The Structure and Function of Macromolecules CHAPTER 6





- Slides: 25

The Structure and Function of Macromolecules CHAPTER 6. 4 pages 166 -169 macromolecules intro video “You are what you eat!”

6. 4: The Building Blocks of Life The elements of life: Organisms are made up of cells. Cells contain molecules made up of the following elements: CHNOPS Carbon (C) Hydrogen (H) Nitrogen (N) Oxygen (O) Phosphorus (P) Sulfur (S) These elements come from the foods we eat.

Matter Cannot be Created nor Destroyed! Matter is recycled!!!! CHNOPS get into our cells through the Cycles of Matter!!

Carbon: All life contains Carbon! Examples: Glucose (C 6 H 12 O 6) & Carbon Dioxide (CO 2) MACROMOLECULES or BIOMOLECULES are LARGE molecules containing carbon.

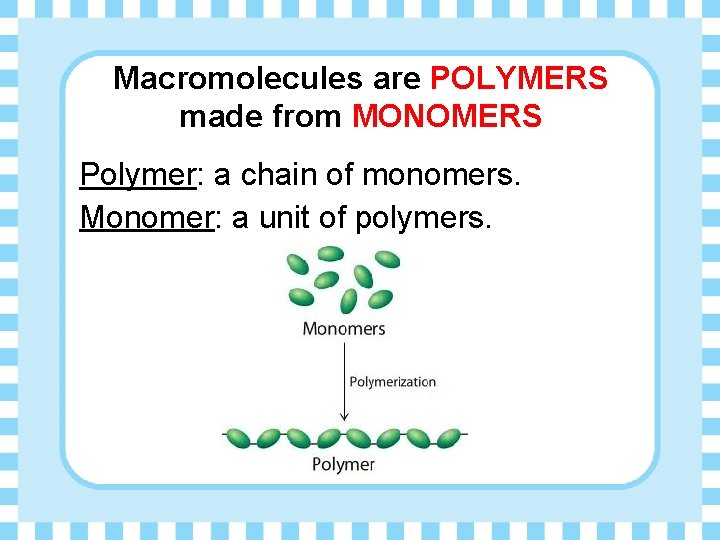
Macromolecules are POLYMERS made from MONOMERS Polymer: a chain of monomers. Monomer: a unit of polymers.

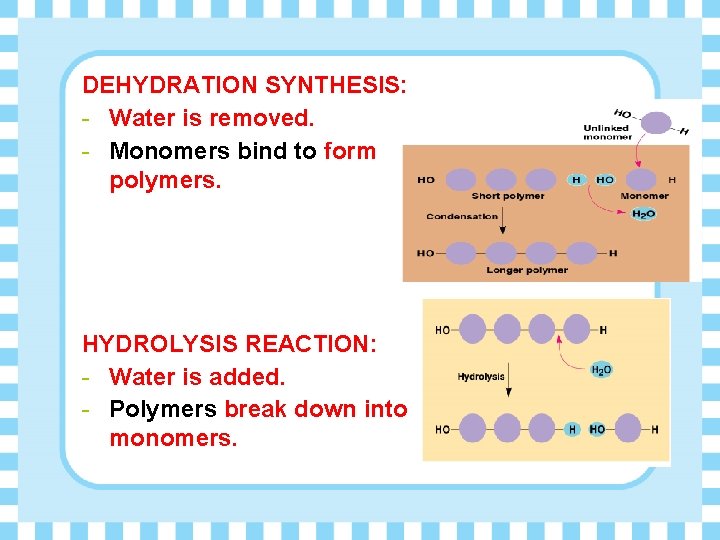
DEHYDRATION SYNTHESIS: - Water is removed. - Monomers bind to form polymers. HYDROLYSIS REACTION: - Water is added. - Polymers break down into monomers.

