DOCUMENTATION ISOIEC 17025 2005 Documentation Welcome Todays Overview



























- Slides: 42

DOCUMENTATION

ISO/IEC 17025: 2005

Documentation

Welcome

Today’s Overview 1 • ISO 17025 What is it? 2 • What are the requirements 3 • Road Map to Accreditation

ISO/IEC 17025 DOCUMENTATIONS DOCUMENTATION PREPARATION 6

ADVANTAGES § Transparency and rationalization § Early recognition of failures, problems etc. § Easier introduction of new employees § Improved co-ordination § Avoidance of duplication of work 8

ADVANTAGES CONT……. . § Improvement of the personnel structure § Transparency of laboratory quality management § Correct allocation of tests § The requirement formal accreditation § The availability of evidence to customers 9

OVERVIEW SYSTEM DOCUMENTATION SPECIFIC DOCUMENTATION • The quality manual • Technical specifications • Procedures • Quality plans • Test instructions • Methods • Technical records (evidence of test) • Work instructions • Technical reports 10

STEP ONE: BUILDING AN EFFECTIVE SYSTEM To have Good Documentation 11

GOOD DOCUMENTATION All elements, requirements and provisions adopted by an organization must be documented in a; § Simple § Systematic § Clear § And orderly manner 12

GOOD DOCUMENTATION Cont…. and should basically be; § Understandable § Adequately detailed § Accurate § Complete § Traceable so that they are auditable 13

AVOID TOO MUCH DETAIL • “The purpose of this procedure is to document the aforementioned activities, herein after referred to as the prescribed tasks in terms that prelude their execution in an inconsistent manner, wherein such inconsistency may potentially result in the prescribed tasks delivering a result that is not repeatable or reproducible” ? ? ? ? 14

POORLY WRITTEN PROCEDURE Why use ten words when one will do? § “The items hereunder referenced in some cases fell excessively outside normal parameters. ” 15

USE OF DOCUMENTATION § To consult § For training purposes § External assessment § To show evidence on operation 16

PROVISION § § § § Identification Approval Issue Distribution Controlling Removal of obsolete ones Maintaining documents List of records operated by organization 17

DOCUMENT CHANGES Changes to documents must be authorized & provide a method for; § Initiation § Development § Reviewing § Control § Issue and § Recording NB: Provide for use of un-controlled copies for proposal & customer use 18

LABORATORY MANAGEMENT SYSTEM § Quality manual § Documented procedures as required by the standard § Documentation needed by organization to ensure effectiveness in operation § Maintain records required by standard 19

DEFINE CONTROLS § § § Identity Storage Protection Retrieval Retention time Disposal 20

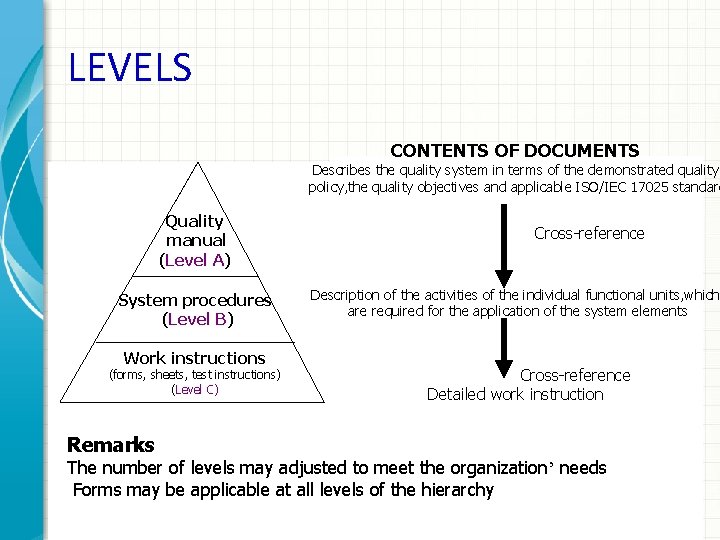
LEVELS CONTENTS OF DOCUMENTS Describes the quality system in terms of the demonstrated quality policy, the quality objectives and applicable ISO/IEC 17025 standard Quality manual (Level A) System procedures (Level B) Work instructions (forms, sheets, test instructions) (Level C) Cross-reference Description of the activities of the individual functional units, which are required for the application of the system elements Cross-reference Detailed work instruction Remarks The number of levels may adjusted to meet the organization’ needs Forms may be applicable at all levels of the hierarchy 21

