the structure and function of macromolecules macromolecules lipids












- Slides: 43

the structure and function of macromolecules

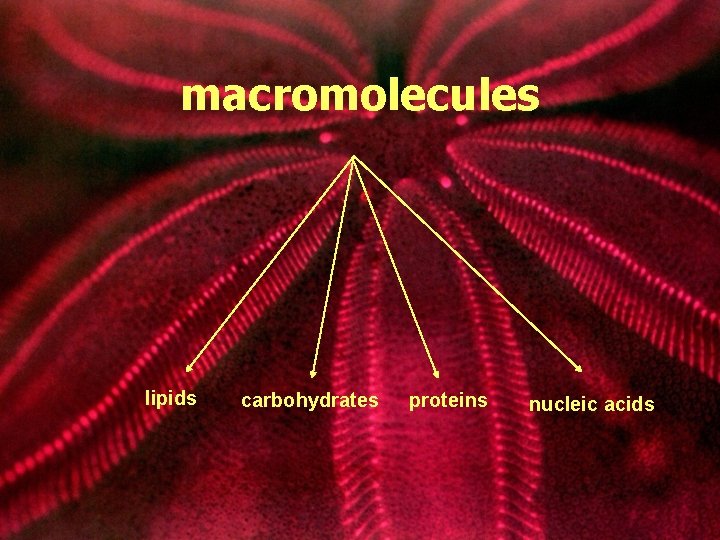
macromolecules lipids carbohydrates proteins nucleic acids

what is a polymer? poly = many, meris = part a chain-like molecule made up of repeating parts each link in the chain is called a monomer

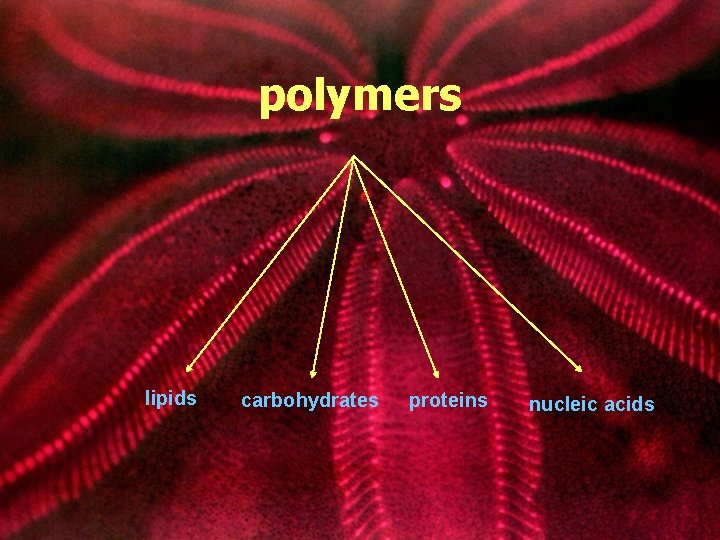
polymers lipids carbohydrates proteins nucleic acids

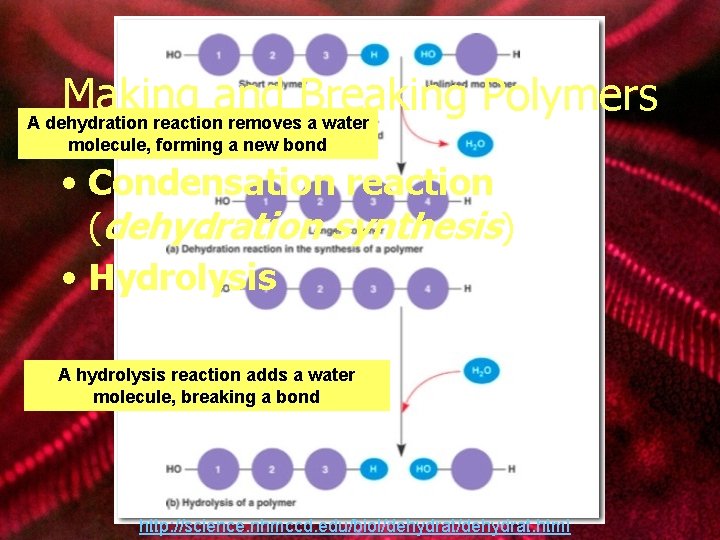
Making and Breaking Polymers A dehydration reaction removes a water molecule, forming a new bond • Condensation reaction (dehydration synthesis) • Hydrolysis A hydrolysis reaction adds a water molecule, breaking a bond http: //science. nhmccd. edu/biol/dehydrat. html

lipids • are not polymers • have little to no affinity for water • three most biologically important kinds are: • Fats • Phospholipids • Steroids

