CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES


- Slides: 20

CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES Polymer principles And Macromolecules Page 62 1

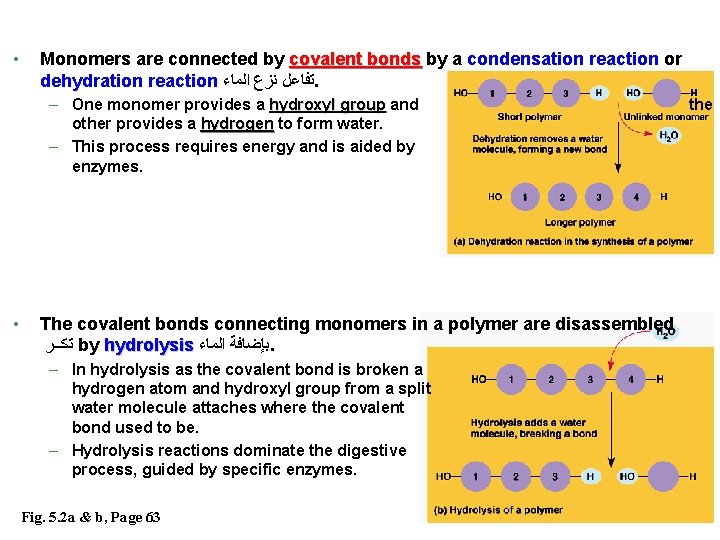
Introduction (Polymers principles) • Cells join ﺗﺮﺑﻂ smaller organic molecules together to form larger molecules. • These larger molecules called macromolecules, macromolecules may be composed of thousands of atoms and weigh over 100, 000 daltons. • The four major classes of macromolecules are: carbohydrates, lipids, proteins, and nucleic acids ( Will be studied later) • Three of the four classes of macromolecules form chainlike ﺷﻜﻞ ﺍﻟﺴﻠﺴﻠﺔ molecules called polymers. • Most macromolecules are polymers – Polymers consist of many similar or identical building blocks linked by covalent bonds • The repeated units are small molecules called monomers 2

• Monomers are connected by covalent bonds by a condensation reaction or dehydration reaction ﺗﻔﺎﻋﻞ ﻧﺰﻉ ﺍﻟﻤﺎﺀ. – One monomer provides a hydroxyl group and other provides a hydrogen to form water. – This process requires energy and is aided by enzymes. • the The covalent bonds connecting monomers in a polymer are disassembled ﺗﻛــﺮ by hydrolysis ﺑﺈﺿﺎﻓﺔ ﺍﻟﻤﺎﺀ. – In hydrolysis as the covalent bond is broken a hydrogen atom and hydroxyl group from a split water molecule attaches where the covalent bond used to be. – Hydrolysis reactions dominate the digestive process, guided by specific enzymes. Fig. 5. 2 a & b, Page 63 3

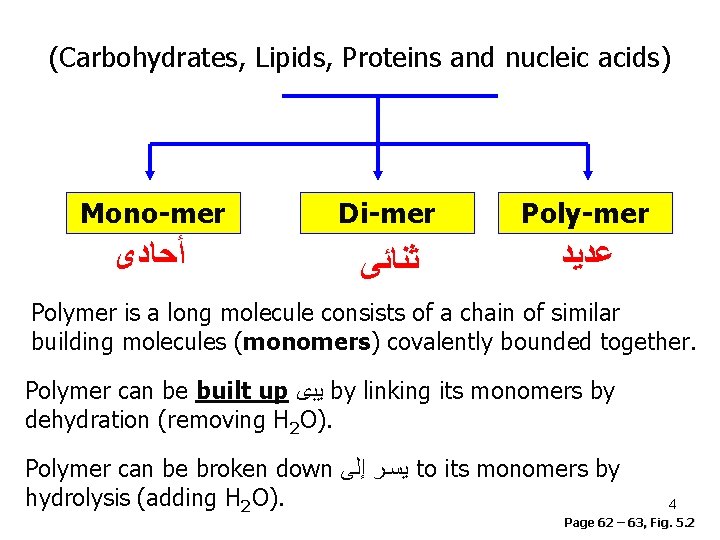
(Carbohydrates, Lipids, Proteins and nucleic acids) Mono-mer Di-mer Poly-mer ﺃﺤﺎﺩﻯ ﺛﻨﺎﺋﻰ ﻋﺪﻳﺪ Polymer is a long molecule consists of a chain of similar building molecules (monomers) covalently bounded together. Polymer can be built up ﻳﺑﻯ by linking its monomers by dehydration (removing H 2 O). Polymer can be broken down ﻳﺳـﺭ ﺇﻟﻰ to its monomers by hydrolysis (adding H 2 O). 4 Page 62 – 63, Fig. 5. 2