QUALITY MANUAL Elements § Title and scope § Table of contents § Review, approval and revision § Quality policy and objectives § Organization, responsibility and authority § References § Laboratory management system description § Appendices 22

PROCEDURES A procedure is a sequence for undertaking various activities. Shall detail; § What shall be done § Who shall do it § How it shall be done § When it shall be done § Where it shall be done § Which (materials, documents etc) shall be used § What if 23

PROCEDURES cont…. . Contents § Title § Purpose § Scope § Responsibility and authority § Description of activities § Records § Appendices 24

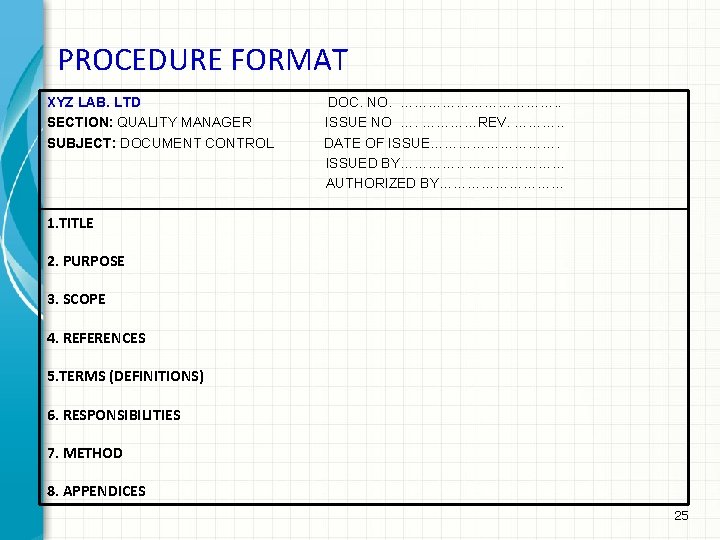
PROCEDURE FORMAT XYZ LAB. LTD SECTION: QUALITY MANAGER SUBJECT: DOCUMENT CONTROL DOC. NO. ………………. . ISSUE NO …. …………REV. ………. . DATE OF ISSUE……………. ISSUED BY…………. . ………………… AUTHORIZED BY…………… 1. TITLE 2. PURPOSE 3. SCOPE 4. REFERENCES 5. TERMS (DEFINITIONS) 6. RESPONSIBILITIES 7. METHOD 8. APPENDICES 25

STEP TWO: HAVE THE RIGHT AMOUNT OF DOCUMENTATION How many ? But how much documentation do I need 26

NUMBER OF PROCEDURES The number will depend on; § § Complexity of the operations The organization of the business The need of the organization The standard requirements 27

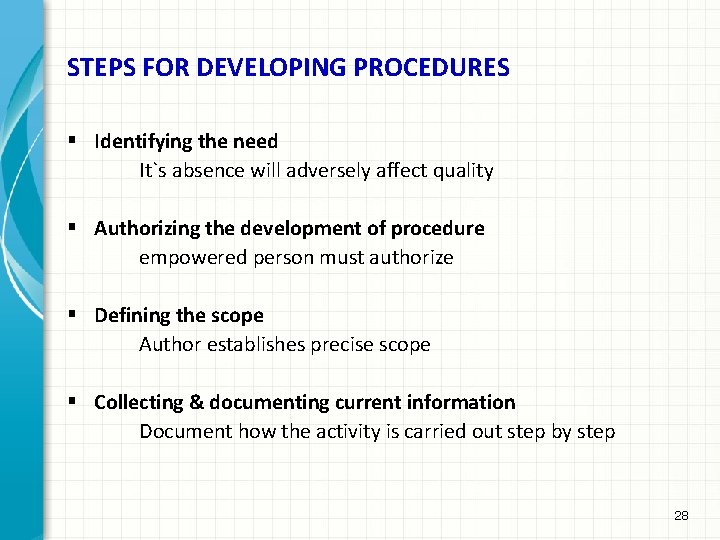
STEPS FOR DEVELOPING PROCEDURES § Identifying the need It`s absence will adversely affect quality § Authorizing the development of procedure empowered person must authorize § Defining the scope Author establishes precise scope § Collecting & documenting current information Document how the activity is carried out step by step 28

