DOCUMENTATION THE FOUNDATION OF QUALITY DOCUMENTATION n n



![ISO 9000 Quality System, ISO 4. 2. 1 General n The. . . [company] ISO 9000 Quality System, ISO 4. 2. 1 General n The. . . [company]](https://slidetodoc.com/presentation_image_h2/209f65968d36691cae5fa79bca15dcba/image-5.jpg)







































- Slides: 49

DOCUMENTATION: THE FOUNDATION OF QUALITY

DOCUMENTATION n n One aspect of quality Most often cited by employers as important lseidman@matcmadison. edu


DOCUMENTATION IS IMPORTANT! n Do what you say; say what you do. n If it isn’t documented, it wasn’t done. n Companies make two products, the one that is sold and the documents that go with it. lseidman@matcmadison. edu
![ISO 9000 Quality System ISO 4 2 1 General n The company ISO 9000 Quality System, ISO 4. 2. 1 General n The. . . [company]](https://slidetodoc.com/presentation_image_h2/209f65968d36691cae5fa79bca15dcba/image-5.jpg)
ISO 9000 Quality System, ISO 4. 2. 1 General n The. . . [company] shall establish, document, and maintain a quality system as a means of ensuring that product conforms to specified requirements. Quality Planning 4. 2. 3. The. . . [company] shall define and document how the requirements for quality will be met. . . [Emphasis added] lseidman@matcmadison. edu

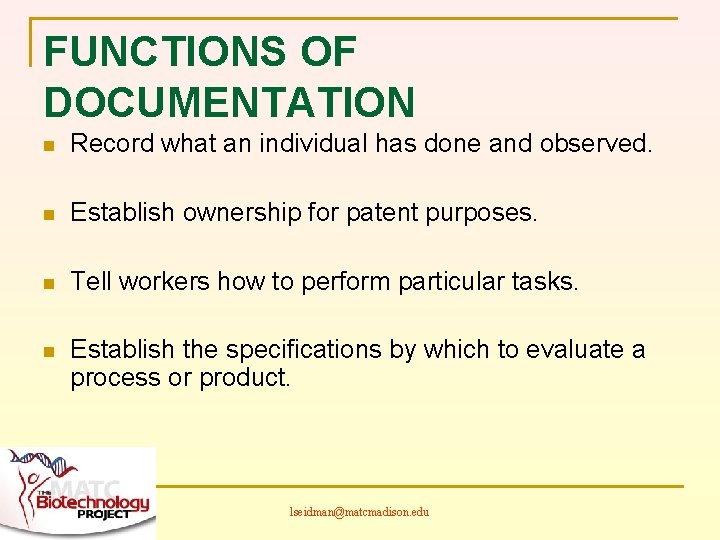
FUNCTIONS OF DOCUMENTATION n Record what an individual has done and observed. n Establish ownership for patent purposes. n Tell workers how to perform particular tasks. n Establish the specifications by which to evaluate a process or product. lseidman@matcmadison. edu

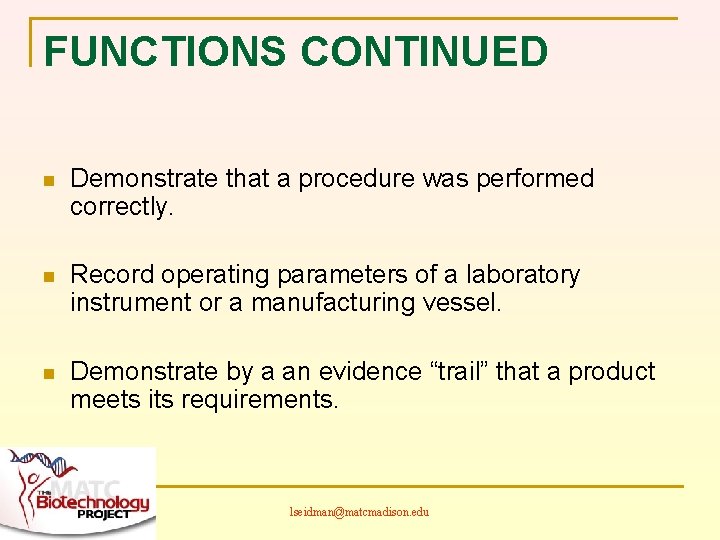
FUNCTIONS CONTINUED n Demonstrate that a procedure was performed correctly. n Record operating parameters of a laboratory instrument or a manufacturing vessel. n Demonstrate by a an evidence “trail” that a product meets its requirements. lseidman@matcmadison. edu

