Chemistry 10 1 10 1 The Mole A




























- Slides: 43

Chemistry 10. 1

10. 1 The Mole: A Measurement of Matter u You could measure the amount of sand in a sand sculpture by counting each grain of sand, but it would be much easier to weigh the sand. You’ll discover how chemists measure the amount of a substance using a unit called a mole, which relates the number of particles to the mass.

10. 1 Measuring Matter u Measuring Matter – What are three methods for measuring the amount of something?

10. 1 Measuring Matter u You often measure the amount of something by one of three different methods—by count, by mass, and by volume.

10. 1

10. 1

10. 1

10. 1

for Sample Problem 10. 1 Problem Solving 10. 1 Solve Problem 1 with the help of an interactive guided tutorial.

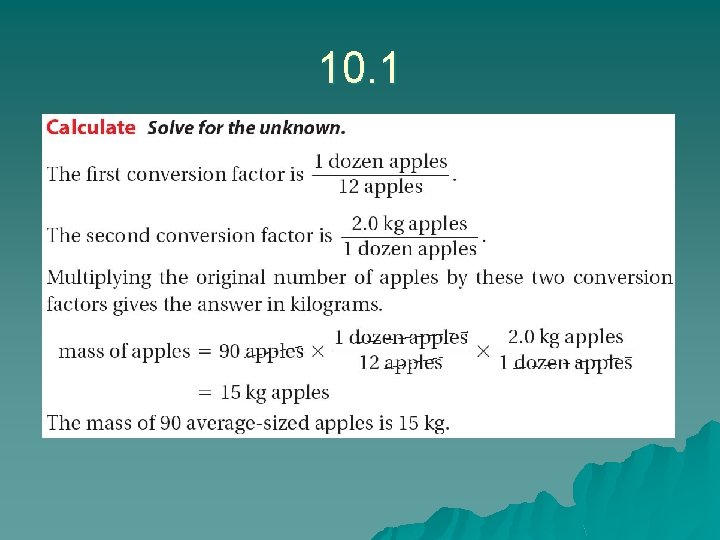
Sample problem 10. 1 u u u We are solving mass of 0. 50 bushels of apples We know: 0. 20 bushels = 1 dozen apples = 2. 0 kg u Answer: u u u 0. 50 bushel x 1 dozen__ 0. 20 bushel = 5. 0 kg x 2. 0 kg__ 1 dozen

10. 1 u What is a mole?

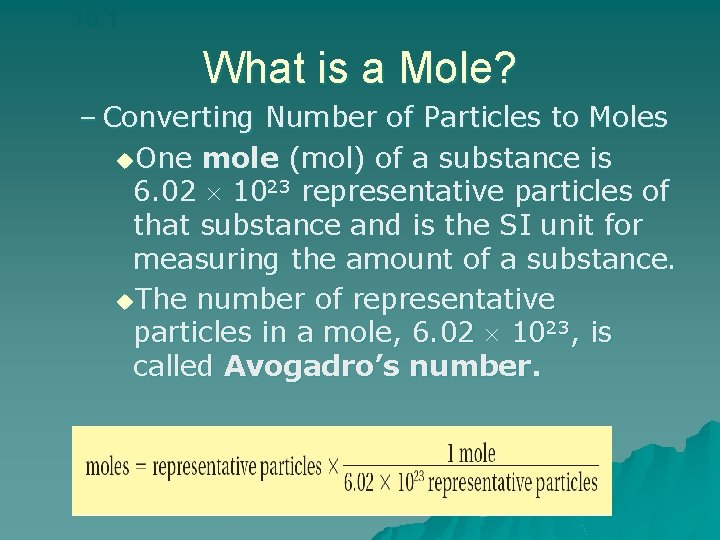
10. 1 What is a Mole? u What Is a Mole? – How is Avogadro’s number related to a mole of any substance?