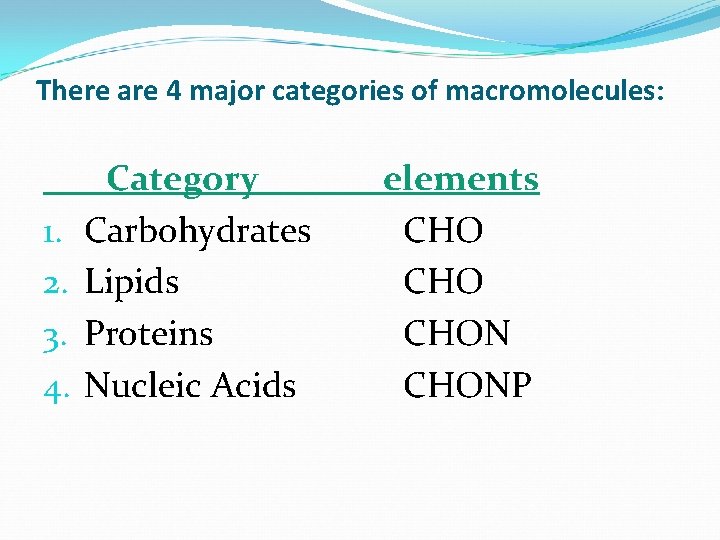
There are 4 major categories of macromolecules: 1. 2. 3. 4. Category Carbohydrates Lipids Proteins Nucleic Acids elements CHO CHONP

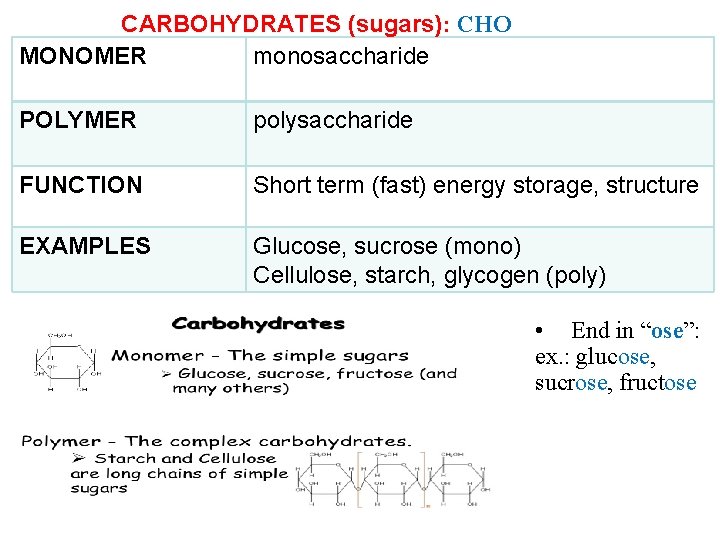
CARBOHYDRATES (sugars): CHO MONOMER monosaccharide POLYMER polysaccharide FUNCTION Short term (fast) energy storage, structure EXAMPLES Glucose, sucrose (mono) Cellulose, starch, glycogen (poly) • End in “ose”: ex. : glucose, sucrose, fructose

9 Carbs not used as primary energy source: Ø Starch: energy storage in plants Ø Cellulose: structure in plants Ø Glycogen: energy storage in animal liver Ø Chitin: structure in animals

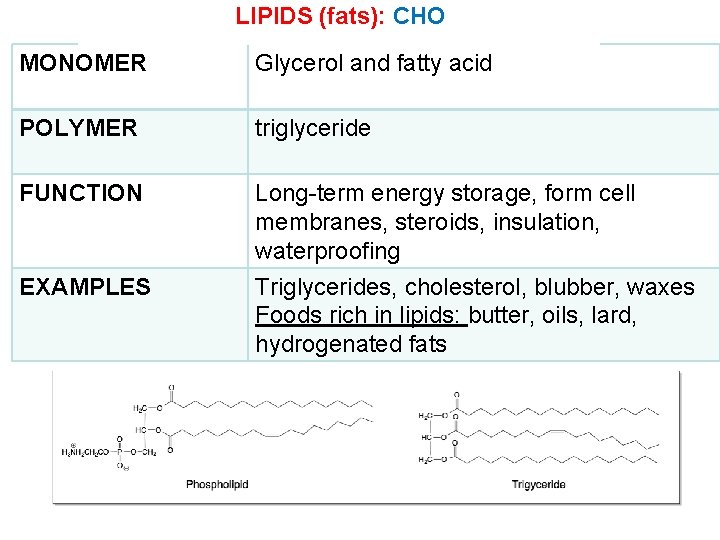
LIPIDS (fats): CHO MONOMER Glycerol and fatty acid POLYMER triglyceride FUNCTION Long-term energy storage, form cell membranes, steroids, insulation, waterproofing EXAMPLES Triglycerides, cholesterol, blubber, waxes Foods rich in lipids: butter, oils, lard, hydrogenated fats

