Chapter 5 The Structure and Function of Macromolecules




































- Slides: 71

Chapter 5 The Structure and Function of Macromolecules

Macromolecules • There are many molecules that comprise living organisms and many of them are quite large. • As such, they are called macromolecules. • There are 4 main macromolecules that comprise living organisms: – 1. Proteins. – 2. Lipids. – 3. Carbohydrates. – 4. Nucleic acids.

Macromolecules • Three of the four classes (carbohydrates, protein, and nucleic acids) are comprised of polymers which are long molecules made up of the same or similar subunits covalently linked together. • The subunits that are linked together are called monomers.

Macromolecules • The synthesis and breakdown of macromolecules is governed by a series of condensation/dehydration or hydrolysis reactions.

Condensation Reactions • In a condensation reaction (aka a dehydration reaction) monomers are added to a growing polymer and a water molecule is given off--hence the name dehydration reaction. • These reactions require energy and the action is carried out by enzymes.


Hydrolysis • Hydrolysis is the process that adds a water molecule to a polymer giving one of the monomers. • This occurs during digestion and it makes the large molecules useable by our bodies by producing smaller subunits that our cells can uptake. • The four main types of macromolecules can be broken down further and analyzed.


Polymers • Polymers

Carbohydrates • Carbohydrates are molecules consisting of carbon, hydrogen, and oxygen. • The three classifications are monosaccharides or simple sugars, disaccharides or double sugars, and polysaccharides which are 3 or more sugars linked together forming a sugar polymer.

Monosaccharides • Monosaccharides generally have the formula Cn. H 2 n. On and depending on the location of the carbonyl group, the sugar is either an aldose or a keytone. • When the carbonyl group is the last carbon on the chain, it is an aldose, when it is not, it is a keytone.

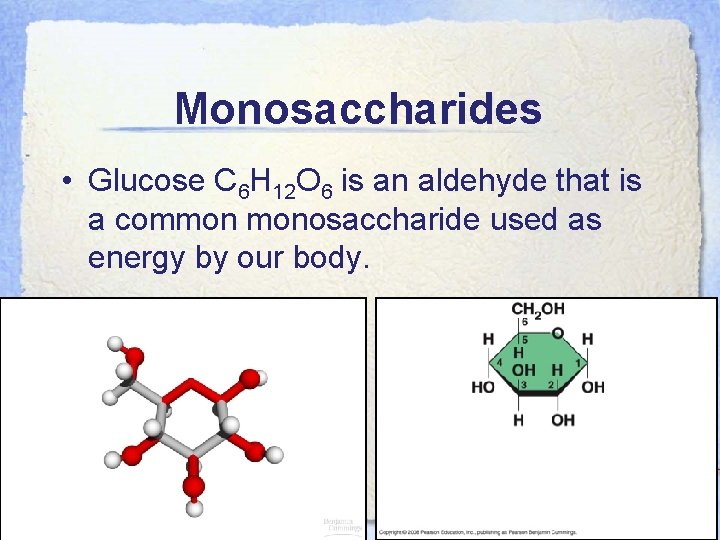
Monosaccharides • Glucose C 6 H 12 O 6 is an aldehyde that is a common monosaccharide used as energy by our body.

Monosaccharides • Not only is it used as an energy source (as are other monosaccharides), but their carbon skeletons serve as building blocks for other major macromolecules within the organism-amino acids and fatty acids.

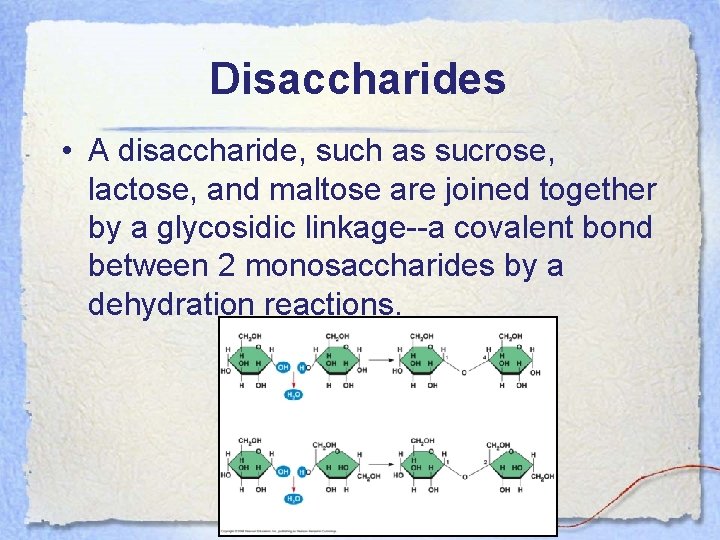
Disaccharides • A disaccharide, such as sucrose, lactose, and maltose are joined together by a glycosidic linkage--a covalent bond between 2 monosaccharides by a dehydration reactions.

Disaccharides • The fruit of a plant is often a great source of sucrose--glucose and fructose (the most common disaccharide) because as the plants form it during photosynthesis, this is where it gets stored.

Disaccharides • Disaccharides

Polysaccharides • When monosaccharides are glycosidically linked together thousands of times, polysaccharides are formed. • There are 2 main types of polysaccharides, structural and storage.