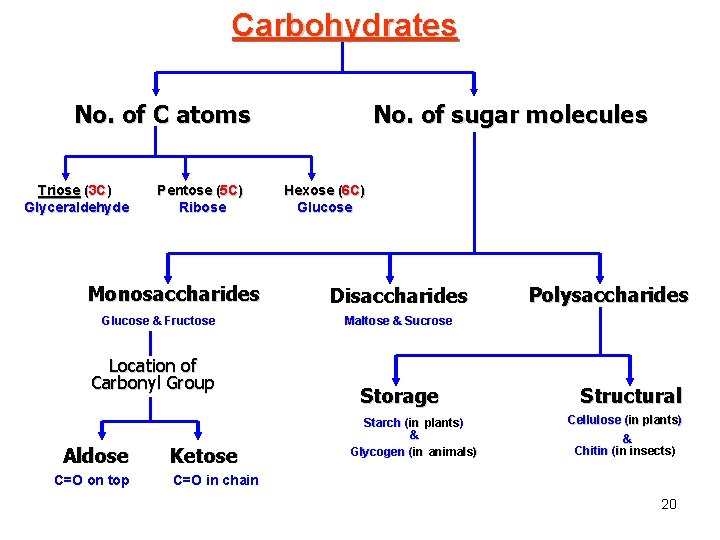
1 - Carbohydrates Fuel and Building Material ( ) ﻣﺎﺩﺓ ﺍﻟﻄﺎﻗﺔ ﻭ ﺍﻟﺒﻨﺎﺀ 1. Sugars, the smallest carbohydrates, serve as fuel and carbon sources 2. Polysaccharides, the polymers of sugars, have storage and structural roles Page 64 -68 5

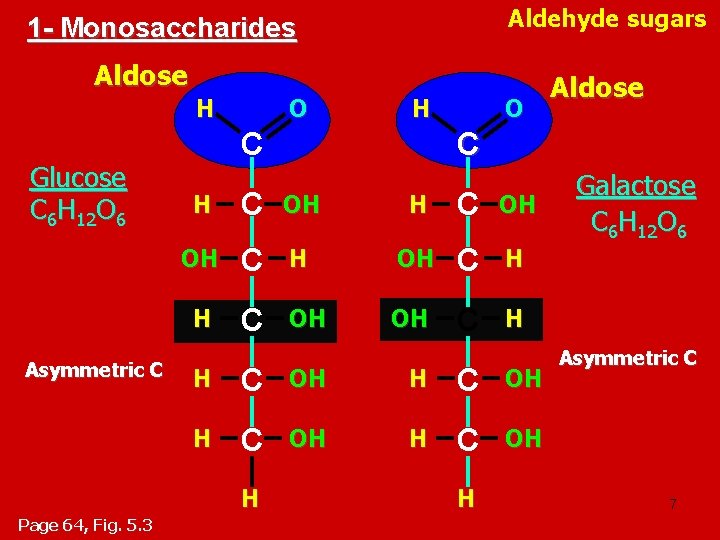
• Carbohydrates include sugars. • Monosaccharides, are the simplest carbohydrates (simple sugars). • Disaccharides, Disaccharides double sugars, consist of two monosaccharides joined by a condensation reaction (dehydration). • Polysaccharides, are polymers of monosaccharides. • Monosaccharides have a carbonyl group and multiple hydroxyl groups. – If the carbonly group (C=O) is at the end of C chain, the sugar is called aldose (aldehyde sugar), if not, the sugars is called ketose (Ketone sugar). ( ( – Glucose called “aldose”, and fructose called “ketose”. They are structural isomers. • Monosaccharides may also exist as enantiomers For example, glucose and galactose, both six-carbon aldoses, differ in the arrangement around asymmetrical carbons ﺫﺭﺓ ﺍﻟﻜﺮﺑﻮﻥ ﺍﻟﻐﻴﺮ ﻣﺘﻤﺎﺛﻠﺔ. • Monosaccharides, particularly glucose, are a major fuel for cellular work. 6