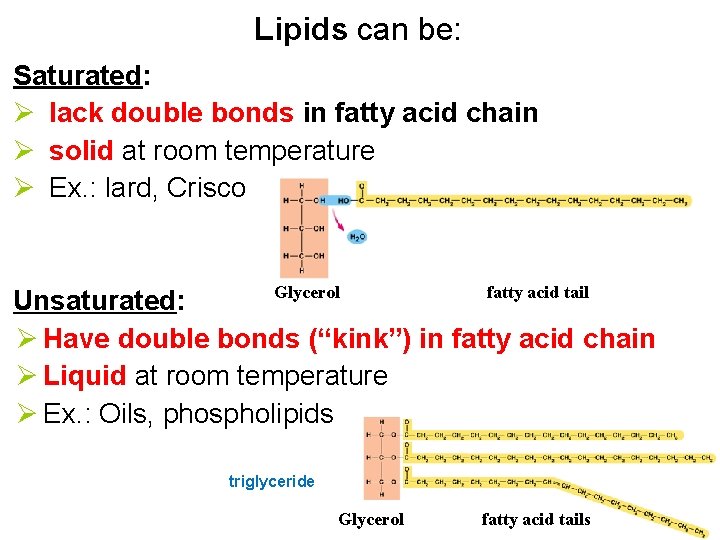
Lipids can be: Saturated: Ø lack double bonds in fatty acid chain Ø solid at room temperature Ø Ex. : lard, Crisco Glycerol fatty acid tail Unsaturated: Ø Have double bonds (“kink”) in fatty acid chain Ø Liquid at room temperature Ø Ex. : Oils, phospholipids triglyceride Glycerol fatty acid tails

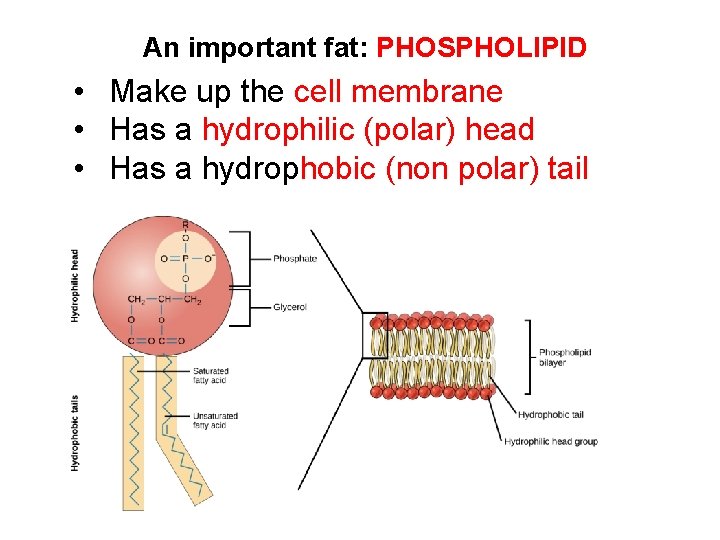
An important fat: PHOSPHOLIPID • Make up the cell membrane • Has a hydrophilic (polar) head • Has a hydrophobic (non polar) tail