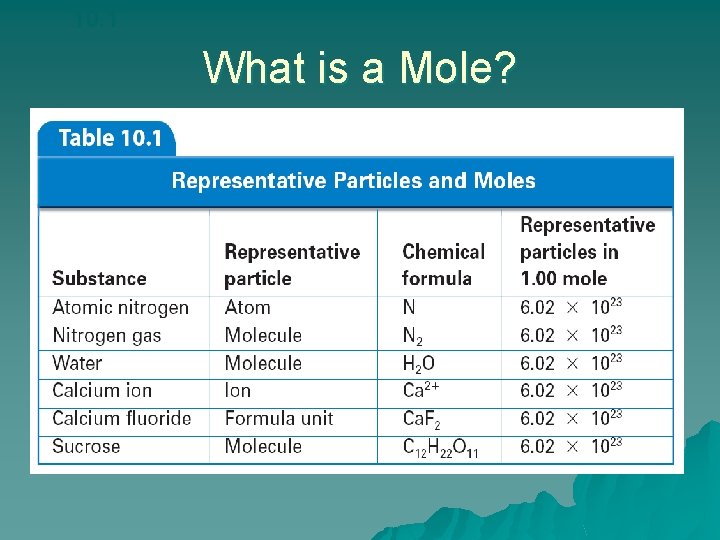
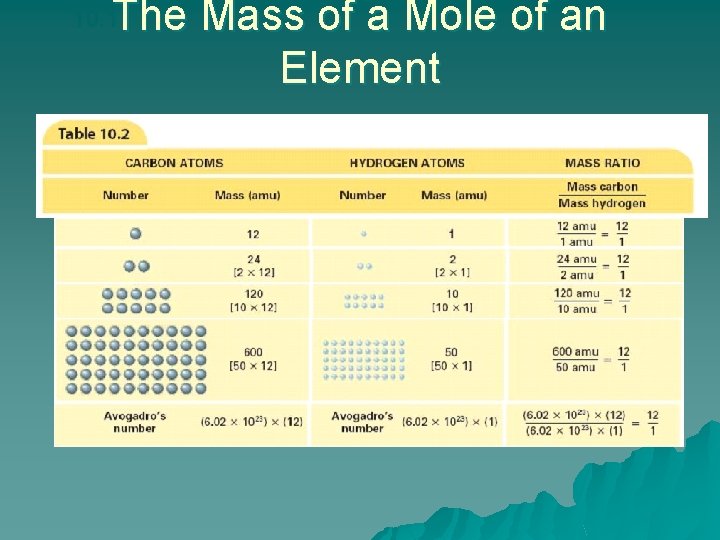
10. 1 What is a Mole? u. A mole of any substance contains Avogadro’s number of representative particles, or 6. 02 1023 representative particles. –The term representative particle refers to the species present in a substance: usually atoms, molecules, or formula units.

10. 1 What is a Mole? – Converting Number of Particles to Moles u. One mole (mol) of a substance is 6. 02 1023 representative particles of that substance and is the SI unit for measuring the amount of a substance. u. The number of representative particles in a mole, 6. 02 1023, is called Avogadro’s number.

10. 1 What is a Mole?

10. 2

10. 2

10. 2

10. 2

for Sample Problem 10. 2 Problem Solving 10. 4 Solve Problem 4 with the help of an interactive guided tutorial.

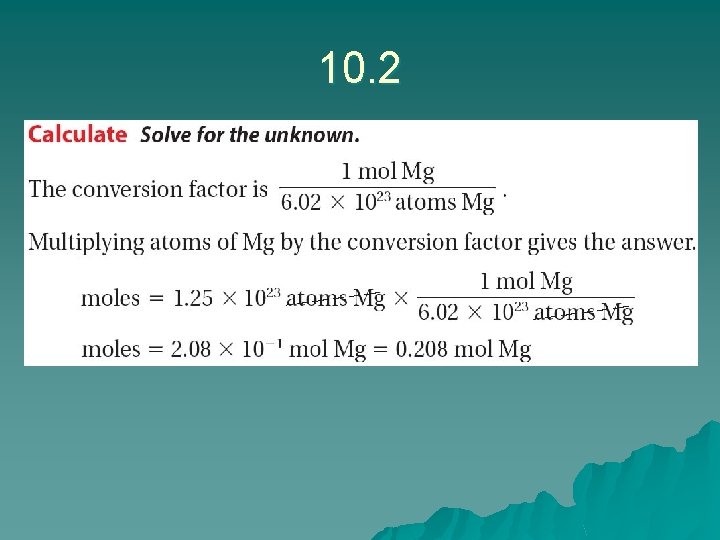
10. 1 What is a Mole? – Converting Moles to Number of Particles

