Unit 9 Outline Review Warmup If 45 0






![Equilibrium • Equilibrium Notes • Unit 9 Equilibrium Review • Equilibrium Expressions K=[Products]/[reactants] • Equilibrium • Equilibrium Notes • Unit 9 Equilibrium Review • Equilibrium Expressions K=[Products]/[reactants] •](https://slidetodoc.com/presentation_image_h/ba0f6d493841a61ba2ce4f12cc864db5/image-12.jpg)
- Slides: 12

Unit 9 Outline Review

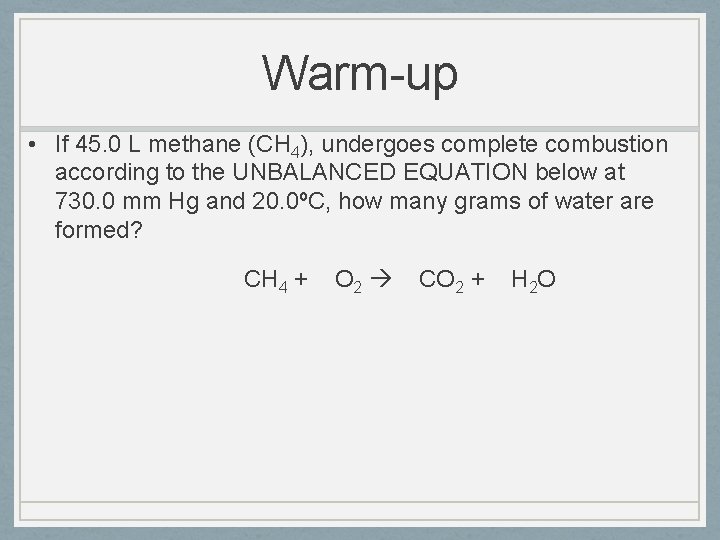
Warm-up • If 45. 0 L methane (CH 4), undergoes complete combustion according to the UNBALANCED EQUATION below at 730. 0 mm Hg and 20. 0ºC, how many grams of water are formed? CH 4 + O 2 CO 2 + H 2 O

Unit 9 Topics • Gas Properties • Ideal Gas Law • Combined Gas Law • Dalton’s Law of Partial Pressure • Gas Stoichiometry • Equilibrium

Gas Properties • Kinetic Molecular Theory POGIL • Understand the characteristics of the phases of matter (spacing, potential for movement, filling a container) • Temperature and Kinetic Energy are directly related • What causes pressure? • How are P, V, T and n related? (direct or inverse relationship? )

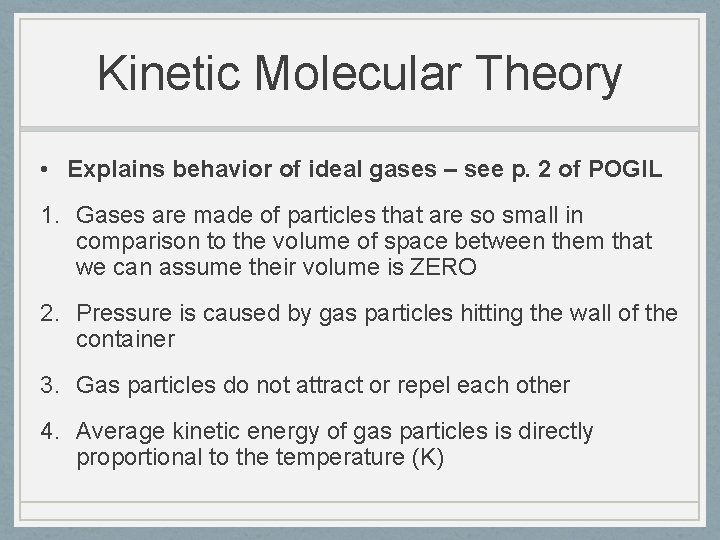
Kinetic Molecular Theory • Explains behavior of ideal gases – see p. 2 of POGIL 1. Gases are made of particles that are so small in comparison to the volume of space between them that we can assume their volume is ZERO 2. Pressure is caused by gas particles hitting the wall of the container 3. Gas particles do not attract or repel each other 4. Average kinetic energy of gas particles is directly proportional to the temperature (K)

Real Gases • Real gases do not obey the ideal gas law at HIGH PRESSURES and LOW TEMPERATURES

Ideal Gas Law • The Ideal Gas Law Notes • One gas with variables remaining constant (P, V, n, T) • PV=n. RT • Use the R value (on pink sheet) that matches unit of Pressure.

Relationships between temp, pressure, and volume • Temperature and pressure are DIRECTLY RELATED • Pressure and volume are INVERSLY RELATED • Volume and temperature are DIRECTLY RELATED

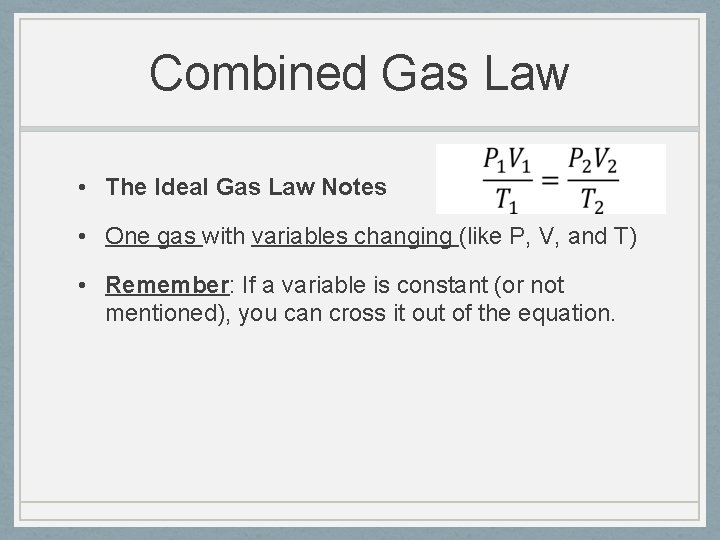
Combined Gas Law • The Ideal Gas Law Notes • One gas with variables changing (like P, V, and T) • Remember: If a variable is constant (or not mentioned), you can cross it out of the equation.

Dalton’s Law of Partial Pressure • Ptotal = P 1 + P 2 + P 3 +…. . • REMEMBER: You may have to first calculate pressure of individual gases BEFORE you can solve

Gas Stoichiometry • Gas Stoichiometry Practice • Draw the Stoichiometry Islands! (This is a great thing to draw on your test in the first minute!) • Must write & balance the chemical reaction! • When at STP: Stoichiometry ONLY! (yay!!!) • Not at STP: Must use PV=n. RT “detour”
![Equilibrium Equilibrium Notes Unit 9 Equilibrium Review Equilibrium Expressions KProductsreactants Equilibrium • Equilibrium Notes • Unit 9 Equilibrium Review • Equilibrium Expressions K=[Products]/[reactants] •](https://slidetodoc.com/presentation_image_h/ba0f6d493841a61ba2ce4f12cc864db5/image-12.jpg)
Equilibrium • Equilibrium Notes • Unit 9 Equilibrium Review • Equilibrium Expressions K=[Products]/[reactants] • Know how to write/identify these, you do not need to do any math • Le Chatelier’s Principle (know how equilibrium shifts left or right if…) • • • Add/Remove Reactant Add/Remove Product Add/Remove heat (endothermic or exothermic) Increase/Decrease Pressure Increase/Decrease Volume