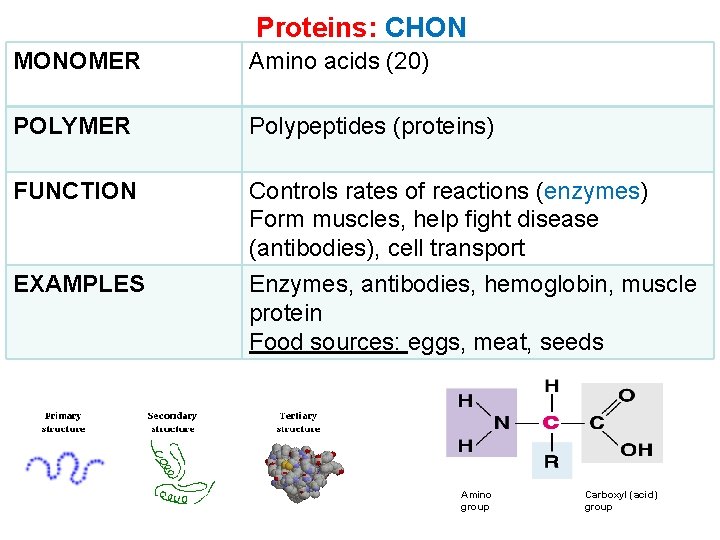
Proteins: CHON MONOMER Amino acids (20) POLYMER Polypeptides (proteins) FUNCTION Controls rates of reactions (enzymes) Form muscles, help fight disease (antibodies), cell transport EXAMPLES Enzymes, antibodies, hemoglobin, muscle protein Food sources: eggs, meat, seeds Amino group Carboxyl (acid) group

Ø Amino acids are joined by peptide bonds to form proteins. Ø Proteins differ in the number and order of amino acids Ø Amino acids interact to give a protein its shape Ø The shape determines the function of a protein. Ø Incorrect amino acids change a protein’s structure and function Ø Proteins can denature (fall apart) if: • p. H is too high or too low • Temperature is too high • Salinity is too high Ø Denatured proteins are biologically inactive

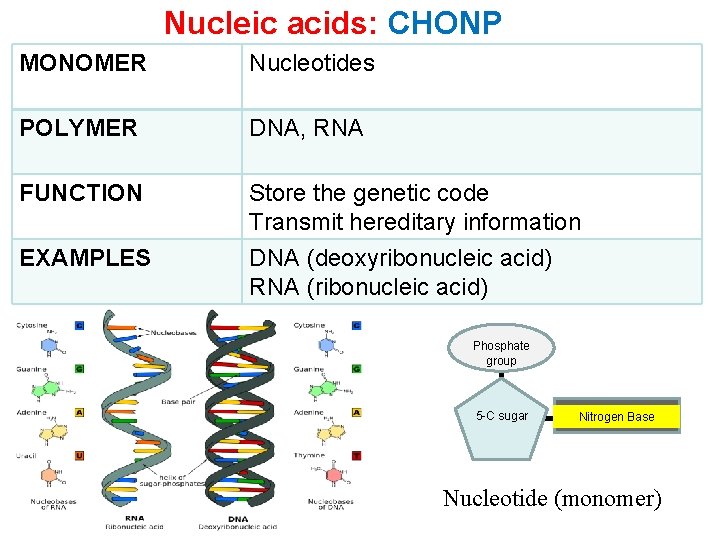
Nucleic acids: CHONP MONOMER Nucleotides POLYMER DNA, RNA FUNCTION Store the genetic code Transmit hereditary information EXAMPLES DNA (deoxyribonucleic acid) RNA (ribonucleic acid) Phosphate group 5 -C sugar Nitrogen Base Nucleotide (monomer)