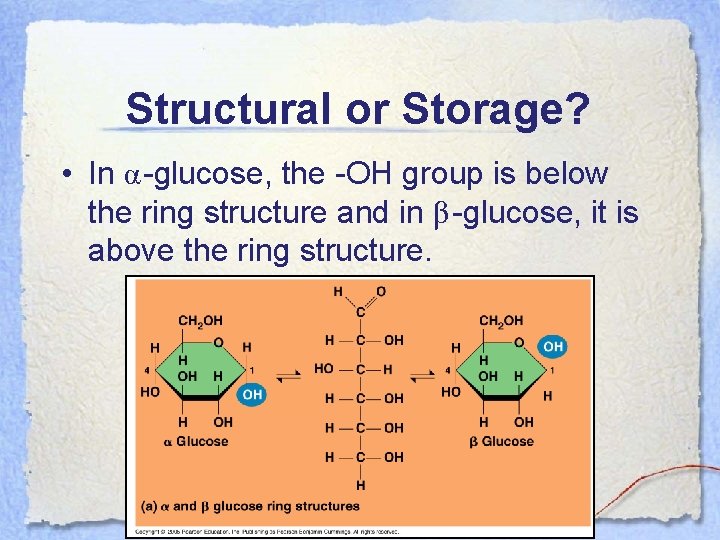
Structural or Storage? • There actually 2 forms of glucose, α and β, and the form is determined by the placement of a hydroxyl group.

Structural or Storage? • In α-glucose, the -OH group is below the ring structure and in β-glucose, it is above the ring structure.

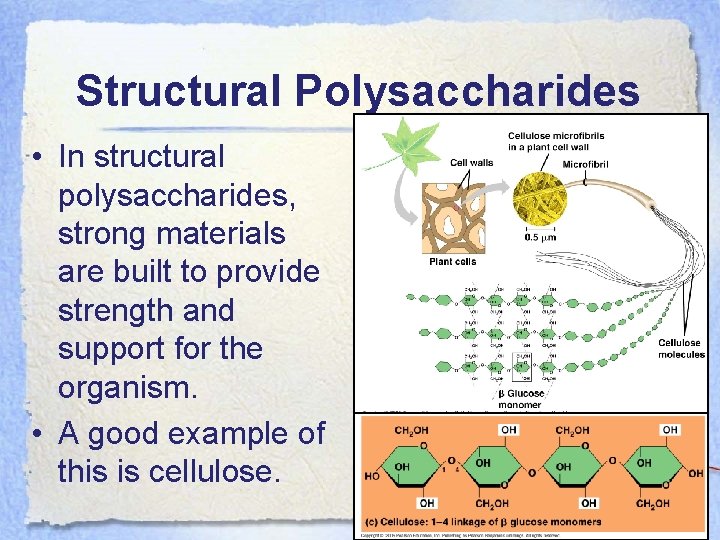
Structural Polysaccharides • In structural polysaccharides, strong materials are built to provide strength and support for the organism. • A good example of this is cellulose.

Structural Polysaccharides • Plant cell walls are comprised of cellulose which gives them strength. Some animals such as cows can digest cellulose, but humans can’t. • For us, it is called “insoluble fiber” and is found in many fruits and vegetables.

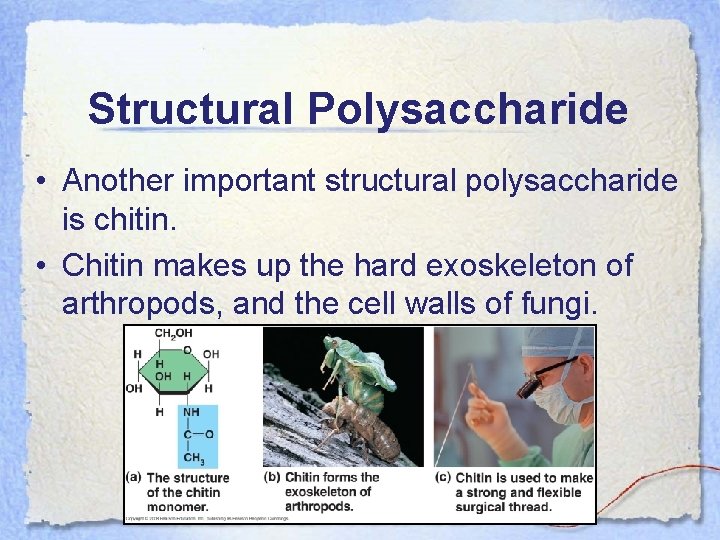
Structural Polysaccharide • Another important structural polysaccharide is chitin. • Chitin makes up the hard exoskeleton of arthropods, and the cell walls of fungi.

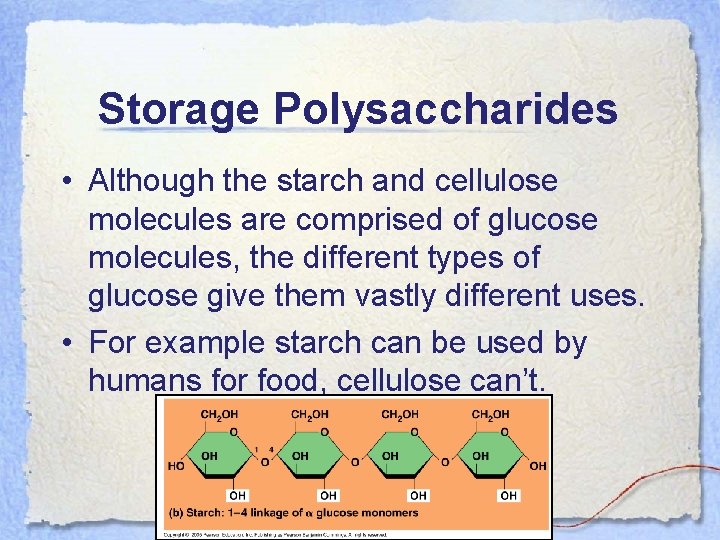
Storage Polysaccharides • Although the starch and cellulose molecules are comprised of glucose molecules, the different types of glucose give them vastly different uses. • For example starch can be used by humans for food, cellulose can’t.