FUNCTIONS CONTINUED n Ensure traceability q q q History and location of a product The components of a product Helps ensure that if problems arise, origin of the problem can be traced to components Product can be found and recalled if necessary Traceability depends on an organized, well-designed system of documentation lseidman@matcmadison. edu

FUNCTIONS CONTINUED n Establish a contract between a company and consumers via written specifications, labels, and other documents. n Establish a contract between a company and regulatory agencies. lseidman@matcmadison. edu

TYPES OF DOCUMENTS n Directive documents tell personnel how to do a task, identify a material, or define something. q Example: Protocols, which guide researchers in investigating a question, are directive documents. Information is not added to protocols when work is performed. lseidman@matcmadison. edu

n Data collection documents facilitate the recording of data and provide evidence that a directive document has been properly followed. Information is added to data collection documents during operations. q Examples include forms, logbooks, and laboratory notebooks. lseidman@matcmadison. edu

n Commitment documents lay out the organization’s goals, standards, and commitments. lseidman@matcmadison. edu

CONTROLLED VERSUS UNCONTROLLED DOCUMENTS n n Most are controlled Are prepared and distributed in a formal process lseidman@matcmadison. edu

“GOOD DOCUMENTATION PRACTICES” n n n Must be accurate, legible, understandable Must have verifiable dates Must be safe from disaster, theft, damage, access by unauthorized persons Must be attributable to one individual Must provide information for traceability Should not be capable of being destroyed by accident or on purpose lseidman@matcmadison. edu

AUDIT n Auditor comes to the facility lseidman@matcmadison. edu

n n First thing they check is that all documentation is present That documentation is internally consistent Documentation reflects what people really do Auditors look closely at documentation lseidman@matcmadison. edu

TYPES OF DOCUMENTS: LABORATORIES n n n n Laboratory notebooks. Standard Operating Procedures (SOPs) Forms Protocols Reports Equipment/Instrument logbooks Recordings from instruments lseidman@matcmadison. edu

MORE LAB DOCUMENTS n n n Electronic documents Analytical laboratory documents Numbering systems Labels Chain of custody forms Training reports lseidman@matcmadison. edu

SOME TYPES OF DOCUMENTS: PRODUCTION n Batch records n Regulatory submissions n Release of final product record lseidman@matcmadison. edu

EXAMPLE n Technician in a company prepares a 1 M solution of Na. Cl: 1. The technician will follow an approved standard operating procedure. 2. The raw materials -- clean glassware, Na. Cl, and purified water -- will all have documents associated with them that show they were tested and satisfactory. lseidman@matcmadison. edu

3. The instruments used to weigh Na. Cl and measure water have logbooks showing they were properly maintained. lseidman@matcmadison. edu

4. The technician will record information about the solution. Preparer’s name Date Steps Quantities and source of raw materials Storage location and conditions Amount ID number lseidman@matcmadison. edu

5. Solution identifying number and documentation associated with assigning number. 6. Distinguishing label. Label lseidman@matcmadison. edu

LABORATORY NOTEBOOKS Assigned to individuals. n Chronological log of everything that individual does and observes. n Primary form of documentation in a research laboratory. n lseidman@matcmadison. edu

n Legal document q Used to establish a patent claim q Assign credit for a discovery q Document the honesty and integrity of data q Trouble-shooting lseidman@matcmadison. edu

Can be subpoenaed q Can be examined by regulatory agency q lseidman@matcmadison. edu