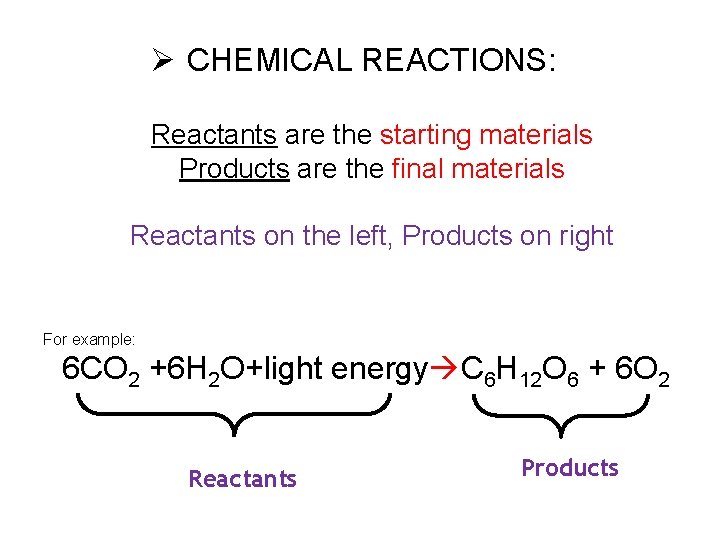
Ø CHEMICAL REACTIONS: Reactants are the starting materials Products are the final materials Reactants on the left, Products on right For example: 6 CO 2 +6 H 2 O+light energy C 6 H 12 O 6 + 6 O 2 Reactants Products

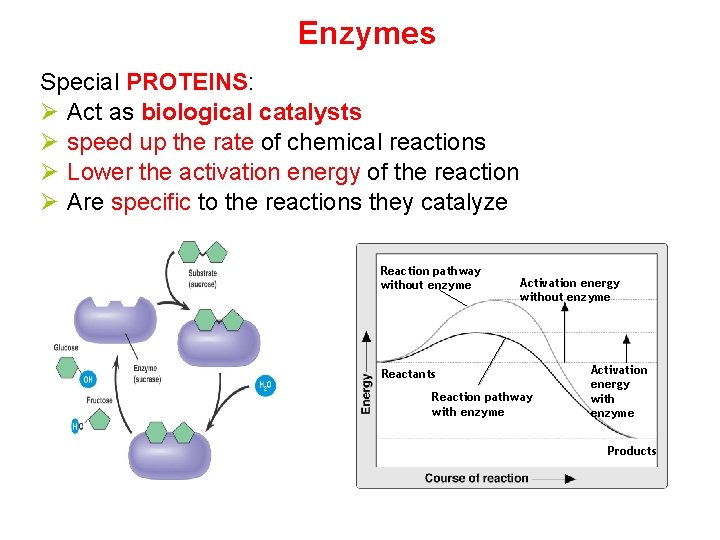
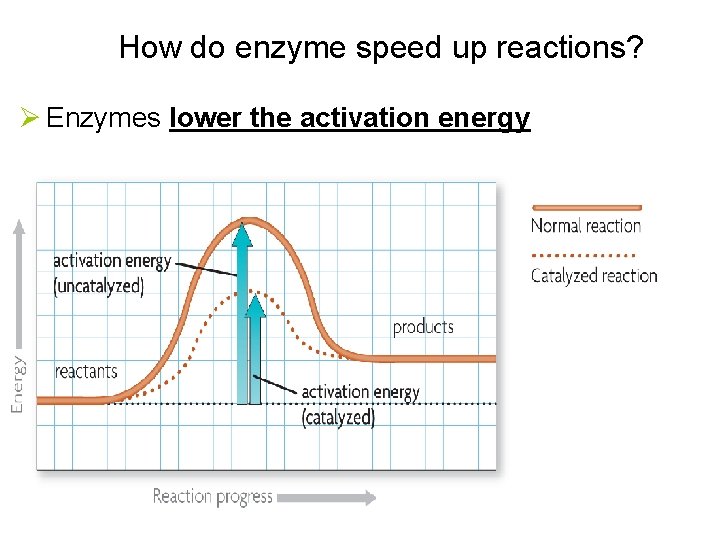
Enzymes Special PROTEINS: Ø Act as biological catalysts Ø speed up the rate of chemical reactions Ø Lower the activation energy of the reaction Ø Are specific to the reactions they catalyze Reaction pathway without enzyme Activation energy without enzyme Reactants Reaction pathway with enzyme Activation energy with enzyme Products