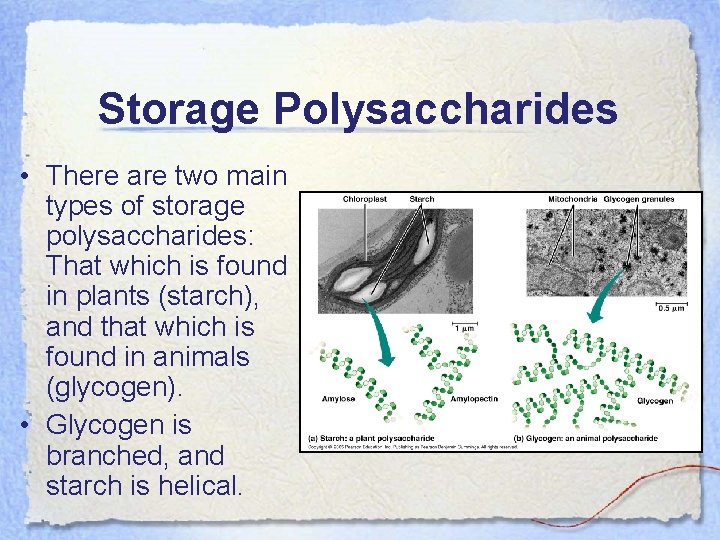
Storage Polysaccharides • There are two main types of storage polysaccharides: That which is found in plants (starch), and that which is found in animals (glycogen). • Glycogen is branched, and starch is helical.

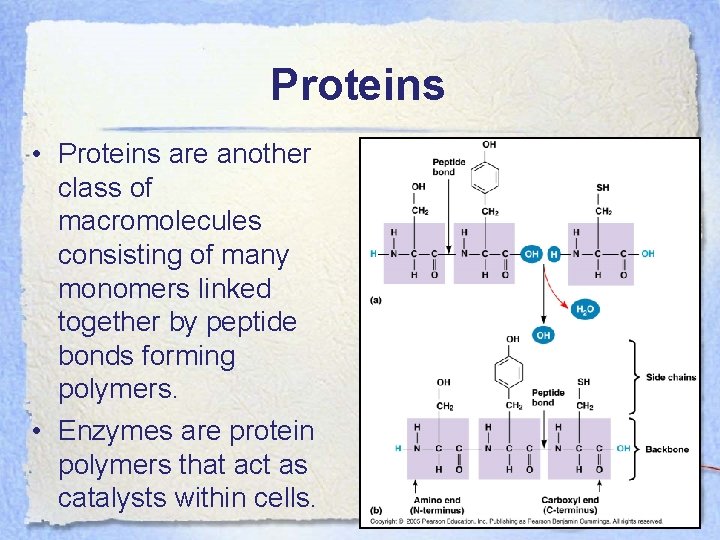
Proteins • Proteins are another class of macromolecules consisting of many monomers linked together by peptide bonds forming polymers. • Enzymes are protein polymers that act as catalysts within cells.

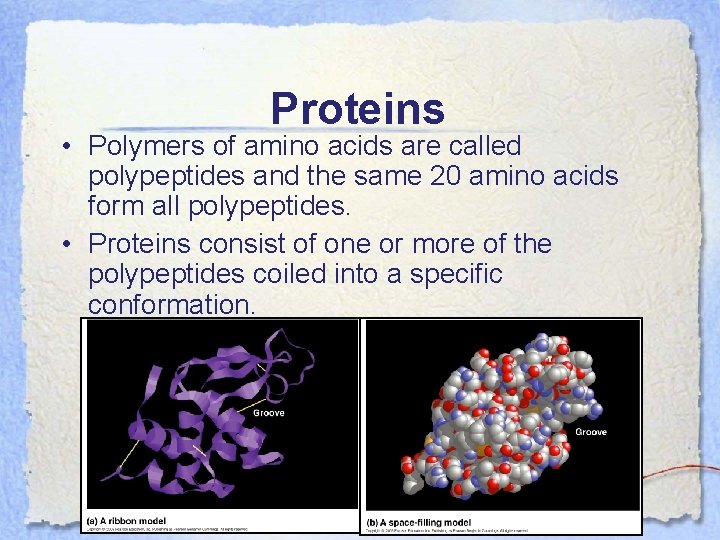
Proteins • Polymers of amino acids are called polypeptides and the same 20 amino acids form all polypeptides. • Proteins consist of one or more of the polypeptides coiled into a specific conformation.

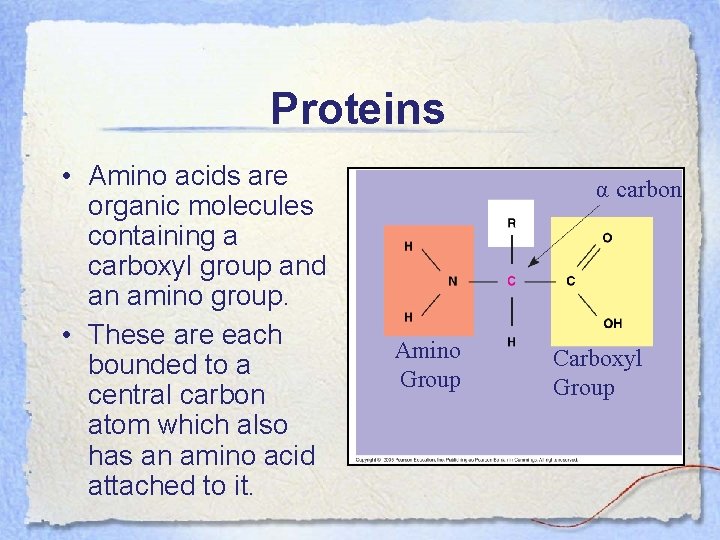
Proteins • Amino acids are organic molecules containing a carboxyl group and an amino group. • These are each bounded to a central carbon atom which also has an amino acid attached to it. α carbon Amino Group Carboxyl Group

Proteins • There are 2 groups that are linked together to form a peptide bond. • A peptide bond is a dehydration reaction which links the amino group of one amino acid to the carboxyl group of a different amino acid yielding water in the process.


