Chapter 24 Amines Amines Organic Nitrogen Compounds n








- Slides: 83

Chapter 24. Amines

Amines – Organic Nitrogen Compounds n Organic derivatives of ammonia, NH 3, n Nitrogen atom with a lone pair of electrons, making amines both basic and nucleophilic n Occur in plants and animals Caffeine 2

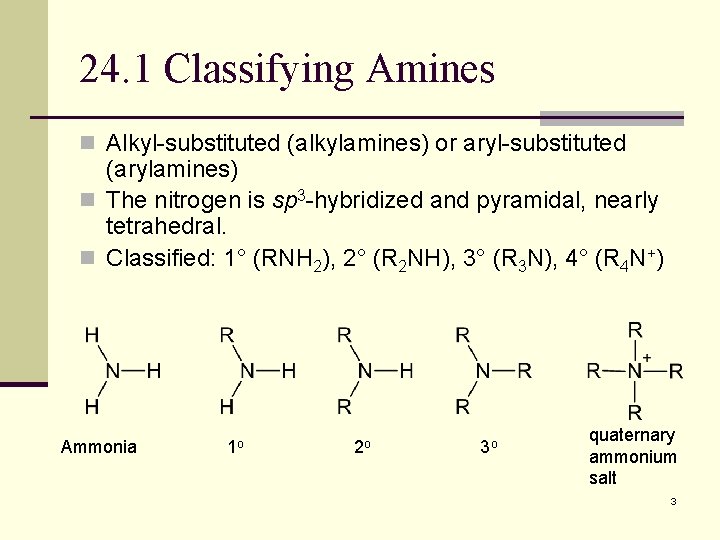
24. 1 Classifying Amines n Alkyl-substituted (alkylamines) or aryl-substituted (arylamines) n The nitrogen is sp 3 -hybridized and pyramidal, nearly tetrahedral. n Classified: 1° (RNH 2), 2° (R 2 NH), 3° (R 3 N), 4° (R 4 N+) Ammonia 1 o 2 o 3 o quaternary ammonium salt 3

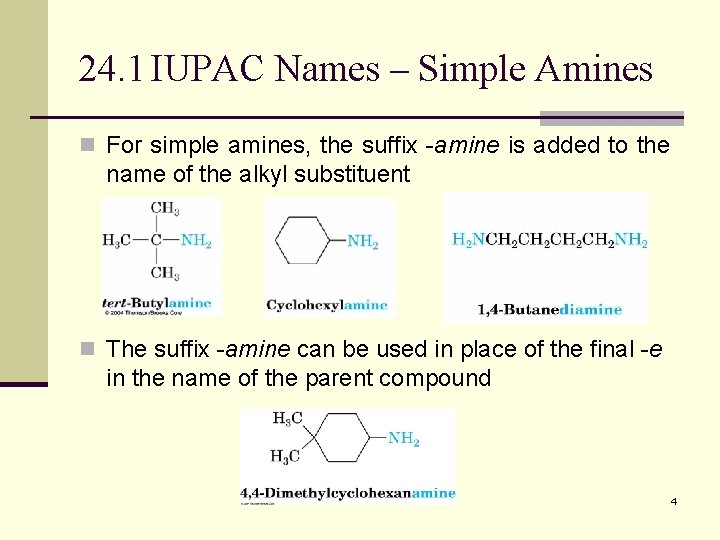
24. 1 IUPAC Names – Simple Amines n For simple amines, the suffix -amine is added to the name of the alkyl substituent n The suffix -amine can be used in place of the final -e in the name of the parent compound 4

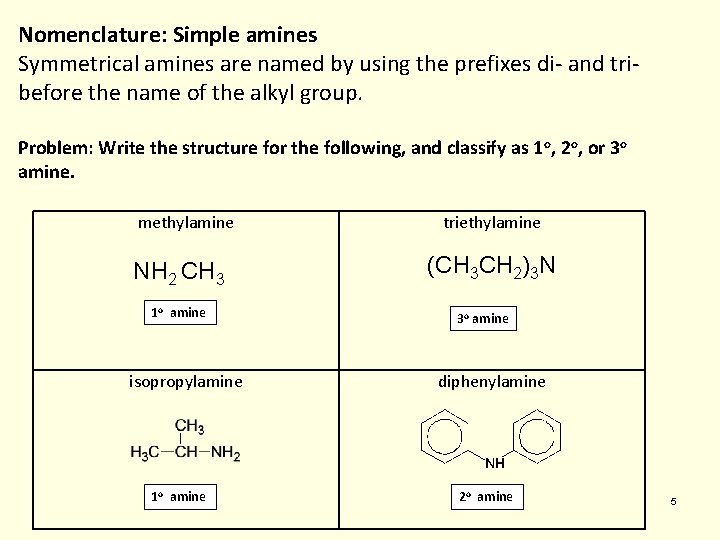
Nomenclature: Simple amines Symmetrical amines are named by using the prefixes di- and tribefore the name of the alkyl group. Problem: Write the structure for the following, and classify as 1 o, 2 o, or 3 o amine. methylamine NH 2 CH 3 1 o amine isopropylamine 1 o amine triethylamine (CH 3 CH 2)3 N 3 o amine diphenylamine 2 o amine 5