RAW DATA n n Raw data are the first records of an original observation. May be recorded into the laboratory notebook with pen May be a paper output from an instrument May be recorded directly into a computer medium. lseidman@matcmadison. edu

n n Outputs from instruments, photos, and other paper data may be labeled and securely taped into a laboratory notebook. It is essential that a scientist or operator save raw data, that it never be altered or edited, and that raw data be retrievable. lseidman@matcmadison. edu

RULES AND GUIDELINES n Laboratory manual and textbook have “rules” and guidelines lseidman@matcmadison. edu

A LAB NOTEBOOK IS NOT A REPORT n A laboratory notebook is not the same as a report. q Lab notebook is chronological n n q May not be entirely “neat” May have procedures materials mixed with results Report is prepared neatly on a word processor. n Uses format, like Introduction, Materials and Methods, Results, etc. lseidman@matcmadison. edu

MISTAKES n Sometimes people record information on separate sheets of paper q q Forgot lab notebook Want to keep the lab notebook neat and orderly n DON’T!! lseidman@matcmadison. edu

Annotated Lab Notebook Page, in Lab Manual

SOPS ARE VERY IMPORTANT n n n Phrase often repeated in GMP: “there shall be procedures for…” This means that by law, companies that are compliant with GMP must have SOPs are documents that spell out how to do something lseidman@matcmadison. edu

n Production facilities and many laboratories use documents SOPS q q q Instruct personnel in how to perform particular tasks. Step-by step outline of how a task is to be performed. Indicate in which situations the task is done Who is qualified or responsible for the work Etc. lseidman@matcmadison. edu

q Since everyone follows the same SOPs, they help ensure that work is performed correctly and consistently over time. lseidman@matcmadison. edu

SOME TYPES OF SOPS n Administrative SOPs q n Processes q n Such as how to assign lab notebooks Such as how to investigate problem Motor tasks q Such as how to operate a balance lseidman@matcmadison. edu

WRITING SOPS n A lot of art to this. What is the purpose of the procedure? What will be the outcome or product of the procedure? What resources and materials will the person performing the SOP require in order to achieve the desired result? lseidman@matcmadison. edu

What equipment will they need? Does the model of equipment need to be specified? What supplies and reagents are needed? Is a raw material produced by a particular manufacturer needed? lseidman@matcmadison. edu

What steps will the person following the SOP perform to achieve the desired product? What must the person do to ensure that the product of the SOP is good? Are there any potential problems that the person may run into while performing the SOP, and, if so, how can these problems be avoided? lseidman@matcmadison. edu

ACTIVITIES n n n Laboratory Exercise 2 introduces SOPs for tasks like operating balances. Many examples in text, website. lseidman@matcmadison. edu

Form

Form

BATCH RECORDS n n Document that accompanies product as it is made Directs operators in how to make product Provides blanks that are filled in as product is made For critical steps, witness watches and signs lseidman@matcmadison. edu

RESEARCH LABS n n 1986 Teresa Imanishi-Kari published paper in “Cell” She worked in David Baltimore’s lab Margaret O’Toole questioned whether the data supported the paper First investigation was at the departmental level lseidman@matcmadison. edu

n n n Initially dismissed it, but later went to investigation by MIT A member of the US congress heard about the incident and took it up as a campaign Subpoenaed all data and lab notebooks lseidman@matcmadison. edu

n n n Had US secret service look at ink, paper, lab notebooks, instrument printouts to see if they were falsified Looked a lot at dates and order of data Were several investigations, hearings, eventual trial Found evidence that dates were altered, data from 1986 was transcribed before data from 1984 In her defense, she said lseidman@matcmadison. edu

n “I am not a neat person” lseidman@matcmadison. edu

n n n Found guilty of fraud at one point, but was later acquitted in a later trial This went on for more than 10 years Disrupted many lives Led to public perception that scientists cheat Affected funding lseidman@matcmadison. edu

MORAL OF THE STORY n n n Keep good records, wherever you work Don’t obscure problems or mistakes Dating is critical, keep records chronological, that is, in order by date lseidman@matcmadison. edu