Proteins • Once amino acids are added to the polypeptide, they begin to interact with the other amino acids on the growing polypeptide. • The sequence then determines the 3 D conformation the protein will take. • The conformation of the protein determines how it will function.

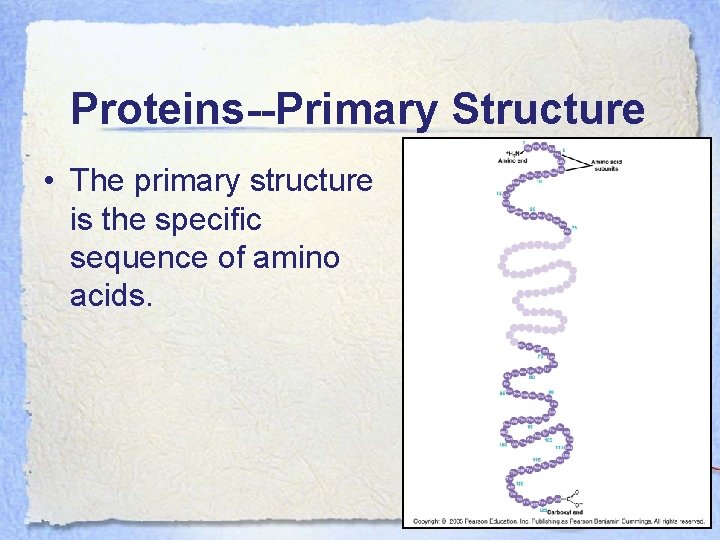
Proteins • An enzyme is an example and the conformation it takes determines how it functions when it interacts with a substrate. • There are 4 different types of protein structure: 1°, 2°, 3°, and 4°.

Proteins--Primary Structure • The primary structure is the specific sequence of amino acids.

Proteins--Primary Structure • Primary Structure

Proteins--Primary Structure • Primary Structure

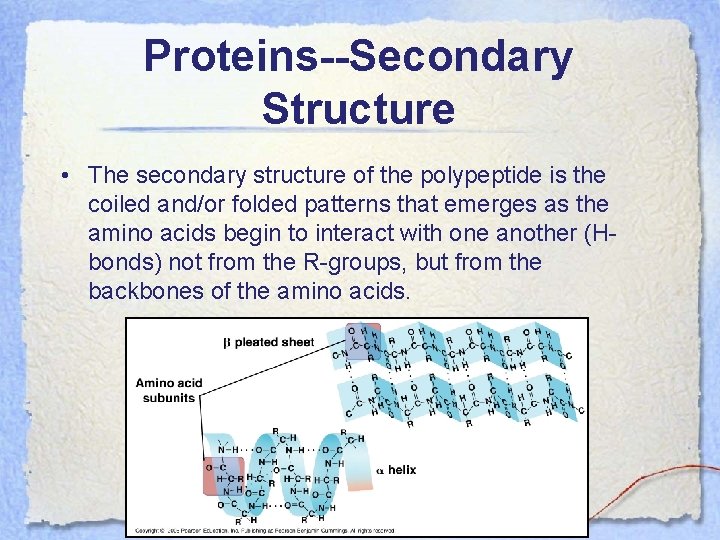
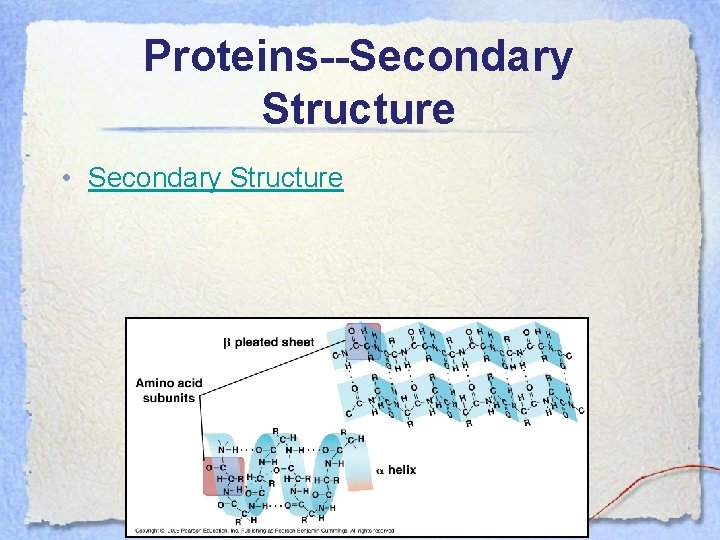
Proteins--Secondary Structure • The secondary structure of the polypeptide is the coiled and/or folded patterns that emerges as the amino acids begin to interact with one another (Hbonds) not from the R-groups, but from the backbones of the amino acids.

Proteins--Secondary Structure • Secondary Structure

Proteins--Secondary Structure • There are two main types of secondary protein structure, the α-helix and the βpleated sheet. • The α-helix is a delicate coil held together by H-bonds. • The β-pleated sheet forms when 2 polypeptides are aligned side by side and hydrogen bond along their lengths.