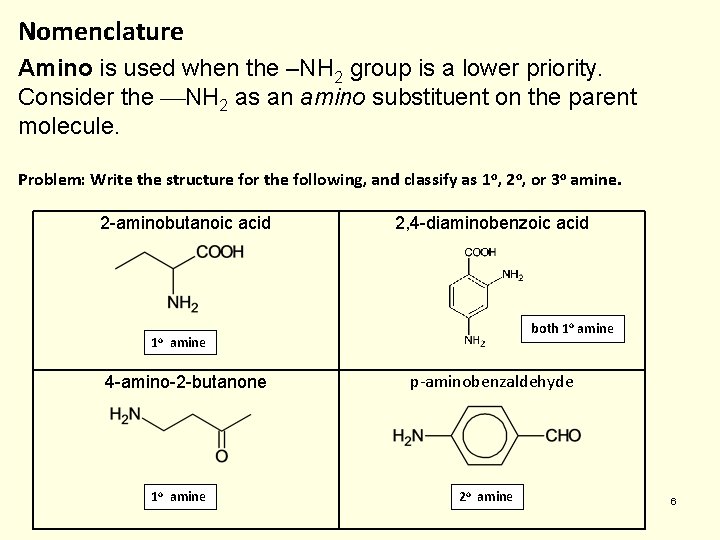
Nomenclature Amino is used when the –NH 2 group is a lower priority. Consider the NH 2 as an amino substituent on the parent molecule. Problem: Write the structure for the following, and classify as 1 o, 2 o, or 3 o amine. 2 -aminobutanoic acid 1 o 2, 4 -diaminobenzoic acid both 1 o amine 4 -amino-2 -butanone 1 o amine p-aminobenzaldehyde 2 o amine 6

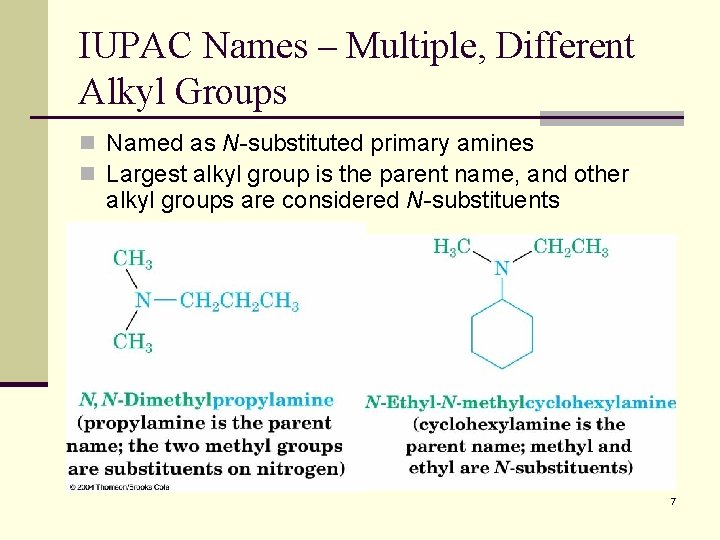
IUPAC Names – Multiple, Different Alkyl Groups n Named as N-substituted primary amines n Largest alkyl group is the parent name, and other alkyl groups are considered N-substituents 7

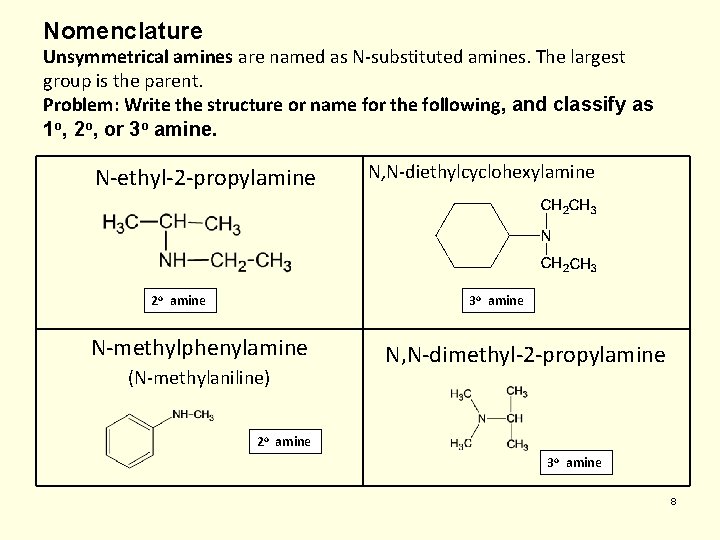
Nomenclature Unsymmetrical amines are named as N-substituted amines. The largest group is the parent. Problem: Write the structure or name for the following, and classify as 1 o, 2 o, or 3 o amine. N-ethyl-2 -propylamine 2 o amine N, N-diethylcyclohexylamine 3 o amine N-methylphenylamine (N-methylaniline) N, N-dimethyl-2 -propylamine 2 o amine 3 o amine 8