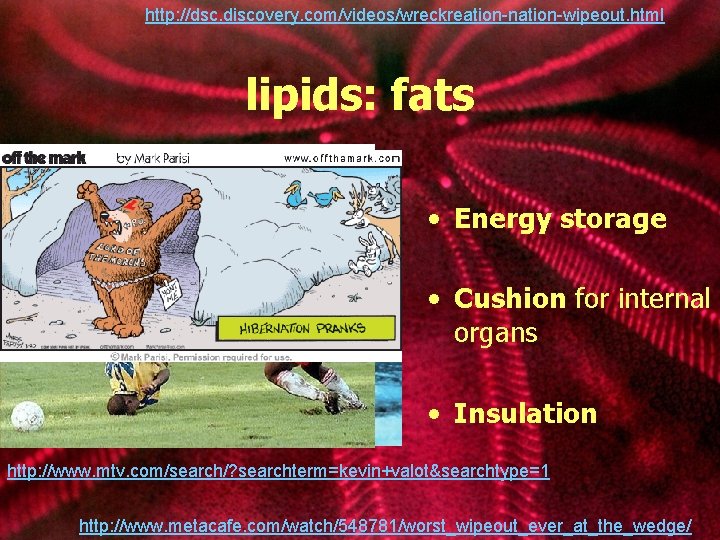
http: //dsc. discovery. com/videos/wreckreation-nation-wipeout. html lipids: fats • Energy storage • Cushion for internal organs • Insulation http: //www. mtv. com/search/? searchterm=kevin+valot&searchtype=1 http: //www. metacafe. com/watch/548781/worst_wipeout_ever_at_the_wedge/

lipids: fats

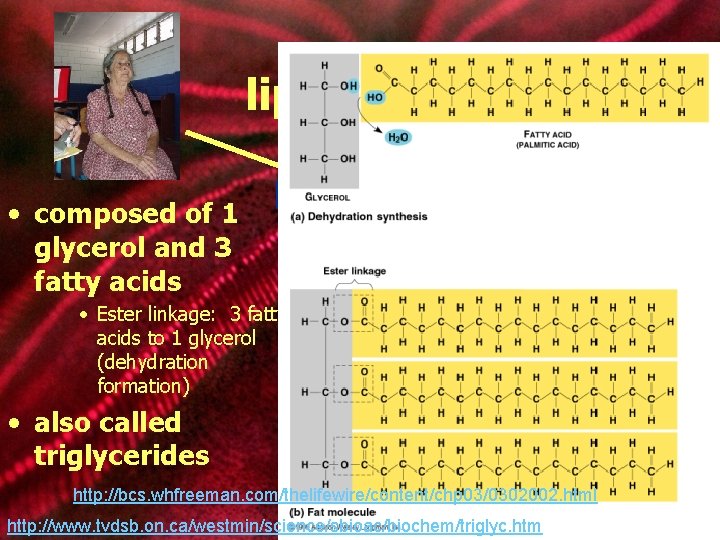
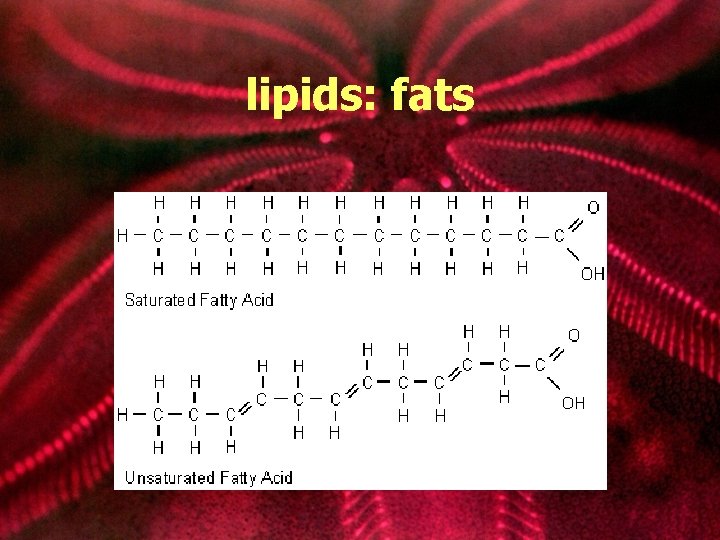
lipids: fats • composed of 1 glycerol and 3 fatty acids ester linkage • Ester linkage: 3 fatty acids to 1 glycerol (dehydration formation) • also called triglycerides glycerol fatty acid chains http: //bcs. whfreeman. com/thelifewire/content/chp 03/0302002. html fat molecule http: //www. tvdsb. on. ca/westmin/science/sbioac/biochem/triglyc. htm