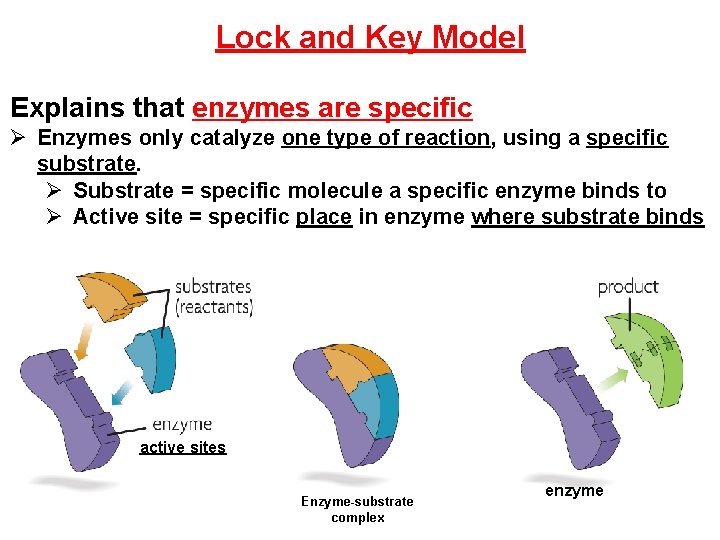
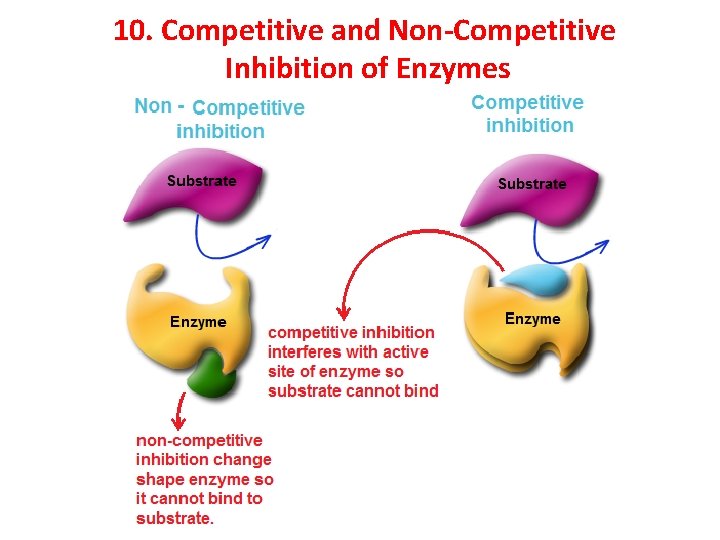
Lock and Key Model Explains that enzymes are specific Ø Enzymes only catalyze one type of reaction, using a specific substrate. Ø Substrate = specific molecule a specific enzyme binds to Ø Active site = specific place in enzyme where substrate binds active sites Enzyme-substrate complex enzyme

‘Lock and Key Model’ – says there is a perfect fit between active site and substrate

Breaking Bonds Making Bonds

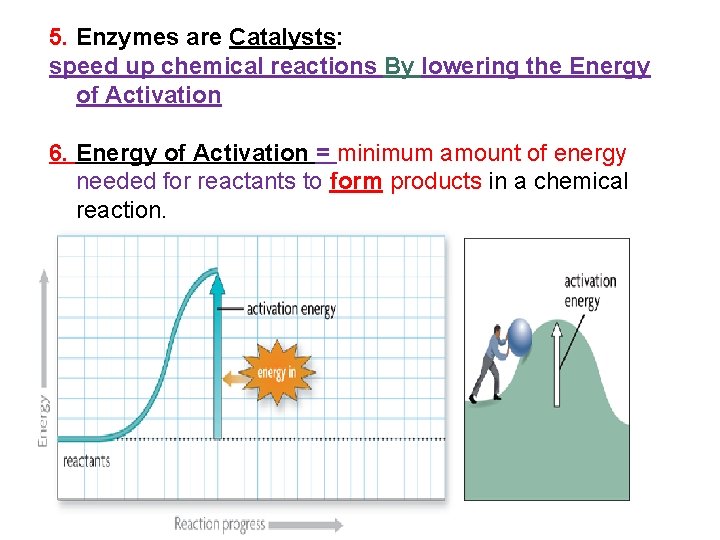
5. Enzymes are Catalysts: speed up chemical reactions By lowering the Energy of Activation 6. Energy of Activation = minimum amount of energy needed for reactants to form products in a chemical reaction.