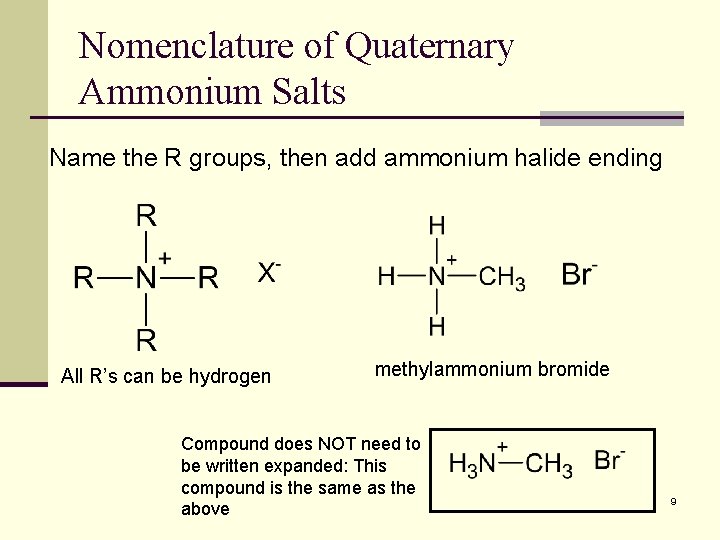
Nomenclature of Quaternary Ammonium Salts Name the R groups, then add ammonium halide ending All R’s can be hydrogen methylammonium bromide Compound does NOT need to be written expanded: This compound is the same as the above 9

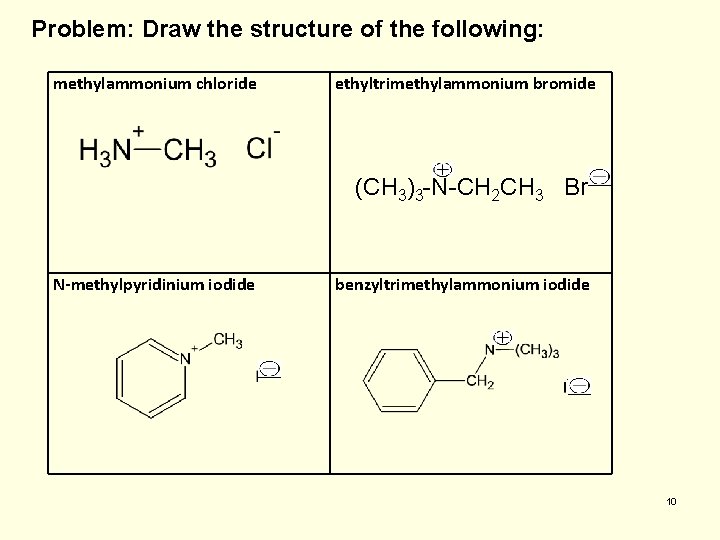
Problem: Draw the structure of the following: methylammonium chloride ethyltrimethylammonium bromide (CH 3)3 -N-CH 2 CH 3 Br- N-methylpyridinium iodide benzyltrimethylammonium iodide 10

11

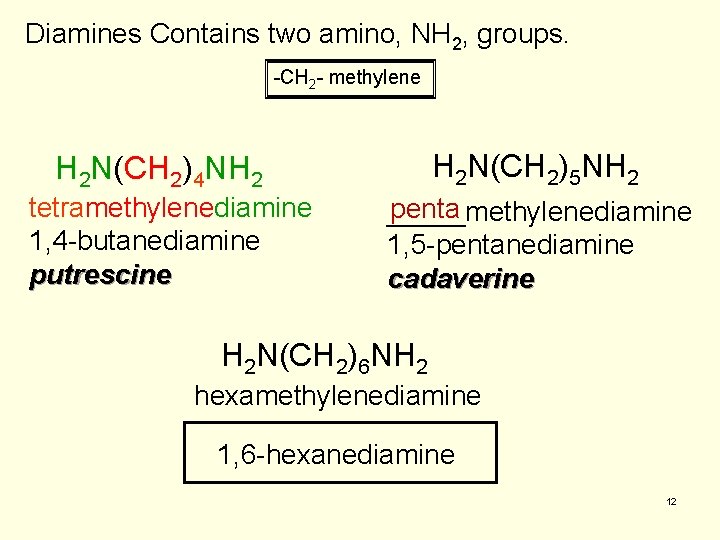
Diamines Contains two amino, NH 2, groups. -CH 2 - methylene H 2 N(CH 2)5 NH 2 H 2 N(CH 2)4 NH 2 tetramethylenediamine 1, 4 -butanediamine putrescine penta _____methylenediamine 1, 5 -pentanediamine cadaverine H 2 N(CH 2)6 NH 2 hexamethylenediamine 1, 6 -hexanediamine 12