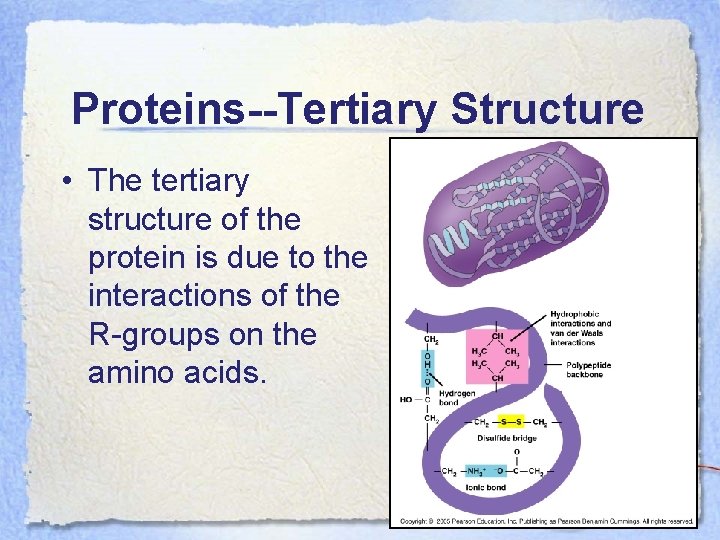
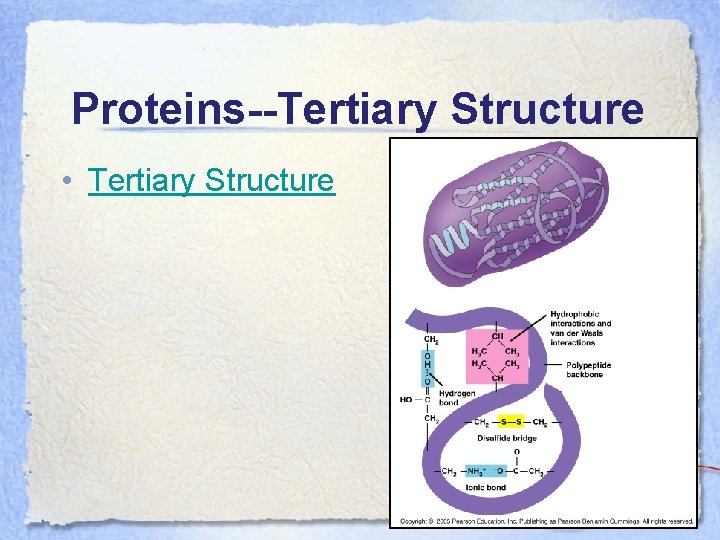
Proteins--Tertiary Structure • The tertiary structure of the protein is due to the interactions of the R-groups on the amino acids.

Proteins--Tertiary Structure • Tertiary Structure

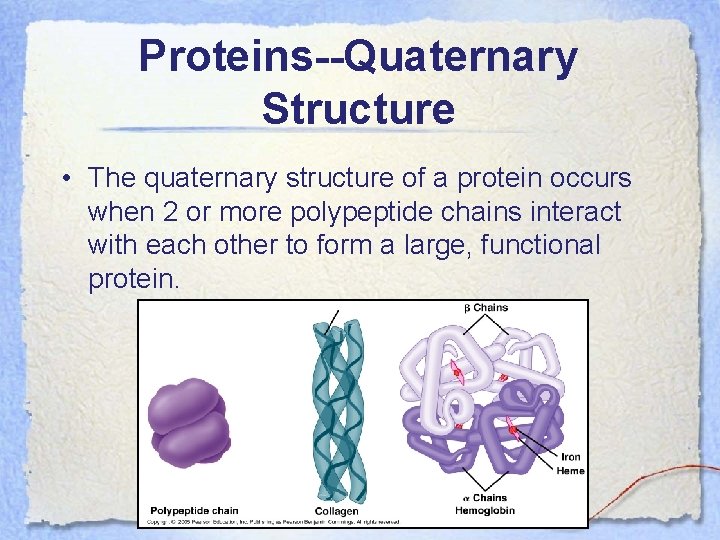
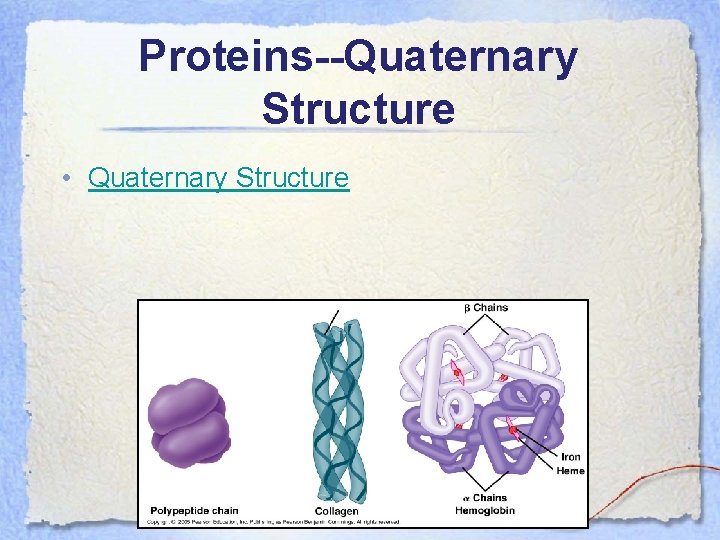
Proteins--Quaternary Structure • The quaternary structure of a protein occurs when 2 or more polypeptide chains interact with each other to form a large, functional protein.

Proteins--Quaternary Structure • Quaternary Structure

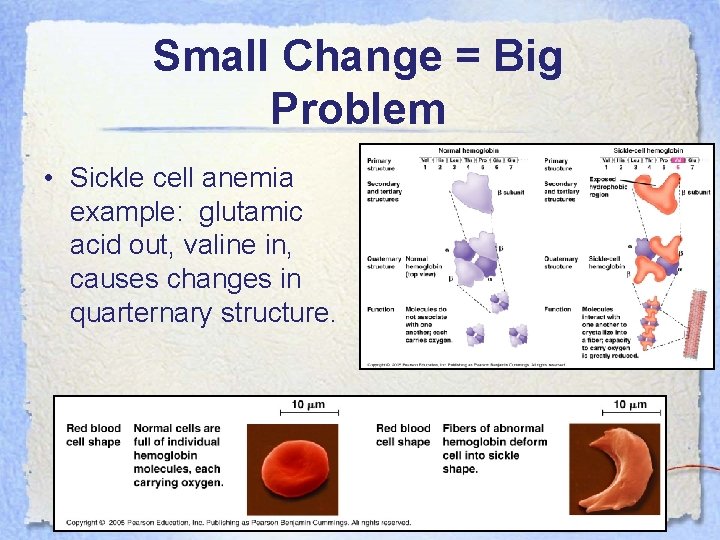
Small Change = Big Problem • Sickle cell anemia example: glutamic acid out, valine in, causes changes in quarternary structure.