Aldehyde sugars 1 - Monosaccharides Aldose H Glucose C 6 H 12 O 6 Asymmetric C O C H C OH OH C H H C OH H Page 64, Fig. 5. 3 H Aldose H Galactose C 6 H 12 O 6 Asymmetric C 7

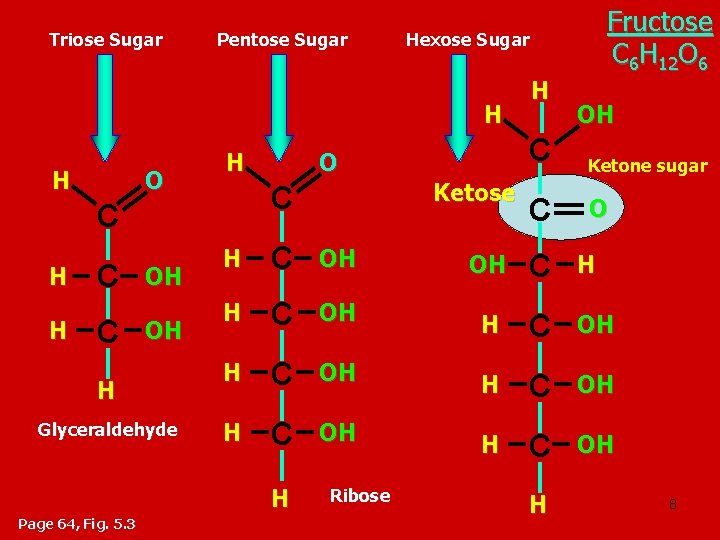
Triose Sugar Pentose Sugar Hexose Sugar H H O H C H H C C OH OH H Glyceraldehyde O H OH C Ketone sugar C O H C OH OH C H H C OH H C OH H Page 64, Fig. 5. 3 Ketose C Fructose C 6 H 12 O 6 Ribose H 8

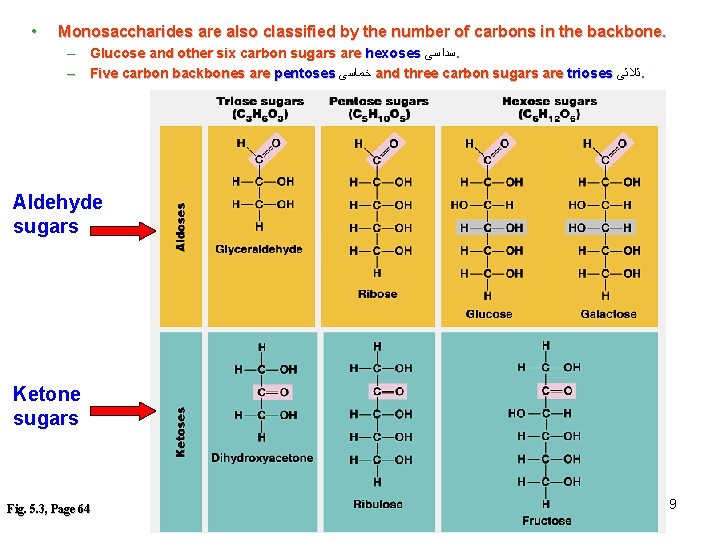
• Monosaccharides are also classified by the number of carbons in the backbone. – Glucose and other six carbon sugars are hexoses ﺳﺪﺍﺳﻰ. – Five carbon backbones are pentoses ﺧﻤﺎﺳﻰ and three carbon sugars are trioses ﺛﻼﺛﻰ. Aldehyde sugars Ketone sugars Fig. 5. 3, Page 64 9

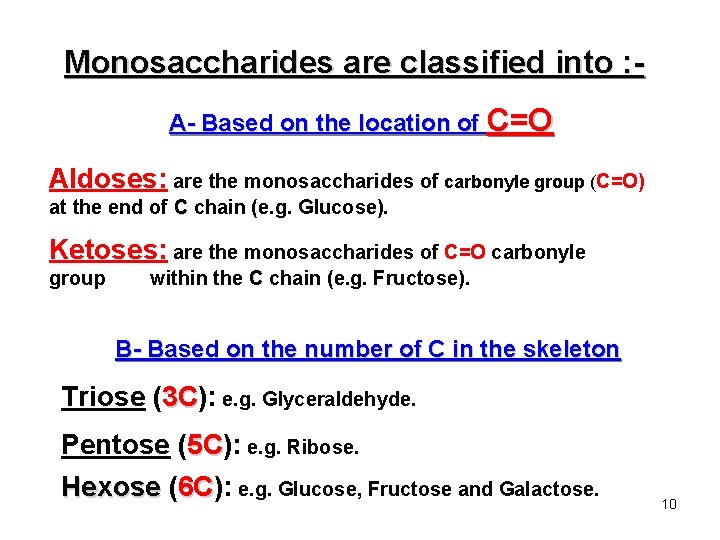
Monosaccharides are classified into : A- Based on the location of C=O Aldoses: are the monosaccharides of carbonyle group (C=O) at the end of C chain (e. g. Glucose). Ketoses: are the monosaccharides of C=O carbonyle group within the C chain (e. g. Fructose). B- Based on the number of C in the skeleton Triose (3 C): 3 C e. g. Glyceraldehyde. Pentose (5 C): 5 C e. g. Ribose. Hexose (6 C): 6 C e. g. Glucose, Fructose and Galactose. 10