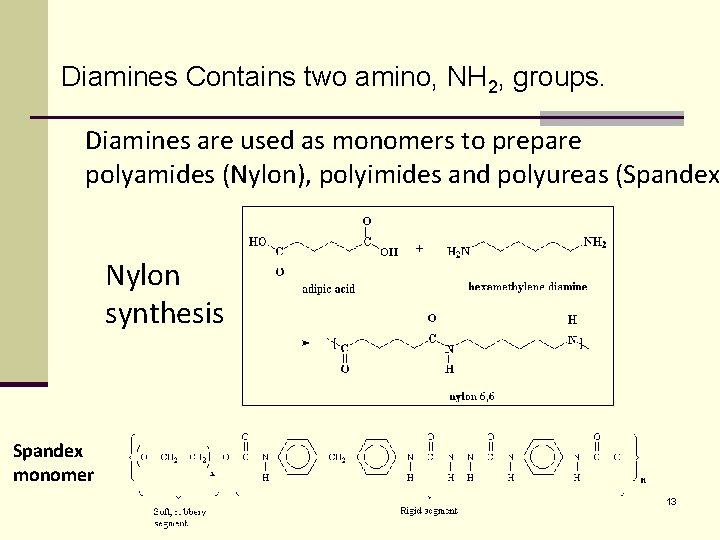
Diamines Contains two amino, NH 2, groups. Diamines are used as monomers to prepare polyamides (Nylon), polyimides and polyureas (Spandex) Nylon synthesis Spandex monomer 13

Making Nylon You. Tube Videos: Press Play to view URL if link doesn’t work: https: //youtu. be/xh. V 2 B-n. E 6 D 0 This one explains the chemistry well. URL if link doesn’t work: https: //youtu. be/sj. Q 00 Ag. Z 2 Go This one is entertaining, with a little chemistry, made by the Crazy. Russian. Hacker http: //www. youtube. com/user/Crazy. Ru ss. . . 14

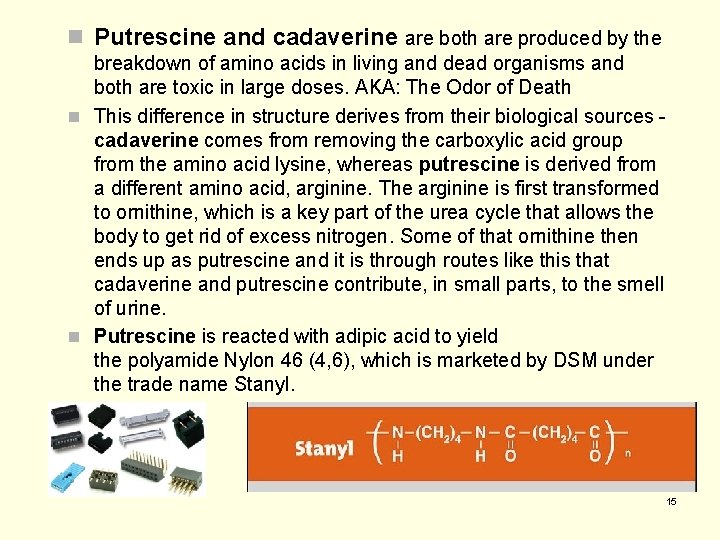
n Putrescine and cadaverine are both are produced by the breakdown of amino acids in living and dead organisms and both are toxic in large doses. AKA: The Odor of Death n This difference in structure derives from their biological sources cadaverine comes from removing the carboxylic acid group from the amino acid lysine, whereas putrescine is derived from a different amino acid, arginine. The arginine is first transformed to ornithine, which is a key part of the urea cycle that allows the body to get rid of excess nitrogen. Some of that ornithine then ends up as putrescine and it is through routes like this that cadaverine and putrescine contribute, in small parts, to the smell of urine. n Putrescine is reacted with adipic acid to yield the polyamide Nylon 46 (4, 6), which is marketed by DSM under the trade name Stanyl. 15

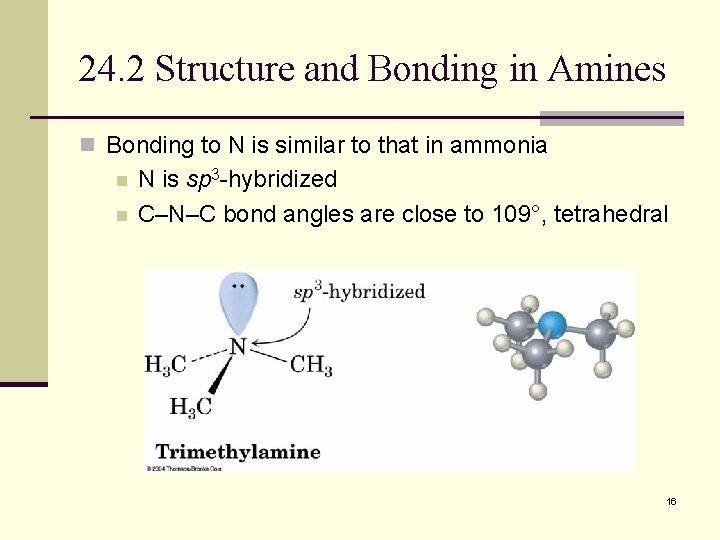
24. 2 Structure and Bonding in Amines n Bonding to N is similar to that in ammonia n n N is sp 3 -hybridized C–N–C bond angles are close to 109°, tetrahedral 16

