PO 4)2? A. B. C. D. Calcium dihydrogen phosphate Calcium (II) hydrogen phosphate Calcium di-dihydrogen phosphate Calcium (II) dihydrogen phosphate

QUESTION Of the following, which provides the most acceptable name for Fe 2(C 2 O 4)3? A. B. C. D. Iron (II) oxalate (III) Iron (III) trioxalate Iron (III) oxalate

ANSWER Of the following, which provides the most acceptable name for Fe 2(C 2 O 4)3? A. B. C. D. Iron (II) oxalate (III) Iron (III) trioxalate Iron (III) oxalate
![Naming Acids Compounds with electropositive Hydrogen atoms Naming Acids [Compounds with electropositive Hydrogen atom(s)]](https://slidetodoc.com/presentation_image_h2/57165a66e8a6571b9ac7e51988ba5513/image-23.jpg)
Naming Acids [Compounds with electropositive Hydrogen atom(s)]

QUESTION Hypochlorous acid is related to the anion found in common household bleach. Which of the following is that common anion? A. B. C. D. Cl. O 4– Cl. O 3– Cl. O 2– Cl. O–

ANSWER Hypochlorous acid is related to the anion found in common household bleach. Which of the following is that common anion? A. B. C. D. Cl. O 4– Cl. O 3– Cl. O 2– Cl. O–

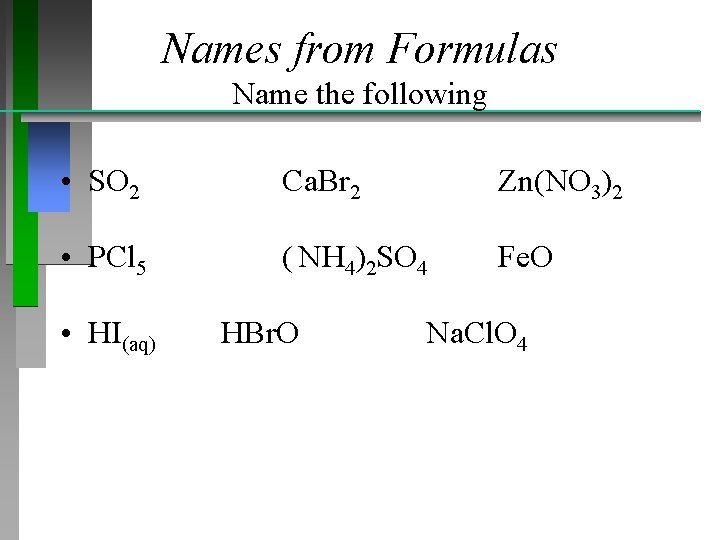
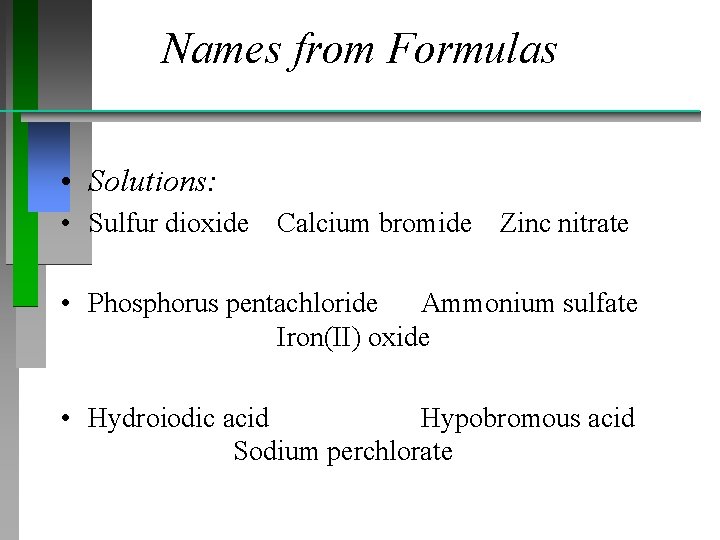
Names from Formulas Name the following • SO 2 Ca. Br 2 Zn(NO 3)2 • PCl 5 ( NH 4)2 SO 4 Fe. O • HI(aq) HBr. O Na. Cl. O 4