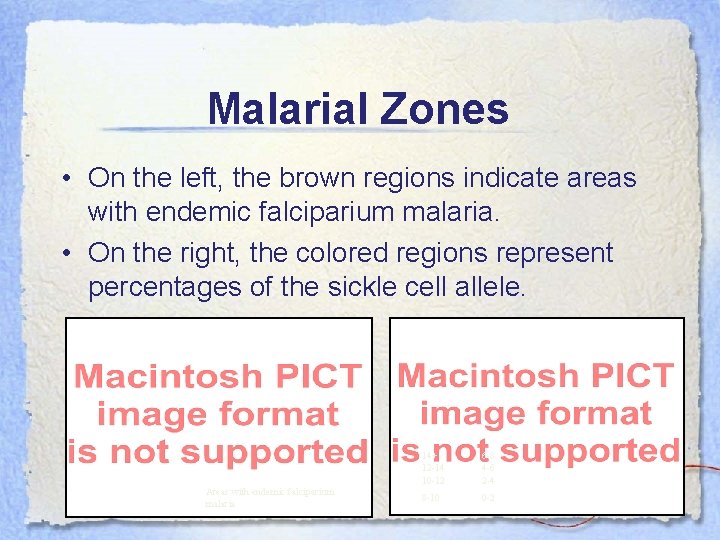
Malarial Zones • On the left, the brown regions indicate areas with endemic falciparium malaria. • On the right, the colored regions represent percentages of the sickle cell allele. Areas with endemic falciparium malaria 14+ 12 -14 10 -12 6 -8 4 -6 2 -4 8 -10 0 -2

Nucleic Acids • Nucleic acids store and transmit information about a protein’s primary structure. • There are 2 types of nucleic acids: RNA and DNA. • DNA provides the necessary information for guiding its own replication. • It also guides RNA synthesis and using RNA controls protein synthesis.

Nucleic Acids • DNA is the information that controls the cell. This information is used to create messenger RNA that interacts with the cells protein synthesizing machinery (ribosomes) to create the proteins that run the cell. • DNA--> RNA --> Protein


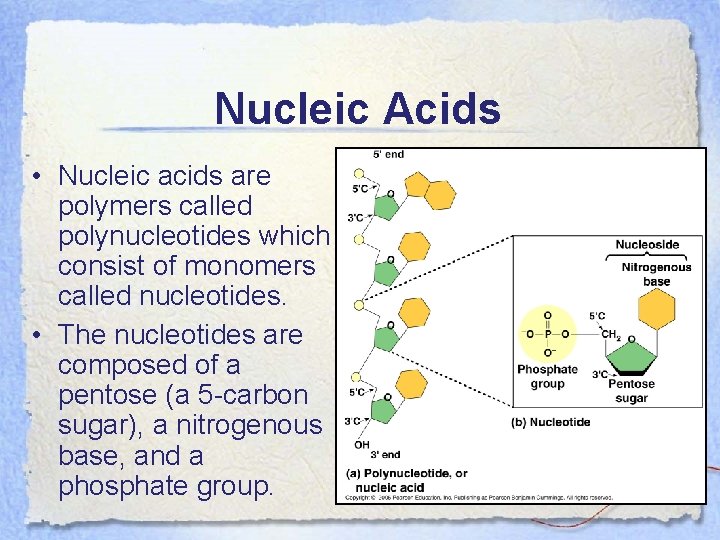
Nucleic Acids • Nucleic acids are polymers called polynucleotides which consist of monomers called nucleotides. • The nucleotides are composed of a pentose (a 5 -carbon sugar), a nitrogenous base, and a phosphate group.

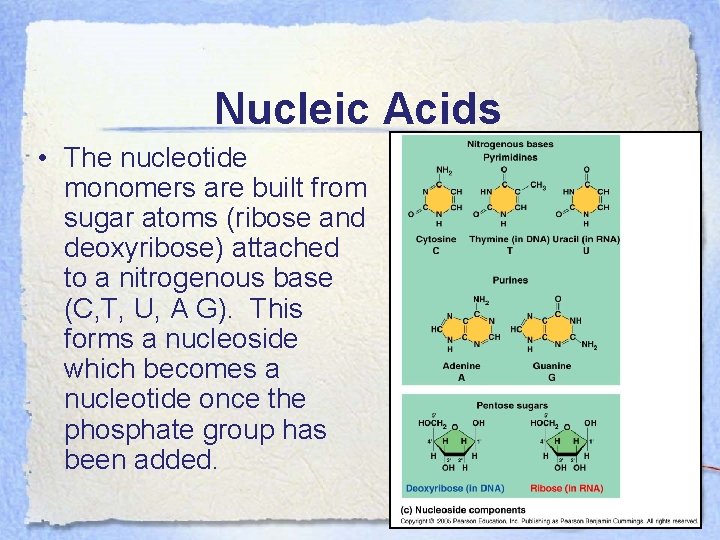
Nucleic Acids • The nucleotide monomers are built from sugar atoms (ribose and deoxyribose) attached to a nitrogenous base (C, T, U, A G). This forms a nucleoside which becomes a nucleotide once the phosphate group has been added.

Nucleic Acids • Nucleotides are linked together by covalent (phosphodiester) bonds between a hydroxyl group on the 3’ carbon of one nucleotide and the phosphate on the 5’ carbon on the next. • This bond starts the repeating sugarphosphate bond. This gives rise to the 3’ and 5’ ends of the DNA molecule. The sequence of each of the bases in the polynucleotide is unique to each gene.