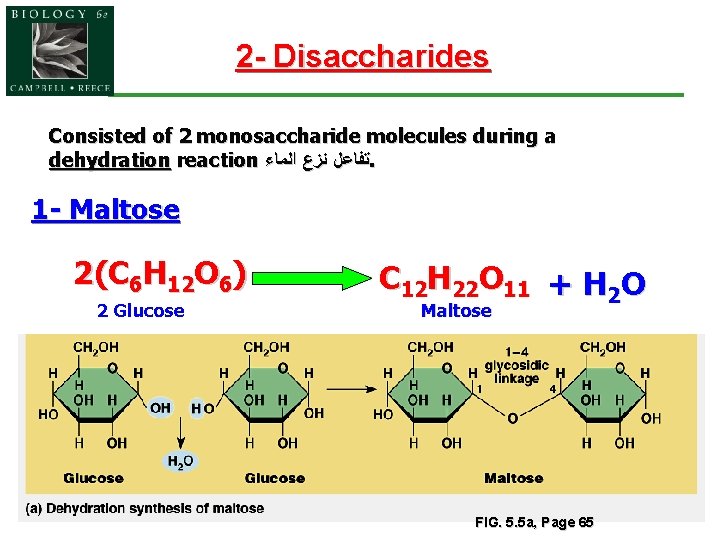
2 - Disaccharides Consisted of 2 monosaccharide molecules during a dehydration reaction ﺗﻔﺎﻋﻞ ﻧﺰﻉ ﺍﻟﻤﺎﺀ. 1 - Maltose 2(C 6 H 12 O 6) 2 Glucose C 12 H 22 O 11 + H 2 O Maltose 11 FIG. 5. 5 a, Page 65

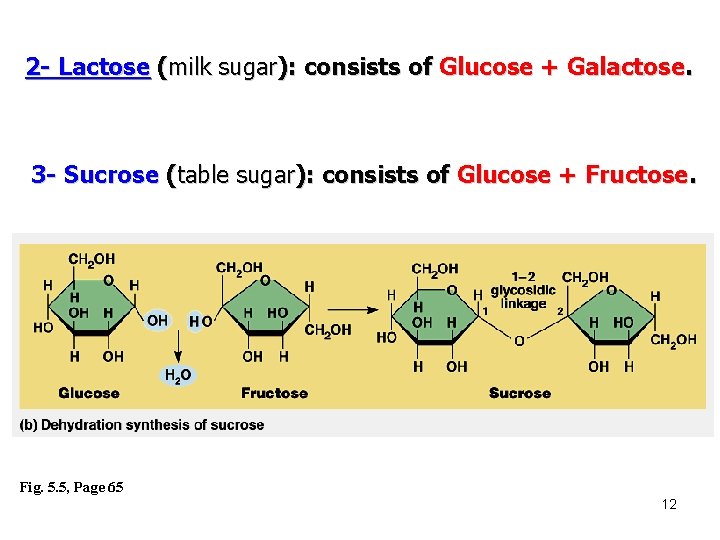
2 - Lactose (milk sugar): consists of Glucose + Galactose. 3 - Sucrose (table sugar): consists of Glucose + Fructose. Fig. 5. 5, Page 65 12

3 - Polysaccharides Consisted of few hundreds to few thousands of monosaccharides. They have storage ﺗﺨﺰﻳﻨﻴﺔ and structural ﺗﺮﻛﻴﺒﻴﺔ roles. Storage polysaccharides provide sugar for cell by hydrolysis. Structural polysaccharides serve as building materials for the organism. 13