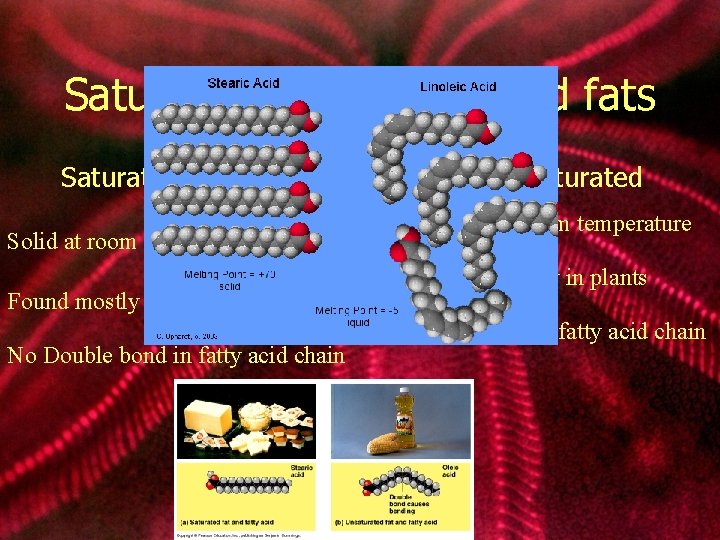
Saturated vs. Unsaturated fats Saturated Solid at room temperature Found mostly in animals No Double bond in fatty acid chain Unsaturated Liquid at room temperature Found mostly in plants Double bound in fatty acid chain

lipids: fats

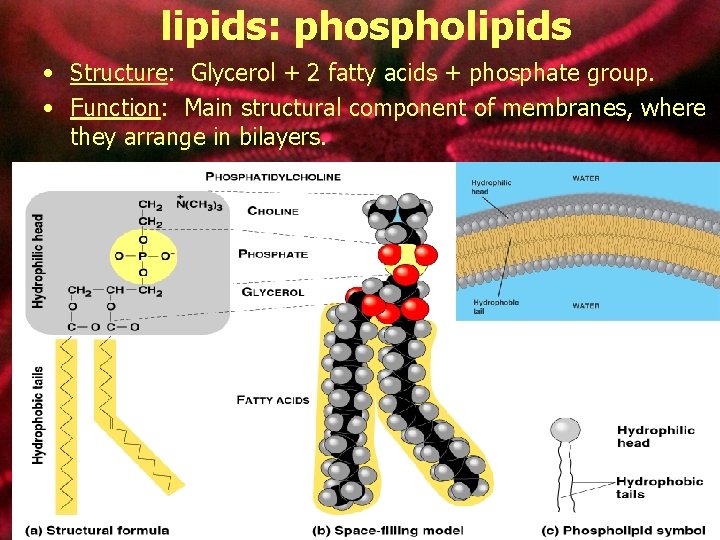
lipids: phospholipids • Structure: Glycerol + 2 fatty acids + phosphate group. • Function: Main structural component of membranes, where they arrange in bilayers.

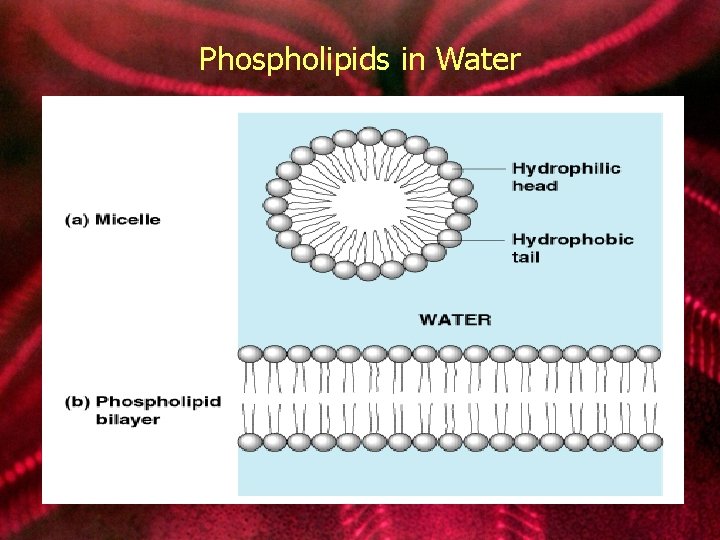
Phospholipids in Water

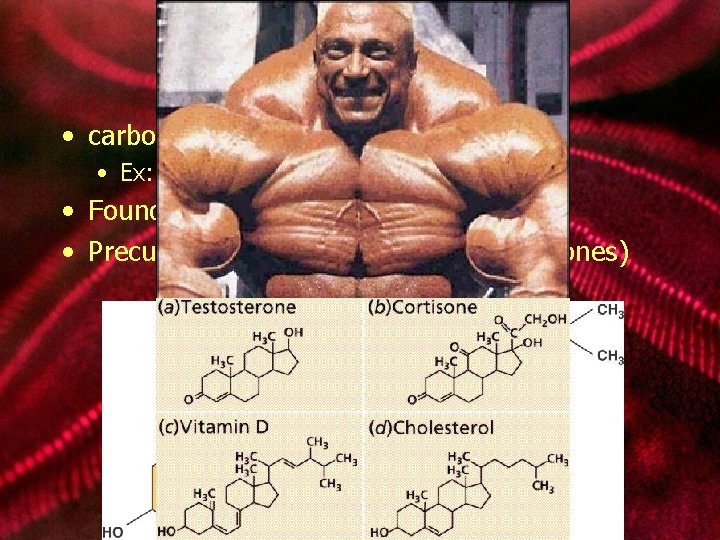
lipids: steroids • carbon skeleton consists of 4 rings • Ex: cholesterol • Found in cell membranes • Precursor for other steroids (sex hormones)

lipids: steroids Examples: • corticosteroids • sex steroids • anabolic steroids

carbohydrates • Sugars • Carbo = carbon, hydrate = water; carbohydrates have the molecular formula (CH 2 O)n • used as cellular “fuel” • used for structure and support monomer = monosaccharide polymer = polysaccharide