STEPS FOR DEVELOPING PROCEDURES Cont…. § Preparing a draft procedure Those involved in the implementation of procedure should be involved in drafting § Obtaining comments on draft procedure Persons & departments concerned should review initial draft to see if it is workable Modifications should be incorporated § Obtain authorization for use of procedure Appropriate person approves procedure once amendments are incorporated & checked Issue procedure § Review the procedure After implementation for some time(say six months) it should be reviewed & if necessary amendments or revised procedure issued 29

WORK INSTRUCTION Work instructions provide detailed activities and requirements for activities defined in procedures (work instruction is a subset of a procedure). Examples – - operating computer, - instruction on sterilization of instruments 30

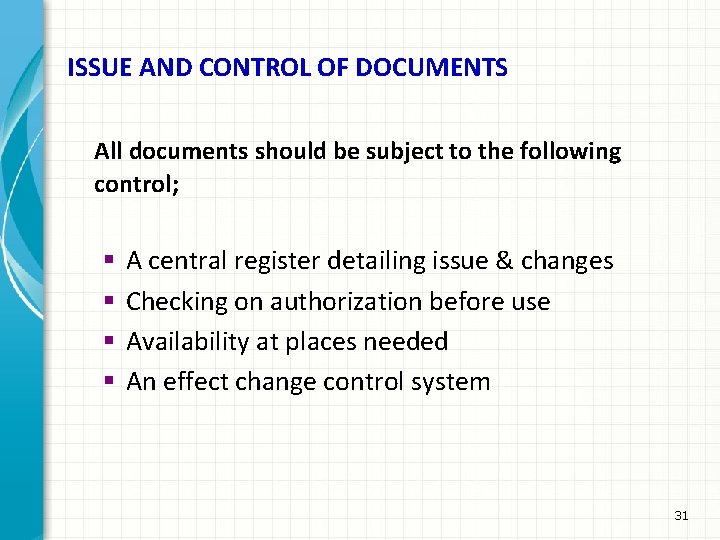
ISSUE AND CONTROL OF DOCUMENTS All documents should be subject to the following control; § § A central register detailing issue & changes Checking on authorization before use Availability at places needed An effect change control system 31

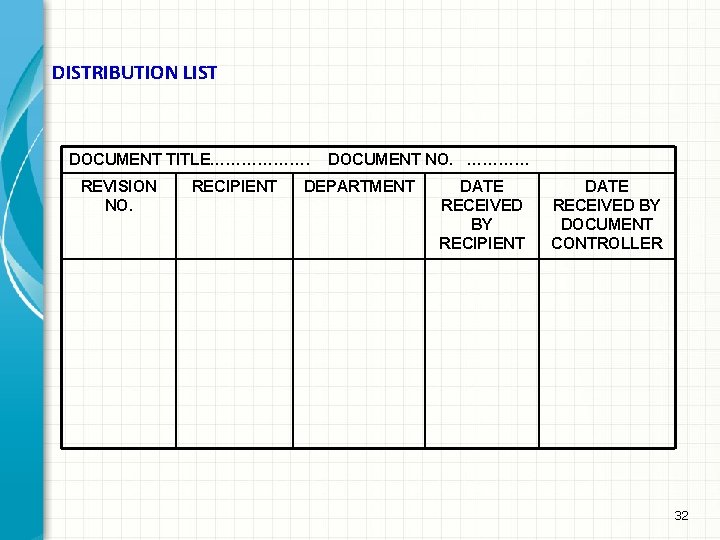
DISTRIBUTION LIST DOCUMENT TITLE………………. REVISION NO. RECIPIENT DOCUMENT NO. ………… DEPARTMENT DATE RECEIVED BY RECIPIENT DATE RECEIVED BY DOCUMENT CONTROLLER 32