Sample problem 10. 2 u? u Moles is 2. 17 x 1023 particles of Br We know 1 mole = 6. 02 x 1023 u Answer: u 2. 17 x 1023 X 1 mole = 0. 360 mol Br 6. 02 x 1023

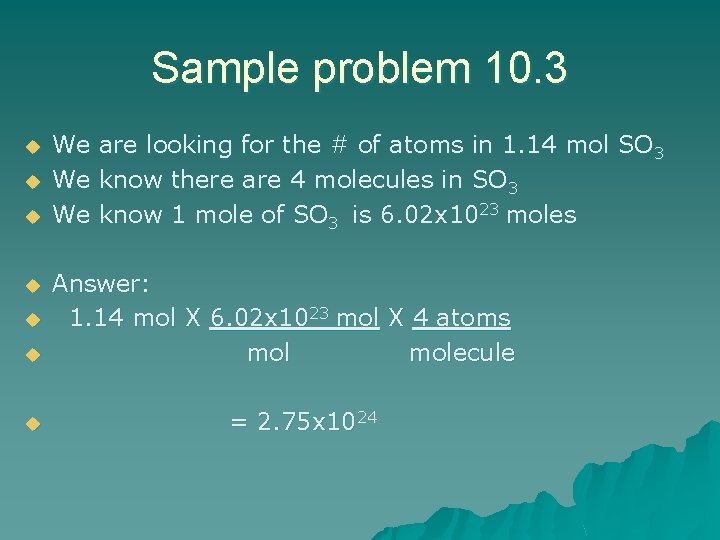
10. 3

10. 3

10. 3

10. 3

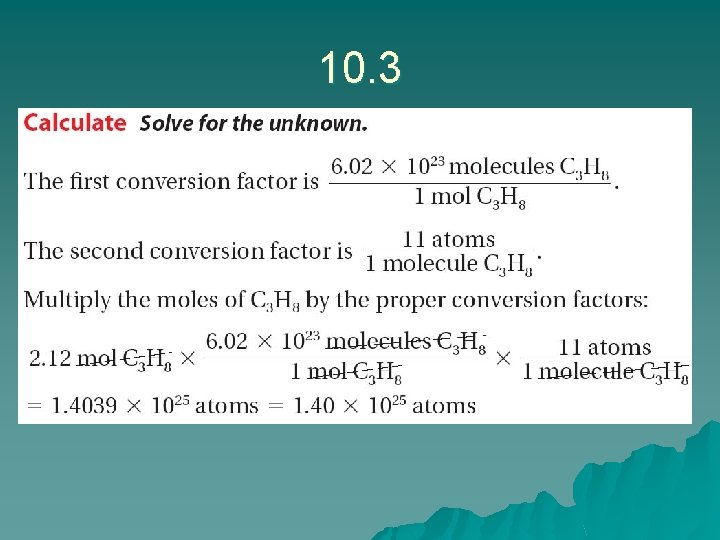
for Sample Problem 10. 3 Problem Solving 10. 5 Solve Problem 5 with the help of an interactive guided tutorial.

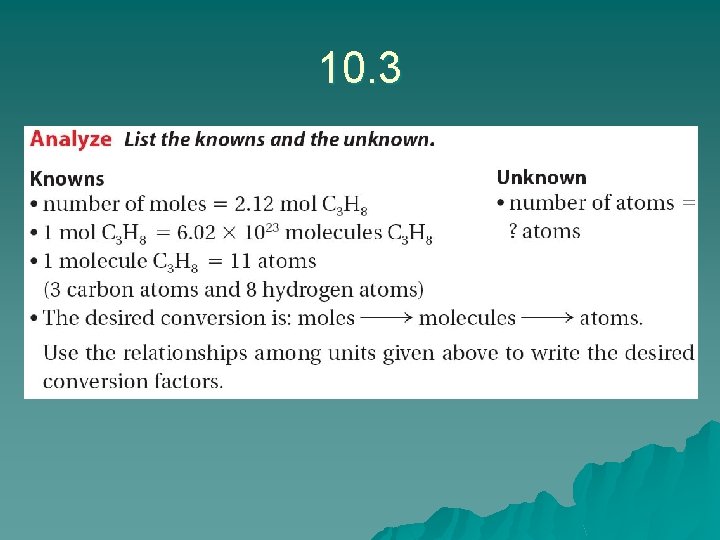
Sample problem 10. 3 u u u u We We We are looking for the # of atoms in 1. 14 mol SO 3 know there are 4 molecules in SO 3 know 1 mole of SO 3 is 6. 02 x 1023 moles Answer: 1. 14 mol X 6. 02 x 1023 mol X 4 atoms molecule = 2. 75 x 1024