Nucleic Acids • RNA consists of a single strand of nucleic acids in a polynucleotide chain. • DNA, on the other hand, consists of 2 polynucleotide chains that run in opposite directions to one another. • This gives rise to the term “antiparallel. ”

Nucleic Acids • The 2 anti-parallel strands of DNA appear like a divided highway and are joined together by hydrogen bonds and Van der Waals interactions. • H-bonds are between paired bases • Van der Waals interactions are between stacked bases.


Nucleic Acids • There are certain bases that you find in DNA and RNA. • DNA has A, T, C, G • RNA has A, U, C, G • When the bases pair together, they only pair one way: A & T, C & G in DNA, and in RNA A pairs with U, C pairs with G.

Nucleic Acids • The two strands are always said to be complementary. That is, if you know the order of one strand of DNA, you can deduce the other. • 5’ ----> 3’ • AGGTCCG • TCCAGGC • 3’<---- 5’

Nucleic Acids • This feature of DNA is what makes precise copying possible. • When cells divide, the DNA serves as a template and the new DNA that is formed is copied from it. • When finished, there are 2 identical copies (ideally) of DNA within the cell and identical daughter cells can now form upon division.

Nucleic Acids • The parts of the DNA molecule that make up the polynucleotides that encode for the amino acids can be used to show closely organisms are related from an evolutionary standpoint. • Molecular biologists can sequence genes and determine how much difference there is between organisms and this helps them form their tree of life.

Nucleic Acids • For example, upon examination of the polypeptide chain of human hemoglobin with that of a gorilla, we only find one amino acid different out of 146. • In gibbons, which are a tree monkey found in SE Asia, there is only a difference of 2 amino acids.

Lipids • • There are three classes of lipids: Fats (Fatty acids) Phospholipids Steroids

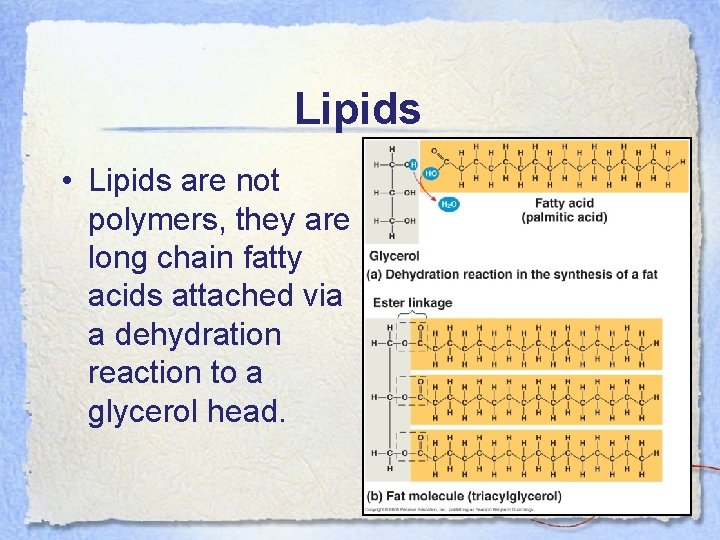
Lipids • Lipids are not polymers, they are long chain fatty acids attached via a dehydration reaction to a glycerol head.

Lipids • Lipids

Lipids • The glycerol molecule is a 3 carbon alcohol with a hydroxyl group attached to each carbon. During the reaction, the carboxyl group at the end of the fatty acid loses its hydroxyl (-OH) and attaches to the oxygen on the glycerol molecule. The -H lost from the glycerol combines with the -OH forming water.

Lipids • The fatty acid portion is a long hydrocarbon usually 16 to 18 carbons in length. • These portions are hydrophobic and this makes fats hydrophobic. • Fatty acids can be classified as saturated on unsaturated as we often hear about in nutrition.

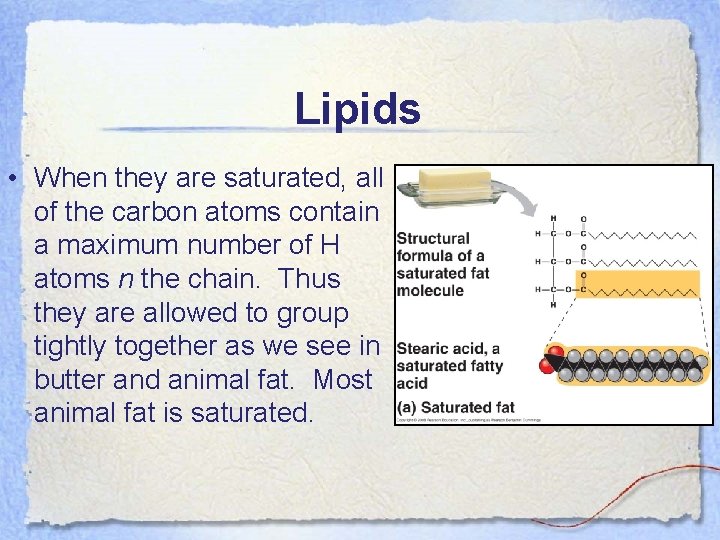
Lipids • When they are saturated, all of the carbon atoms contain a maximum number of H atoms n the chain. Thus they are allowed to group tightly together as we see in butter and animal fat. Most animal fat is saturated.

Lipids • Unsaturated fats contain carbon atoms that are double bonded to other carbon atoms in the fatty acid chain. This creates a kink in the chain and prevents the molecules from packing closely together.

Lipids • Such examples are olive oil and vegetable oil. • The oils of fish and vegetables generally are unsaturated.