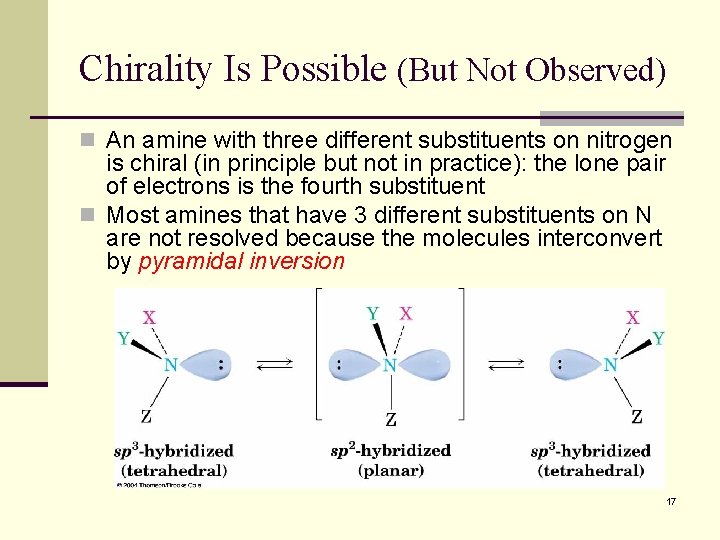
Chirality Is Possible (But Not Observed) n An amine with three different substituents on nitrogen is chiral (in principle but not in practice): the lone pair of electrons is the fourth substituent n Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion 17

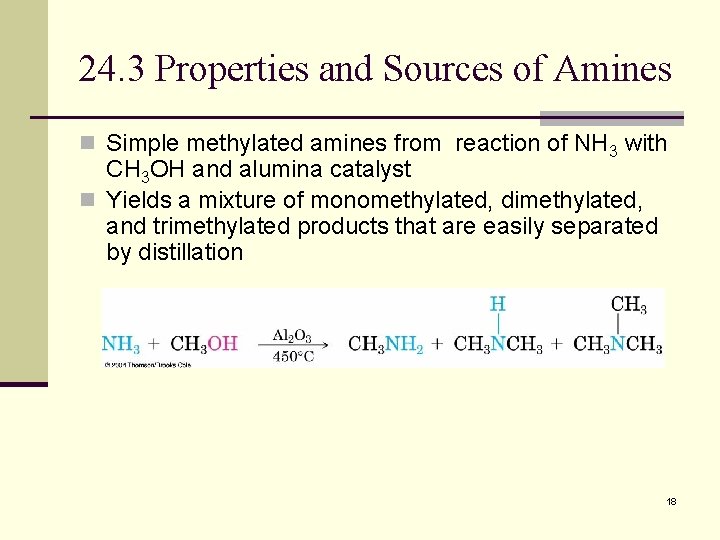
24. 3 Properties and Sources of Amines n Simple methylated amines from reaction of NH 3 with CH 3 OH and alumina catalyst n Yields a mixture of monomethylated, dimethylated, and trimethylated products that are easily separated by distillation 18

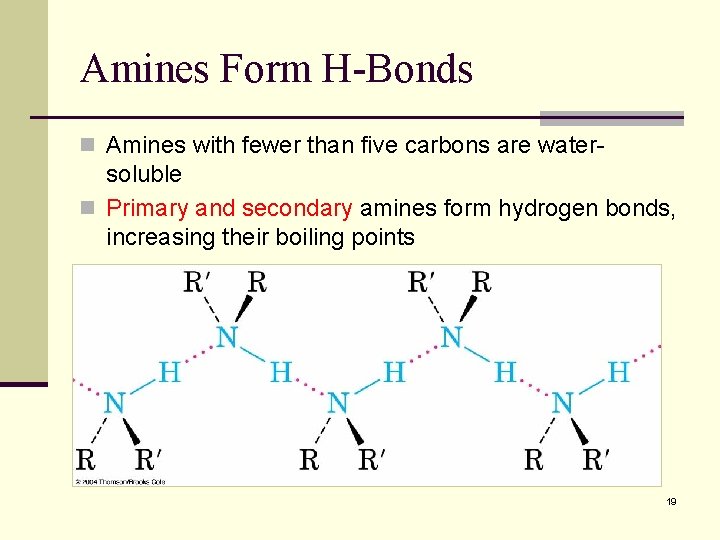
Amines Form H-Bonds n Amines with fewer than five carbons are water- soluble n Primary and secondary amines form hydrogen bonds, increasing their boiling points 19

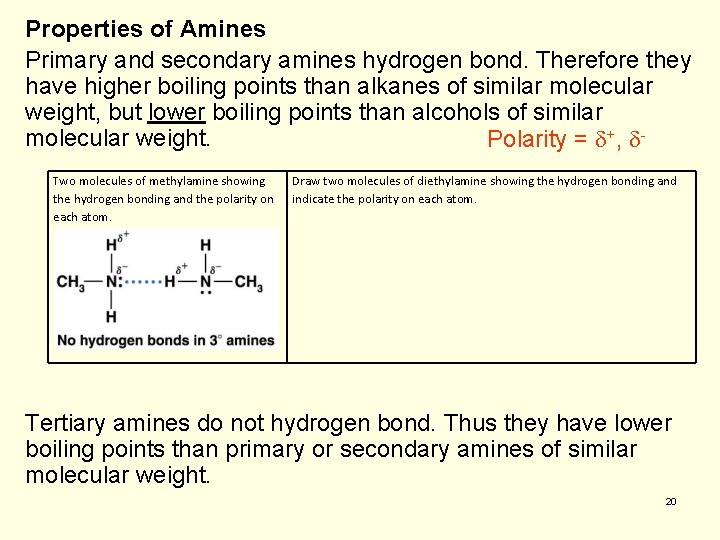
Properties of Amines Primary and secondary amines hydrogen bond. Therefore they have higher boiling points than alkanes of similar molecular weight, but lower boiling points than alcohols of similar molecular weight. Polarity = +, Two molecules of methylamine showing the hydrogen bonding and the polarity on each atom. Draw two molecules of diethylamine showing the hydrogen bonding and indicate the polarity on each atom. Tertiary amines do not hydrogen bond. Thus they have lower boiling points than primary or secondary amines of similar molecular weight. 20