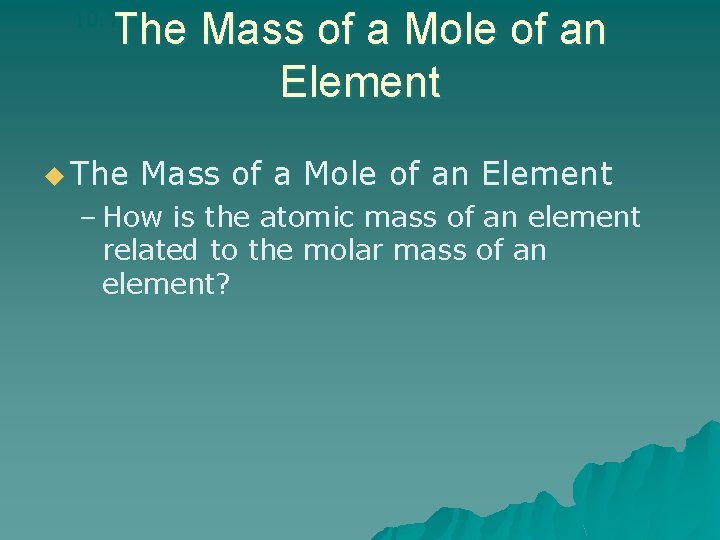
The Mass of a Mole of an Element 10. 1 u The Mass of a Mole of an Element – How is the atomic mass of an element related to the molar mass of an element?

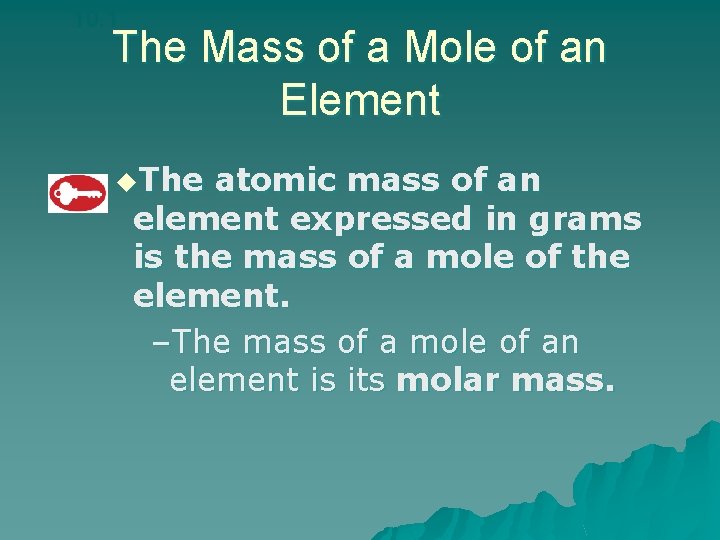
10. 1 The Mass of a Mole of an Element u. The atomic mass of an element expressed in grams is the mass of a mole of the element. –The mass of a mole of an element is its molar mass.