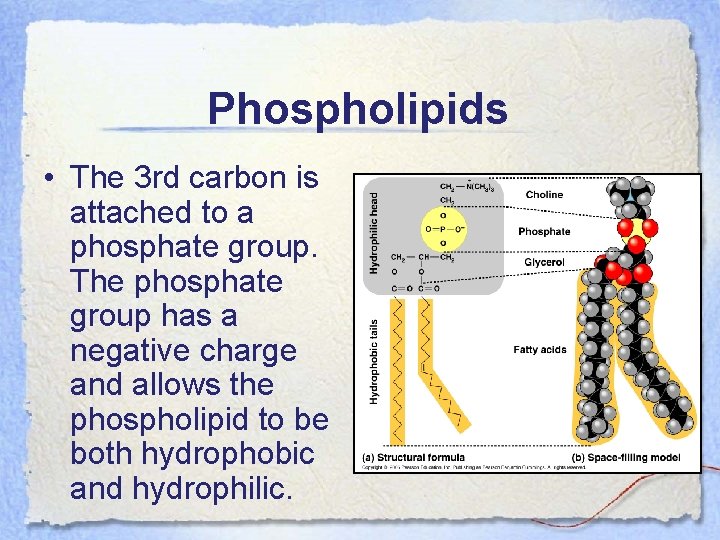
Phospholipids • Phospholipids are a very important molecule that are similar to fact except that they only have 2 fatty acids attached to the glycerol head.

Phospholipids • The 3 rd carbon is attached to a phosphate group. The phosphate group has a negative charge and allows the phospholipid to be both hydrophobic and hydrophilic.

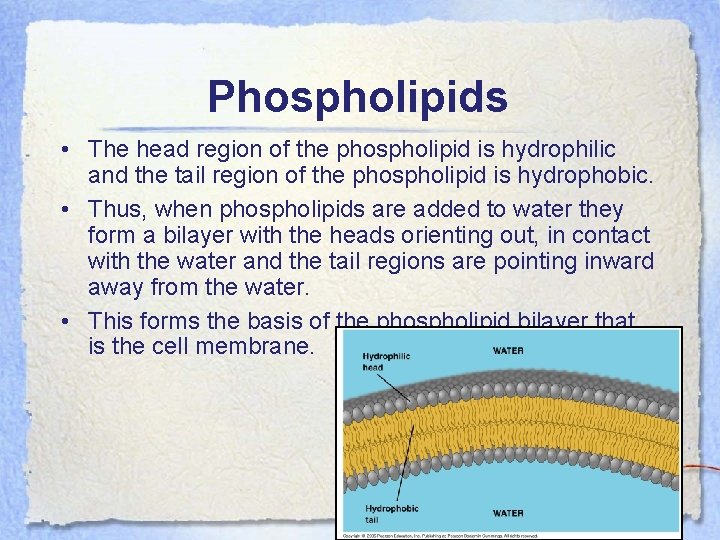
Phospholipids • The head region of the phospholipid is hydrophilic and the tail region of the phospholipid is hydrophobic. • Thus, when phospholipids are added to water they form a bilayer with the heads orienting out, in contact with the water and the tail regions are pointing inward away from the water. • This forms the basis of the phospholipid bilayer that is the cell membrane.

Steroids • Steroids are a final class of lipids that we’ll discuss. They are comprised of 4 fused rings and have various functional groups attached to them.

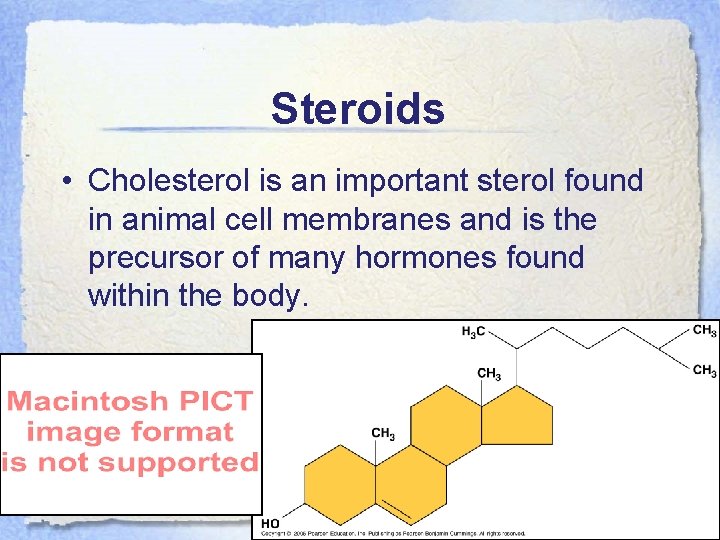
Steroids • Cholesterol is an important sterol found in animal cell membranes and is the precursor of many hormones found within the body.

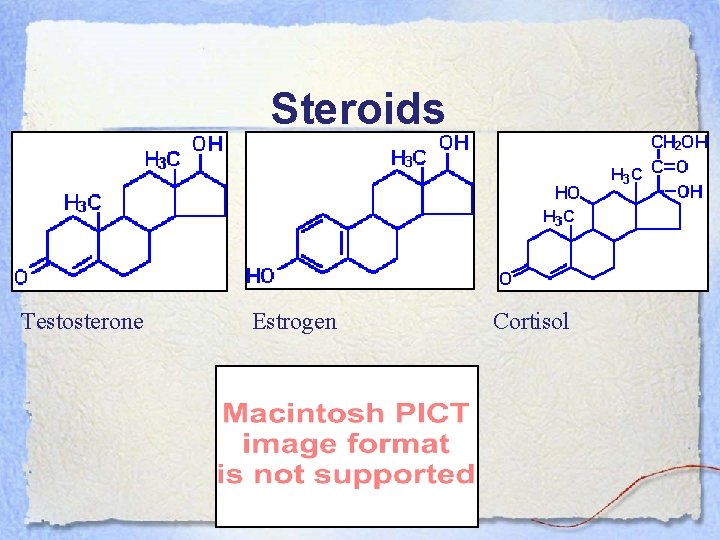
Steroids Testosterone Estrogen Cortisol