Names from Formulas • Solutions: • Sulfur dioxide Calcium bromide Zinc nitrate • Phosphorus pentachloride Ammonium sulfate Iron(II) oxide • Hydroiodic acid Hypobromous acid Sodium perchlorate

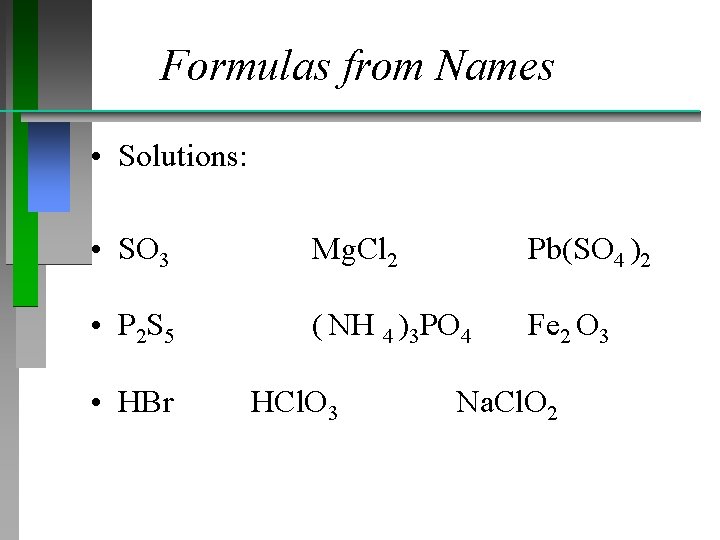
Formulas from Names Provide formulas for the following • • • Sulfur trioxide Magnesium chloride Lead (IV) sulfate Diphosphorus pentasulfide Ammonium phosphate Iron (III) oxide Hydrobromic acid Chloric acid Sodium chlorite

Formulas from Names • Solutions: • SO 3 Mg. Cl 2 Pb(SO 4 )2 • P 2 S 5 ( NH 4 )3 PO 4 Fe 2 O 3 • HBr HCl. O 3 Na. Cl. O 2
 Rusay
Rusay Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Heterocyclic compounds nomenclature
Heterocyclic compounds nomenclature Hetrocycle
Hetrocycle Nomenclature of alkanes
Nomenclature of alkanes Nomenclature of binary ionic compounds
Nomenclature of binary ionic compounds Coordination compound nomenclature
Coordination compound nomenclature Organisms taxonomy
Organisms taxonomy Venn diagram ionic and covalent bonds
Venn diagram ionic and covalent bonds Naming chemical compounds
Naming chemical compounds Names of ionic compounds
Names of ionic compounds Tetraiodine nonaoxide formula
Tetraiodine nonaoxide formula Naming and writing formulas for molecular compounds
Naming and writing formulas for molecular compounds What is the chemical formula for tetranitrogen heptoxide?
What is the chemical formula for tetranitrogen heptoxide? Section 3 writing formulas and naming compounds
Section 3 writing formulas and naming compounds Binary compound
Binary compound Lesson 17 technicolor atoms flame test answers
Lesson 17 technicolor atoms flame test answers Writing formulas from names
Writing formulas from names Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Which two formulas represent compounds
Which two formulas represent compounds Monatomic ions
Monatomic ions Prefixes for hydrates
Prefixes for hydrates Chapter 9 chemical names and formulas
Chapter 9 chemical names and formulas Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key Chapter 9 chemical names and formulas chapter quiz answers
Chapter 9 chemical names and formulas chapter quiz answers Chapter 6 section 3 compound names and formulas answer key
Chapter 6 section 3 compound names and formulas answer key Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key Properties of alkyl halides
Properties of alkyl halides Nomenclature poteau incendie
Nomenclature poteau incendie