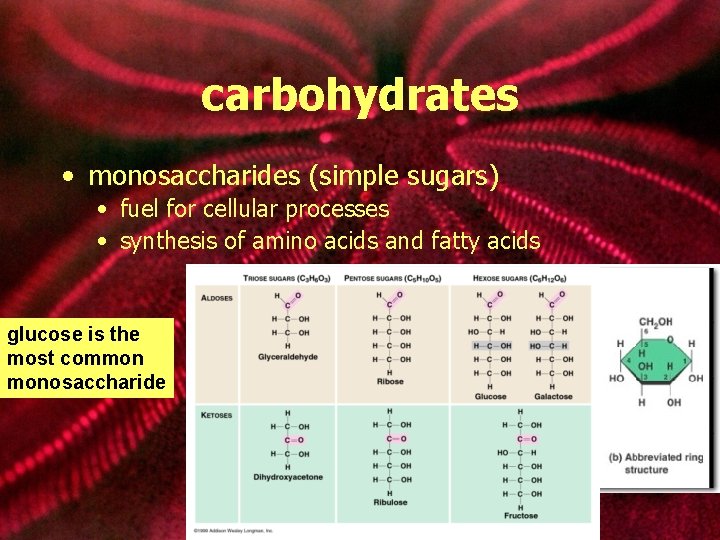
carbohydrates • monosaccharides (simple sugars) • fuel for cellular processes • synthesis of amino acids and fatty acids glucose is the most common monosaccharide

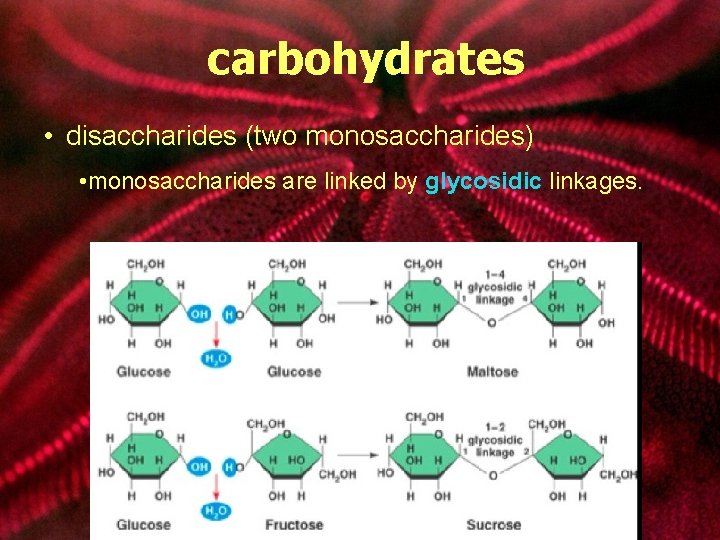
carbohydrates • disaccharides (two monosaccharides) • monosaccharides are linked by glycosidic linkages.

what kind of reaction creates disaccharides from monosaccharides? A) hydrolysis reaction B) covalent reaction C) dehydration reaction

what kind of reaction creates disaccharides from monosaccharides? C) dehydration reaction

after you drink a class of milk, how does your body digest lactose (glucose + galactose)? A) hydrolysis reactions B) covalent reactions C) dehydration reactions

after you drink a class of milk, how does your body digest lactose (glucose + galactose)? A) hydrolysis reactions

carbohydrates • polysaccharides • energy storage • building material for structure

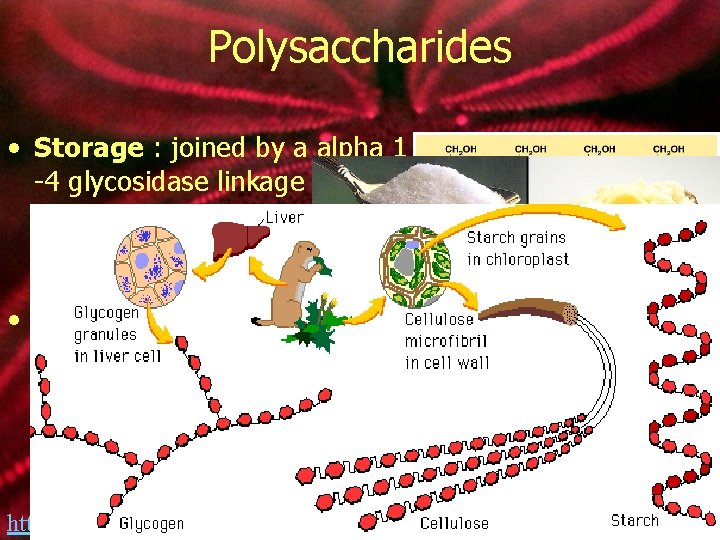
Polysaccharides • Storage : joined by a alpha 1 -4 glycosidase linkage • Starch: Plants • amylose and amylopectin • Glycogen: Animals • Structural: joined by a Beta 1 -4 glycosidase linkage • Cellulose~ most abundant organic compound • Found in the cell wall of plants • Chitin~ exoskeletons of insects http: //bcs. whfreeman. com/thelifewire/content/chp 03/0302002. html