TRANSMITAL FORM TO : ………………. . DEPARTMENT : ………………. DOCUMENT OR PAGE IDENTIFICATION : ………………. NAME/NO/SUBJECT : ……………… NEW : ………………. AMENDED : ……………… REPLACEMENT : ……………… EFFECTIVE DATE : ………………. . DATE DISPATCHED : ……… DISPATCHED BY : ……… DATE RECEIVED : ………. . RECEIVED BY : ………… AMENDMENT EFFECTED ON : ……………. AMENDED/REPLACED PAGE/ DOCUMENT RETURNED ON : ……………. . I CONFIRM THAT I KNOW AND UNDERSTAND THE DOCUMENT AND THAT I HAVE ENSURED THAT ALL PERSONNEL UNDER MY CONTROL HAVE BEEN INFORMED THEREOF AND THAT THEY UNDERSTAND WILL IMPLEMENT IT. SIGNATURE : ……………… DATE……………. 33

Establishing a process o Prepare o Implement o Achieve o Maintain o Review o Improve 34

Expectations • Customers • Suppliers • Employees • Shareholders • Society 35

Success factors • • • Market share Sales Service provision Results Efficiency Recognition 36

Quality Manual Document all requirements of the Standard and Implement – Clause 4. 2. 2 Management system 37

Procedures References clauses - Management requirements – – – Organization – 4. 1. 5(c), 4. 1. 5(d) Document control – 4. 3. 1, 4. 3. 3. 4 Review of requests, tenders and contracts – 4. 4. 1 Purchasing services and supplies – 4. 6. 1 Complaints – 4. 8 Control of nonconforming testing and/or calibration work – 4. 9. 1 Corrective action – 4. 11. 1 Preventive action – 4. 12. 2 Control of records – 4. 13. 1. 1, 4. 13. 1. 4 Internal audits – 4. 1 Management reviews – 4. 15. 1 38

Procedures cont… References clauses – Technical requirements – Personnel – 5. 2. 2 – Test and calibration methods and method validation – 5. 4. 1, 5. 4. 6. 2, 5. 4. 7. 2(b), – Equipment – 5. 5. 6, 5. 5. 10, 5. 5. 11, – Measurement traceability – 5. 6. 1, 5. 6. 3. 2, 5. 6. 3. 4 – Sampling – 5. 7. 1, 5. 7. 3 – Handling of testing and calibration items – 5. 8. 1, 5. 8. 4 – Assuring the quality of test and calibration results – 5. 9 39

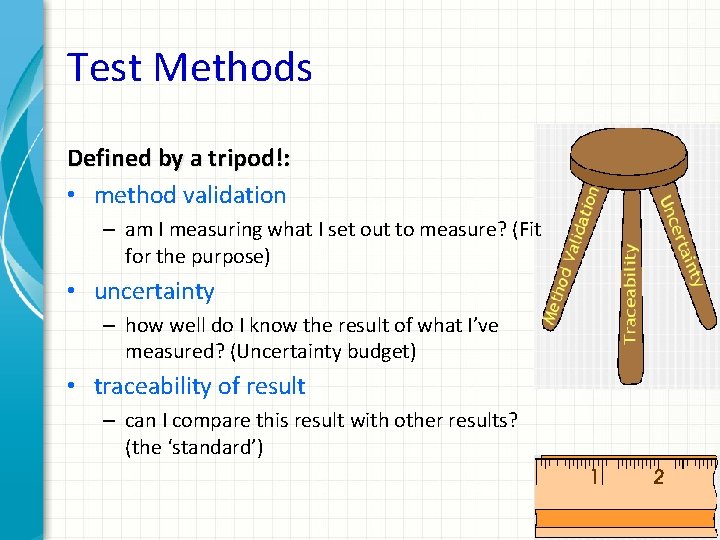
Test Methods Defined by a tripod!: • method validation – am I measuring what I set out to measure? (Fit for the purpose) • uncertainty – how well do I know the result of what I’ve measured? (Uncertainty budget) • traceability of result – can I compare this result with other results? (the ‘standard’) 40

Implementation • We must take the first step. • But…………… need to implement the system and move forward 41

THANK YOU 42

QUESTIONS?