How do enzyme speed up reactions? Ø Enzymes lower the activation energy

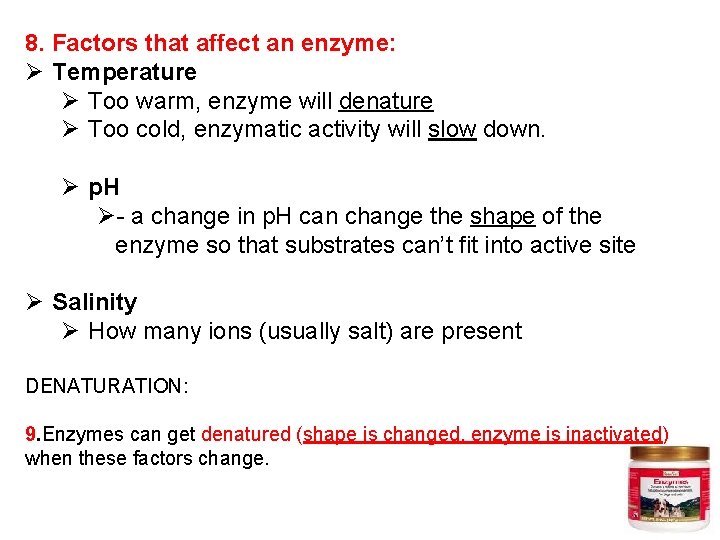
8. Factors that affect an enzyme: Ø Temperature Ø Too warm, enzyme will denature Ø Too cold, enzymatic activity will slow down. Ø p. H Ø- a change in p. H can change the shape of the enzyme so that substrates can’t fit into active site Ø Salinity Ø How many ions (usually salt) are present DENATURATION: 9. Enzymes can get denatured (shape is changed, enzyme is inactivated) when these factors change.

10. Competitive and Non-Competitive Inhibition of Enzymes

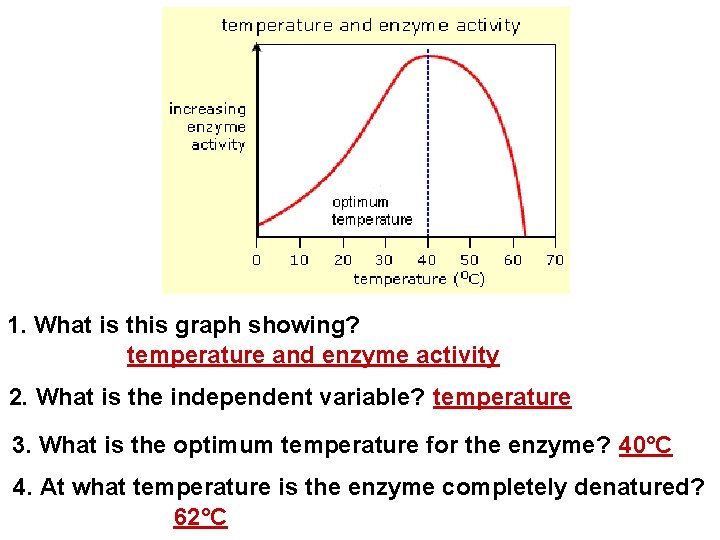
1. What is this graph showing? temperature and enzyme activity 2. What is the independent variable? temperature 3. What is the optimum temperature for the enzyme? 40°C 4. At what temperature is the enzyme completely denatured? 62°C