proteins amino acids polypeptide protein

Proteins • Importance: • Instrumental in nearly everything organisms do • 50% dry weight of cells • Most structurally sophisticated molecules known

Types of proteins: • Structural functions in support • ex. : elastin, collagen, and keratin • Storage food source • ex. : ovalbumin (eggs) • Transport moves other substances • ex. : hemoglobin, myoglobin and cell membrane proteins • Hormonal coordinates bodily activities • ex. : insulin • Contractile movement • ex. : actin and myosin (muscle) • Antibodies defense • ex. : Ig. E, Ig. A, and Ig. G • Enzymes aid in chemical reactions • ex. : amylase and protease

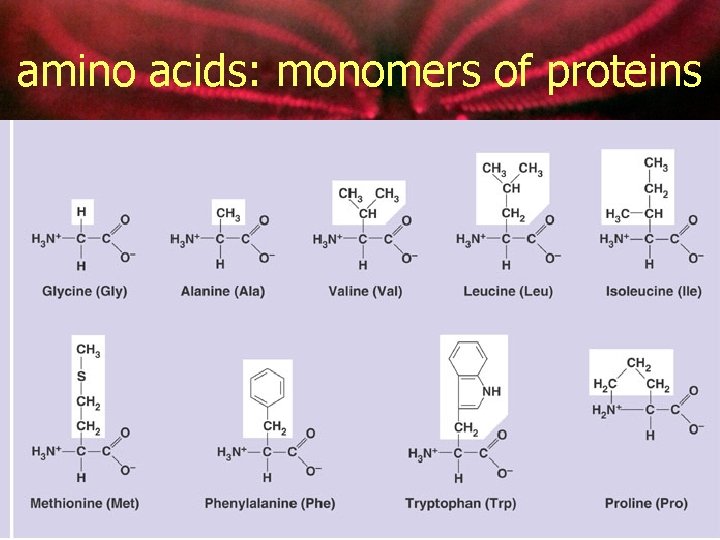
amino acids: monomers of proteins

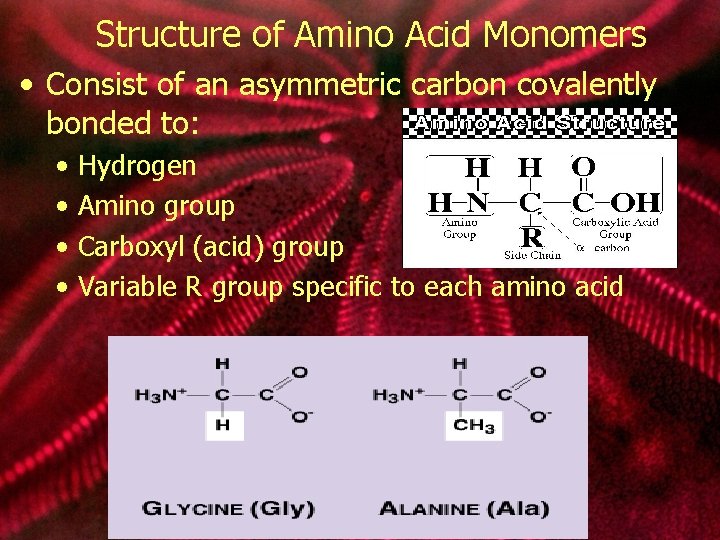
Structure of Amino Acid Monomers • Consist of an asymmetric carbon covalently bonded to: • • Hydrogen Amino group Carboxyl (acid) group Variable R group specific to each amino acid

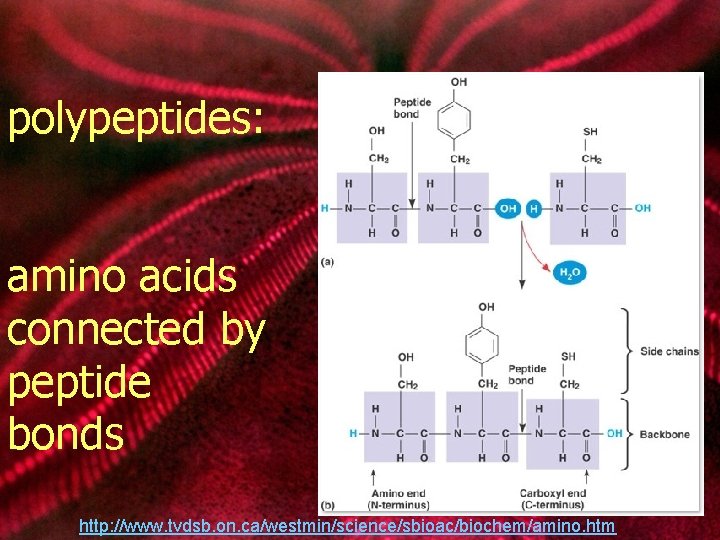
polypeptides: amino acids connected by peptide bonds http: //www. tvdsb. on. ca/westmin/science/sbioac/biochem/amino. htm