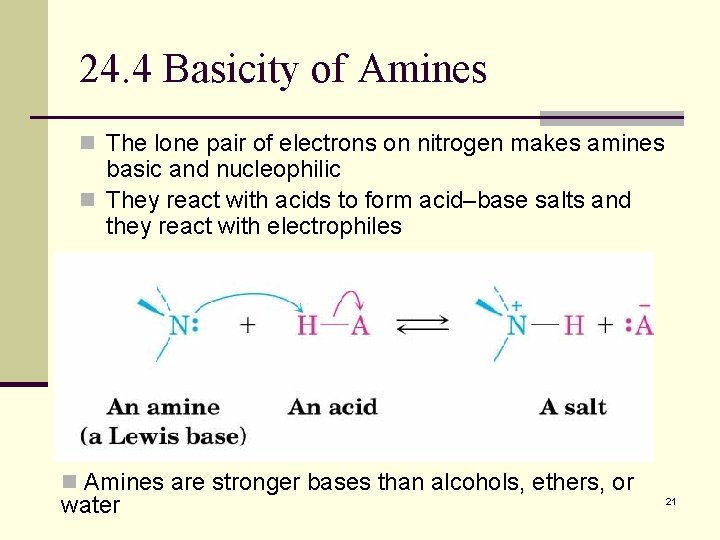
24. 4 Basicity of Amines n The lone pair of electrons on nitrogen makes amines basic and nucleophilic n They react with acids to form acid–base salts and they react with electrophiles n Amines are stronger bases than alcohols, ethers, or water 21

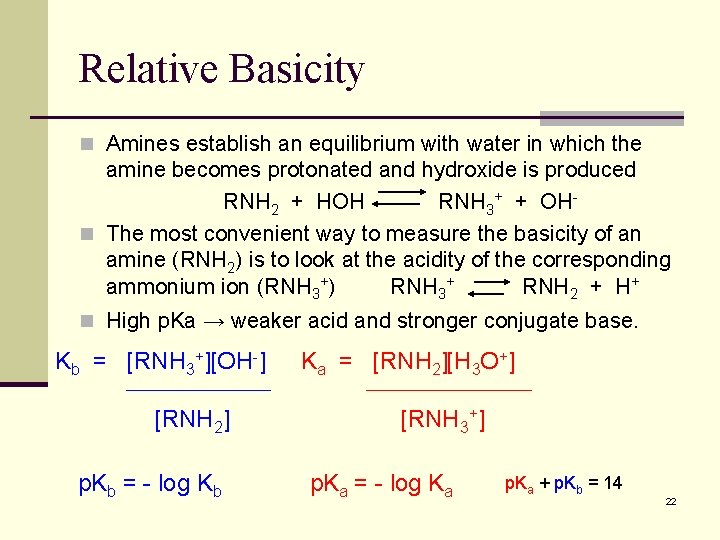
Relative Basicity n Amines establish an equilibrium with water in which the amine becomes protonated and hydroxide is produced RNH 2 + HOH RNH 3+ + OHn The most convenient way to measure the basicity of an amine (RNH 2) is to look at the acidity of the corresponding ammonium ion (RNH 3+) RNH 3+ RNH 2 + H+ n High p. Ka → weaker acid and stronger conjugate base. Kb = [RNH 3+][OH-] [RNH 2] p. Kb = - log Kb Ka = [RNH 2][H 3 O+] [RNH 3+] p. Ka = - log Ka p. Ka + p. Kb = 14 22

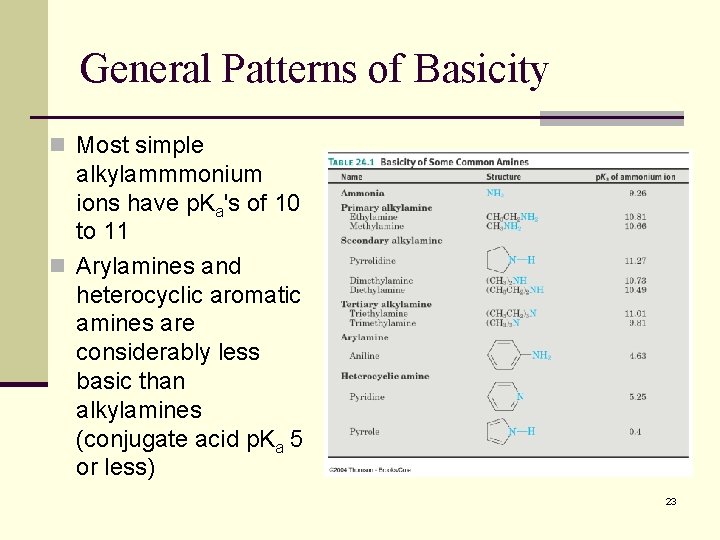
General Patterns of Basicity n Most simple alkylammmonium ions have p. Ka's of 10 to 11 n Arylamines and heterocyclic aromatic amines are considerably less basic than alkylamines (conjugate acid p. Ka 5 or less) 23