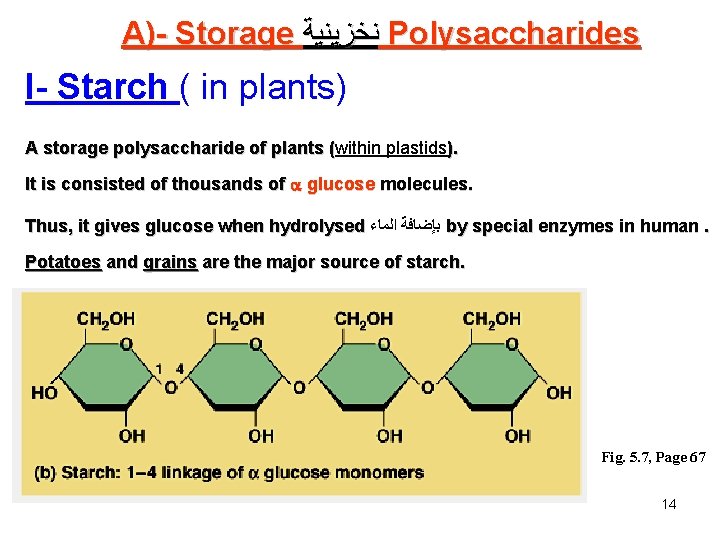
A)- Storage ﻧﺨﺰﻳﻨﻴﺔ Polysaccharides I- Starch ( in plants) A storage polysaccharide of plants (within plastids). ( It is consisted of thousands of glucose molecules. Thus, it gives glucose when hydrolysed ﺑﺈﺿﺎﻓﺔ ﺍﻟﻤﺎﺀ by special enzymes in human. Potatoes and grains are the major source of starch. Fig. 5. 7, Page 67 14

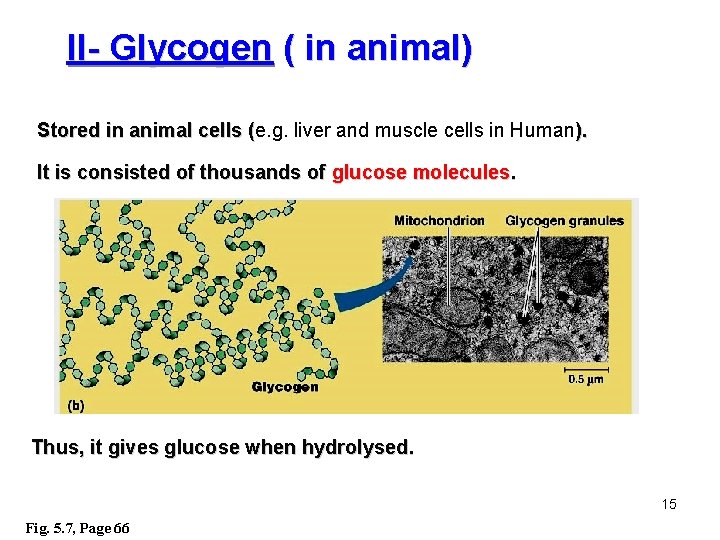
II- Glycogen ( in animal) Stored in animal cells (e. g. liver and muscle cells in Human). ( It is consisted of thousands of glucose molecules. Thus, it gives glucose when hydrolysed. 15 Fig. 5. 7, Page 66

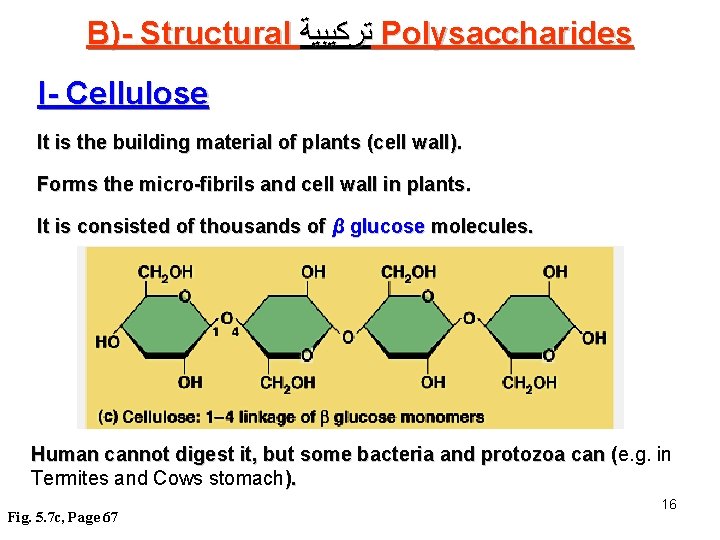
B)- Structural ﺗﺮﻛﻴﺒﻴﺔ Polysaccharides I- Cellulose It is the building material of plants (cell wall). Forms the micro-fibrils and cell wall in plants. It is consisted of thousands of β glucose molecules. Human cannot digest it, but some bacteria and protozoa can (e. g. in ( Termites and Cows stomach). Fig. 5. 7 c, Page 67 16