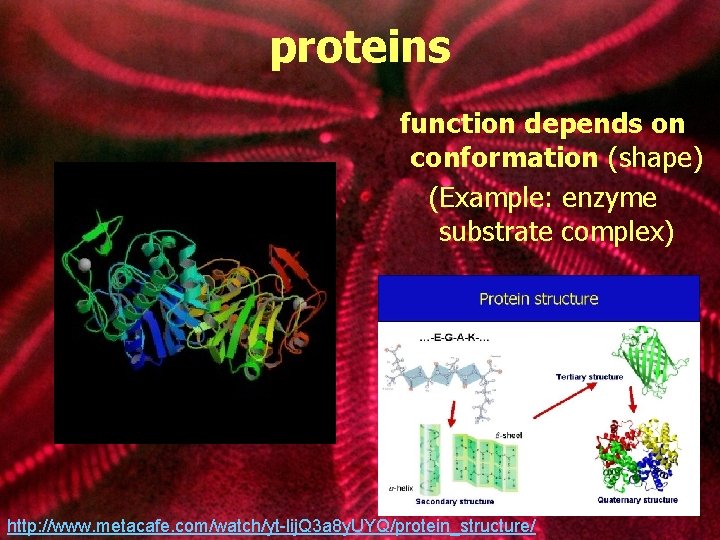
proteins function depends on conformation (shape) (Example: enzyme substrate complex) http: //www. metacafe. com/watch/yt-lij. Q 3 a 8 y. UYQ/protein_structure/

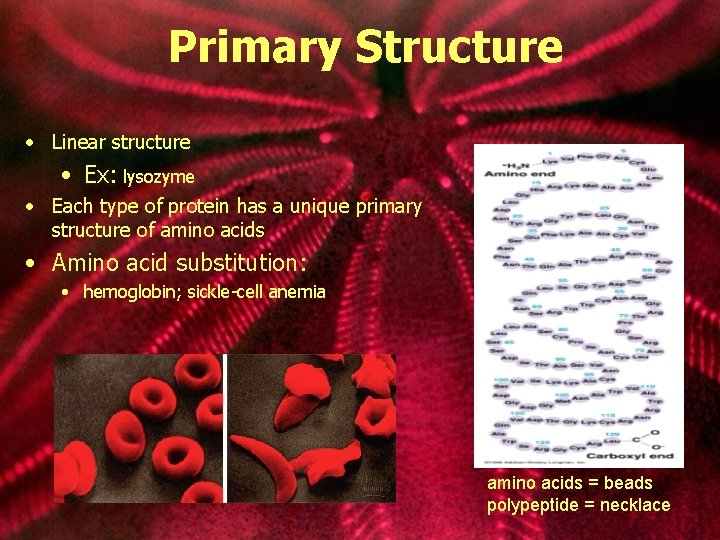
Primary Structure • Linear structure • Ex: lysozyme • Each type of protein has a unique primary structure of amino acids • Amino acid substitution: • hemoglobin; sickle-cell anemia amino acids = beads polypeptide = necklace

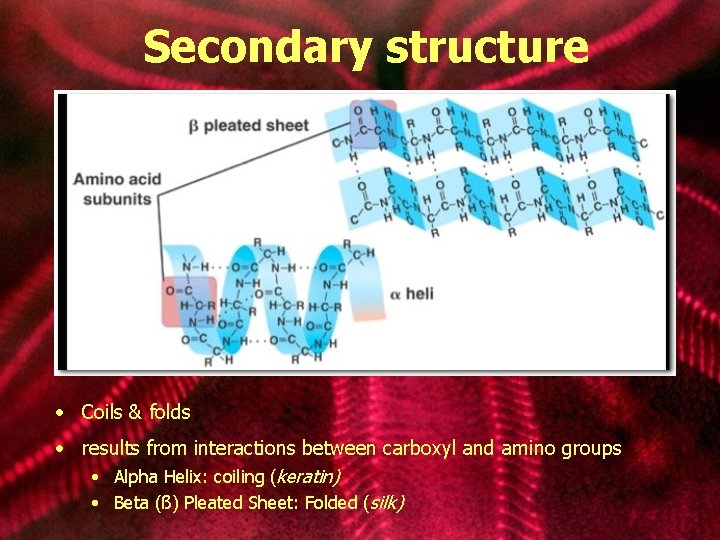
Secondary structure • Coils & folds • results from interactions between carboxyl and amino groups • Alpha Helix: coiling (keratin) • Beta (ß) Pleated Sheet: Folded (silk)