Base Strength n Alkylamines have similar basicities. Arylamines and heterocyclic amines are less basic than alkylamines. n Amides (RCONH 2) are nonbasic. They do not undergo protonation when treated with aqueous acids, and they are poor nucleophiles. n Amine basicity can be used as a means of separating amines. An amine can be converted to its salt, extracted from a solution with water, neutralized, and re-extracted with an organic solvent. 24

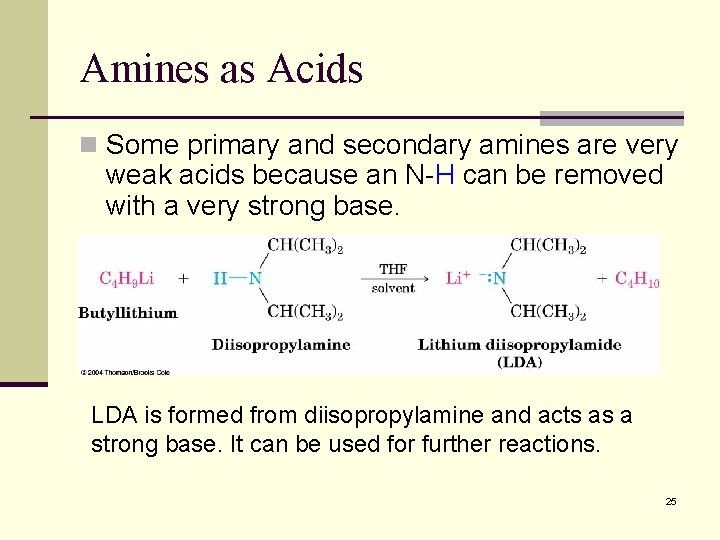
Amines as Acids n Some primary and secondary amines are very weak acids because an N-H can be removed with a very strong base. LDA is formed from diisopropylamine and acts as a strong base. It can be used for further reactions. 25

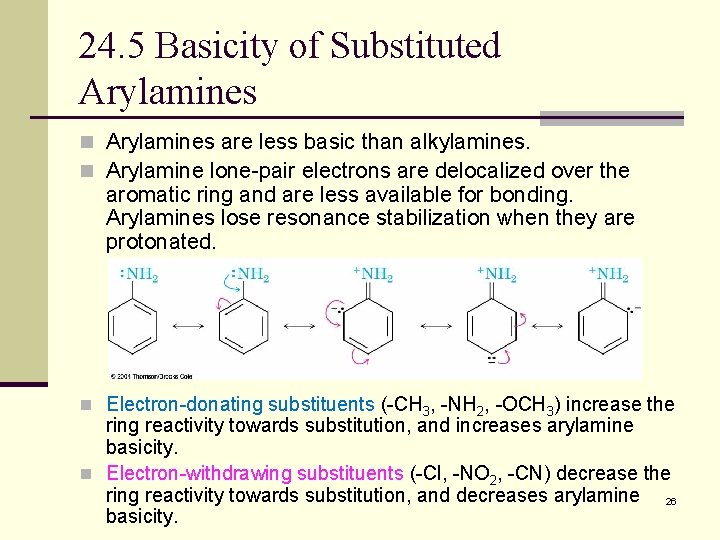
24. 5 Basicity of Substituted Arylamines n Arylamines are less basic than alkylamines. n Arylamine lone-pair electrons are delocalized over the aromatic ring and are less available for bonding. Arylamines lose resonance stabilization when they are protonated. n Electron-donating substituents (-CH 3, -NH 2, -OCH 3) increase the ring reactivity towards substitution, and increases arylamine basicity. n Electron-withdrawing substituents (-Cl, -NO 2, -CN) decrease the ring reactivity towards substitution, and decreases arylamine 26 basicity.

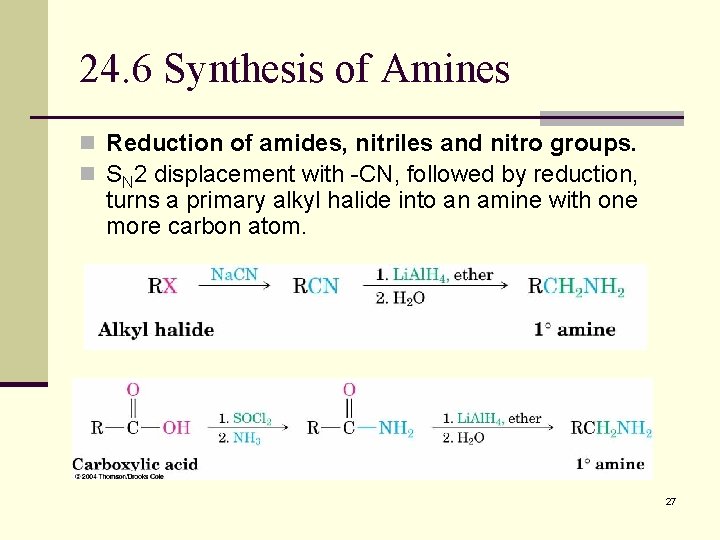
24. 6 Synthesis of Amines n Reduction of amides, nitriles and nitro groups. n SN 2 displacement with -CN, followed by reduction, turns a primary alkyl halide into an amine with one more carbon atom. 27