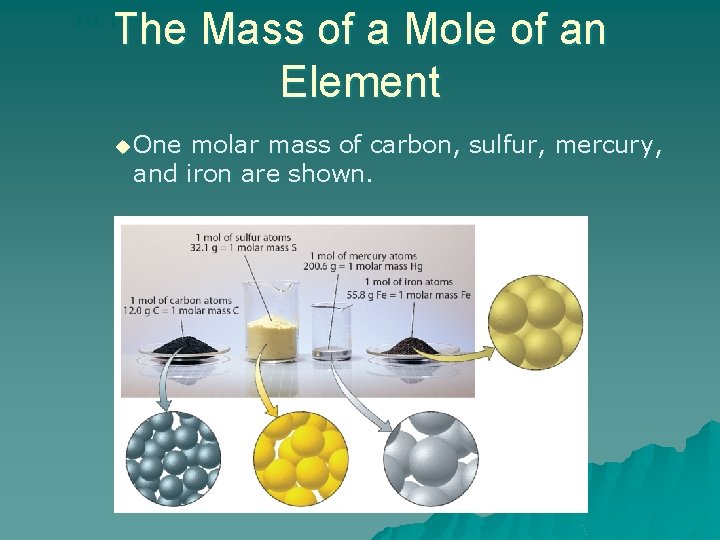
The Mass of a Mole of an Element 10. 1 u One molar mass of carbon, sulfur, mercury, and iron are shown.

Animation 11 – Find out how Avogadro’s number is based on the relationship between the amu and the gram.

The Mass of a Mole of an Element 10. 1

10. 1 The Mass of a Mole of a Compound u The Mass of a Mole of a Compound –How is the mass of a mole of a compound calculated?

10. 1 The Mass of a Mole of a Compound – To calculate the molar mass of a compound, find the number of grams of each element in one mole of the compound. Then add the masses of the elements in the compound.

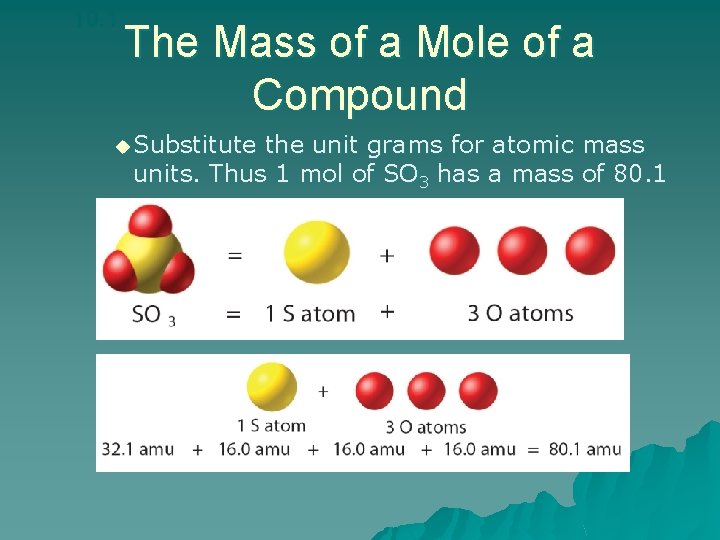
10. 1 The Mass of a Mole of a Compound u Substitute the unit grams for atomic mass units. Thus 1 mol of SO 3 has a mass of 80. 1 g.

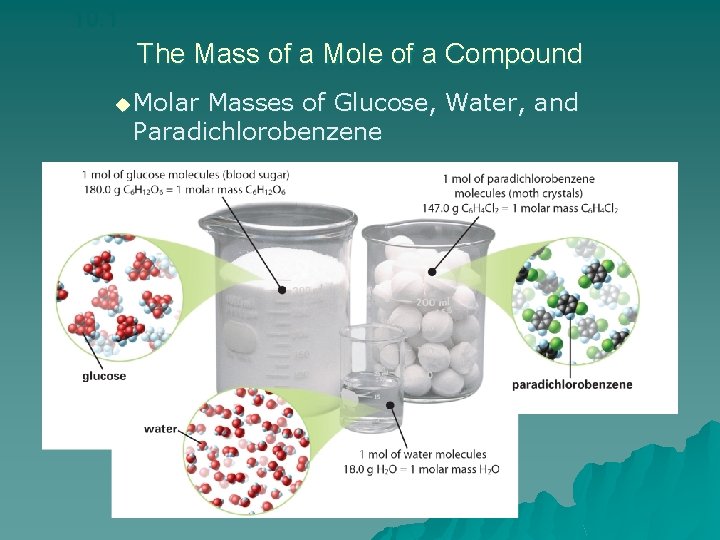
10. 1 The Mass of a Mole of a Compound u Molar Masses of Glucose, Water, and Paradichlorobenzene