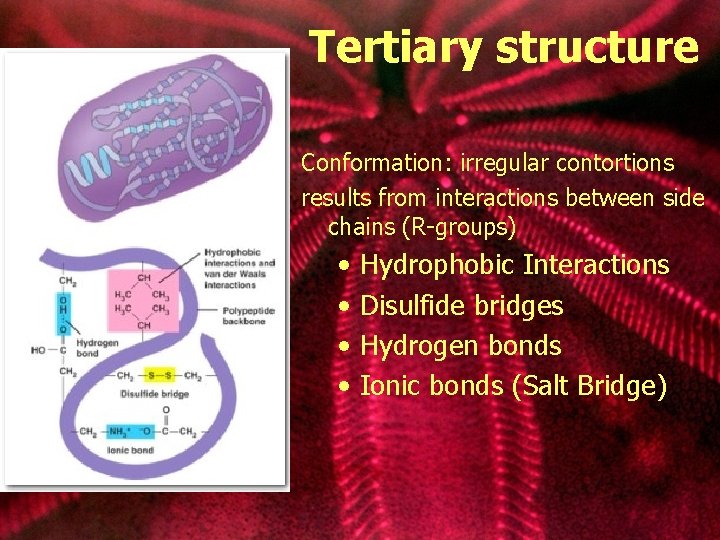
Tertiary structure Conformation: irregular contortions results from interactions between side chains (R-groups) • • Hydrophobic Interactions Disulfide bridges Hydrogen bonds Ionic bonds (Salt Bridge)

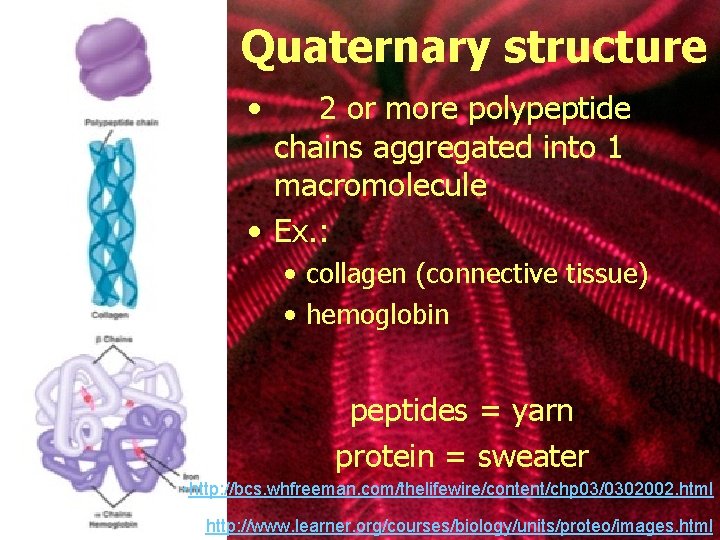
Quaternary structure • 2 or more polypeptide chains aggregated into 1 macromolecule • Ex. : • collagen (connective tissue) • hemoglobin peptides = yarn protein = sweater • http: //bcs. whfreeman. com/thelifewire/content/chp 03/0302002. html http: //www. learner. org/courses/biology/units/proteo/images. html

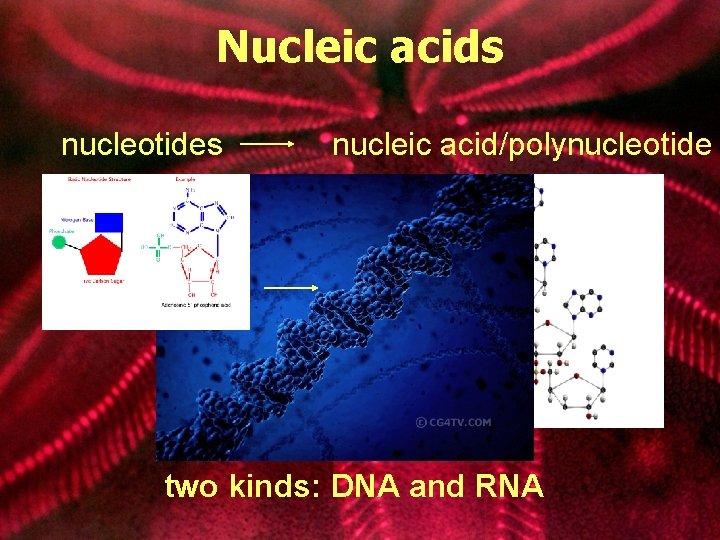
Nucleic acids nucleotides nucleic acid/polynucleotide two kinds: DNA and RNA