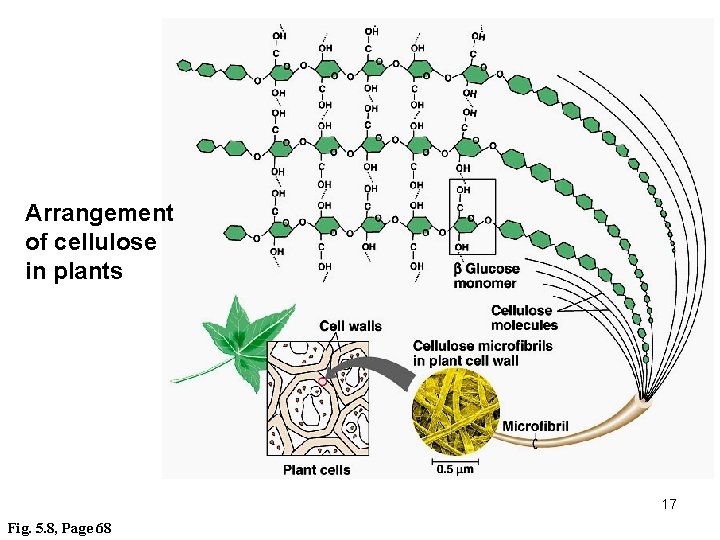
Arrangement of cellulose in plants 17 Fig. 5. 8, Page 68

• The enzymes that digest starch cannot hydrolyze the beta linkages in cellulose. – Cellulose in our food passes through the digestive tract and is eliminated in feces as “insoluble fiber”. – As it travels through the digestive tract, it abrades the intestinal walls and stimulates the secretion of mucus. • Some microbes can digest cellulose to its glucose monomers through the use of cellulase enzymes. • Many eukaryotic herbivores آﻜﻼﺕ ﺍﻟﻌﺸﺐ , like cows and termites, have symbiotic relationships with cellulolytic microbes, allowing them access to this 18 rich source of energy.

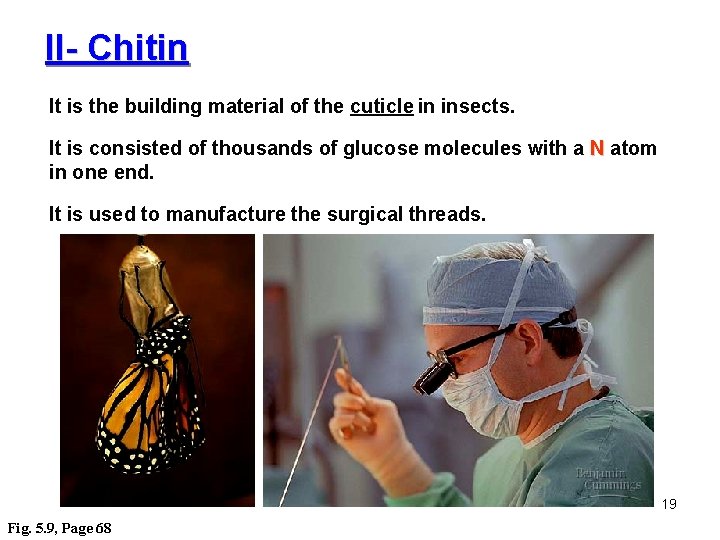
II- Chitin It is the building material of the cuticle in insects. It is consisted of thousands of glucose molecules with a N atom in one end. It is used to manufacture the surgical threads. 19 Fig. 5. 9, Page 68

Carbohydrates No. of C atoms Triose (3 C) Glyceraldehyde Pentose (5 C) Ribose Monosaccharides Glucose & Fructose Location of Carbonyl Group Aldose C=O on top Ketose No. of sugar molecules Hexose (6 C) Glucose Disaccharides Polysaccharides Maltose & Sucrose Storage Starch (in plants) & Glycogen (in animals) Structural Cellulose (in plants) & Chitin (in insects) C=O in chain 20