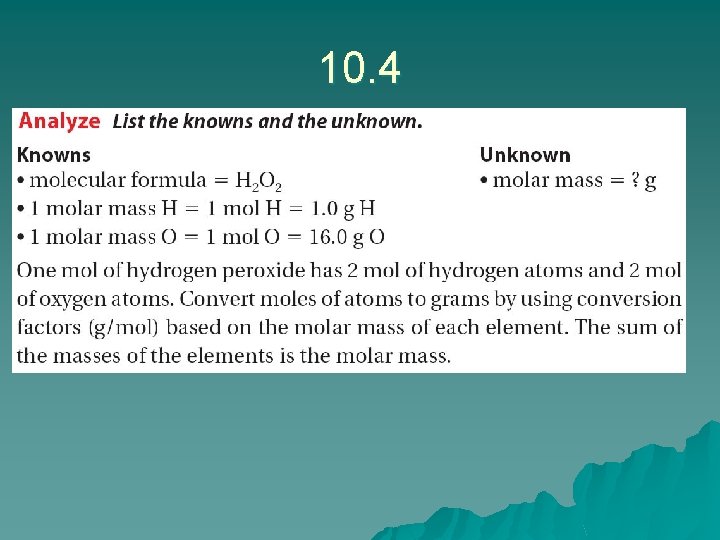
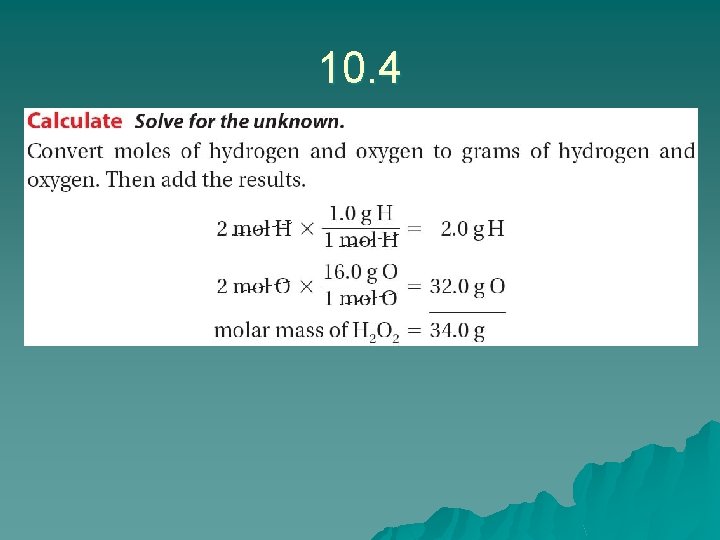
10. 4

10. 4

10. 4

10. 4

for Sample Problem 10. 4 Problem Solving 10. 7 Solve Problem 7 with the help of an interactive guided tutorial.

END OF SHOW
 Gram to gram conversion
Gram to gram conversion Stoichiometry mole-mole
Stoichiometry mole-mole Mole mole factor
Mole mole factor Grams to moles formula
Grams to moles formula Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Mole-mass-volume relationships
Mole-mass-volume relationships Phosphorus + oxygen equation
Phosphorus + oxygen equation Mole cookies chemistry
Mole cookies chemistry Mole cookies chemistry
Mole cookies chemistry What's a mole chemistry
What's a mole chemistry Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Moles to grans
Moles to grans Molar map
Molar map Mole bridge
Mole bridge Limiting reactant
Limiting reactant Moles formula in chemistry
Moles formula in chemistry Mole bridge chemistry
Mole bridge chemistry Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Mole hill chemistry
Mole hill chemistry Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Phép trừ bù
Phép trừ bù Số nguyên tố là số gì
Số nguyên tố là số gì Phối cảnh
Phối cảnh Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em ưu thế lai là gì
ưu thế lai là gì Sơ đồ cơ thể người
Sơ đồ cơ thể người Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Tư thế ngồi viết
Tư thế ngồi viết Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Công thức tiính động năng
Công thức tiính động năng Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Một số thể thơ truyền thống
Một số thể thơ truyền thống Diễn thế sinh thái là
Diễn thế sinh thái là Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Lp html
Lp html