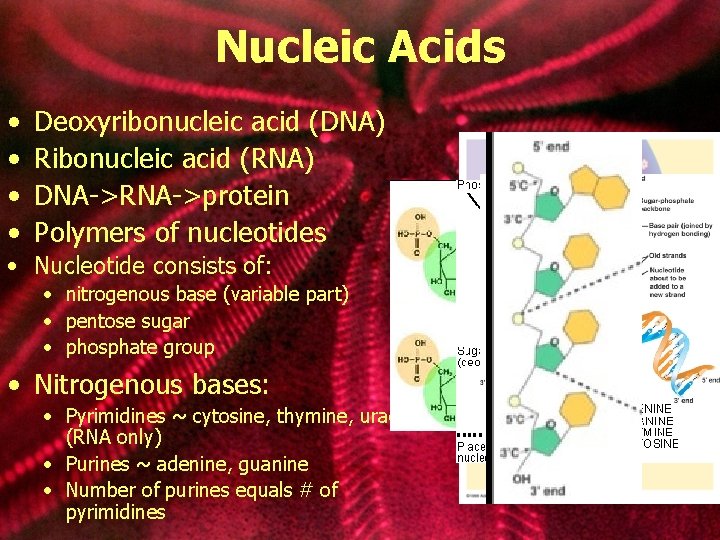
Nucleic Acids • • Deoxyribonucleic acid (DNA) Ribonucleic acid (RNA) DNA->RNA->protein Polymers of nucleotides • Nucleotide consists of: • nitrogenous base (variable part) • pentose sugar • phosphate group • Nitrogenous bases: • Pyrimidines ~ cytosine, thymine, uracil (RNA only) • Purines ~ adenine, guanine • Number of purines equals # of pyrimidines

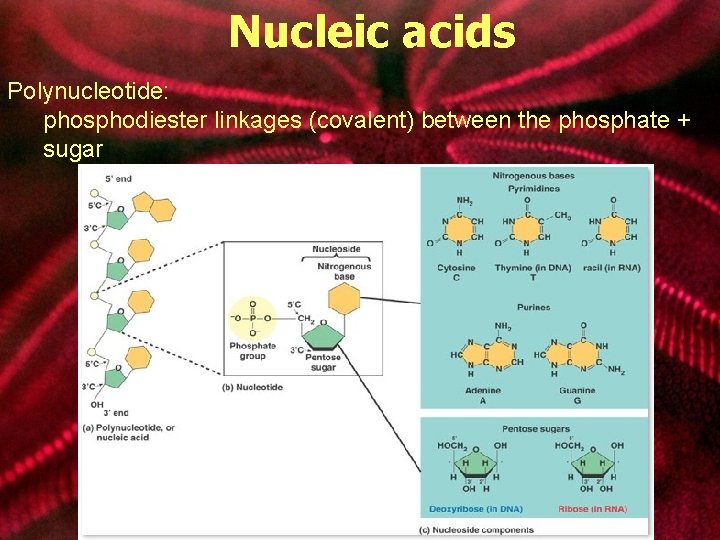
Nucleic acids Polynucleotide: phosphodiester linkages (covalent) between the phosphate + sugar

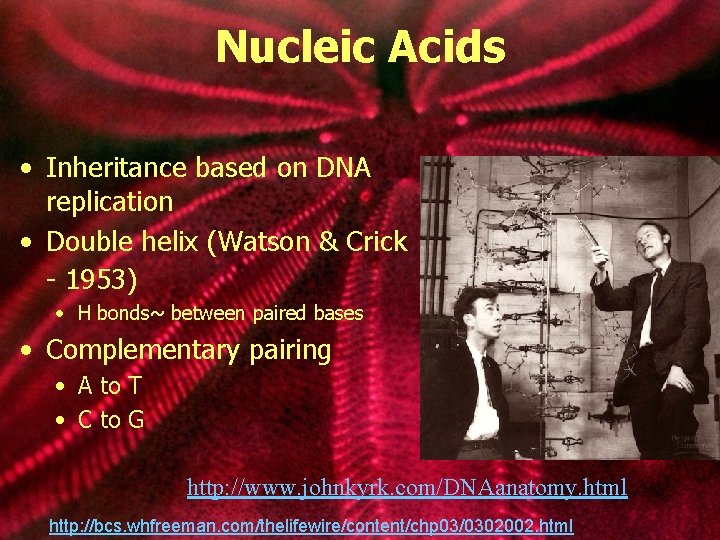
Nucleic Acids • Inheritance based on DNA replication • Double helix (Watson & Crick - 1953) • H bonds~ between paired bases • Complementary pairing • A to T • C to G http: //www. johnkyrk. com/DNAanatomy. html http: //bcs. whfreeman. com/thelifewire/content/chp 03/0302002. html

Check yourself… • Which of the 4 macromolecules is not composed of polymers? • What is the difference between a polypeptide and a protein? • Which type of macromolecule conveys information to the cell?

Check yourself… • Which of the 4 macromolecules is not composed of polymers? lipids

Check yourself… • What is the difference between a polypeptide and a protein? A polypeptide is a string of amino acids while a protein consists of more than one polypeptide.

Check yourself… • Which type of macromolecule conveys information to the cell? Nucleic acid/polynucleotide