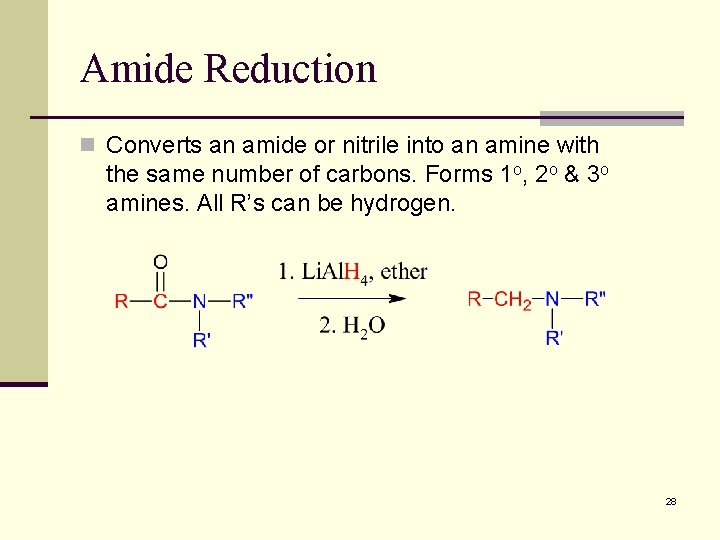
Amide Reduction n Converts an amide or nitrile into an amine with the same number of carbons. Forms 1 o, 2 o & 3 o amines. All R’s can be hydrogen. 28

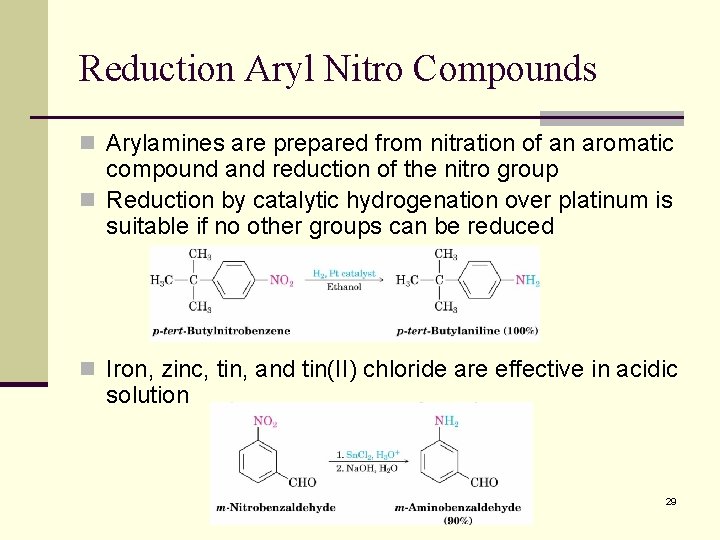
Reduction Aryl Nitro Compounds n Arylamines are prepared from nitration of an aromatic compound and reduction of the nitro group n Reduction by catalytic hydrogenation over platinum is suitable if no other groups can be reduced n Iron, zinc, tin, and tin(II) chloride are effective in acidic solution 29

SN 2 Reactions of Amines and Alkyl Halides n Ammonia and other amines are good nucleophiles 30

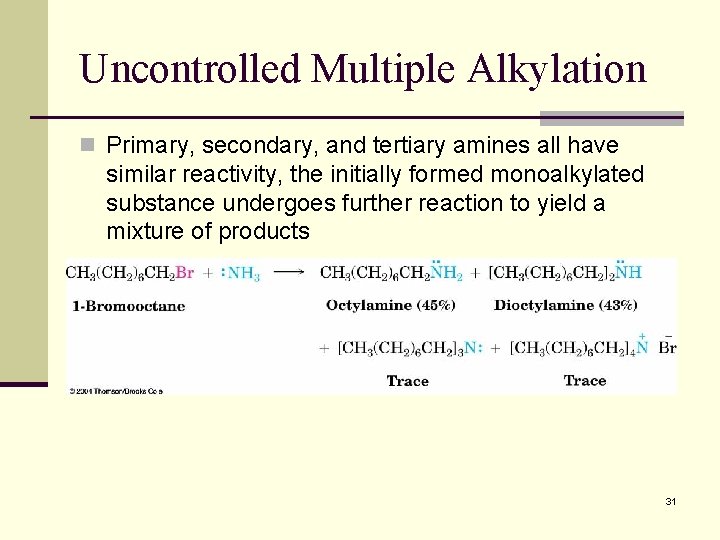
Uncontrolled Multiple Alkylation n Primary, secondary, and tertiary amines all have similar reactivity, the initially formed monoalkylated substance undergoes further reaction to yield a mixture of products 31

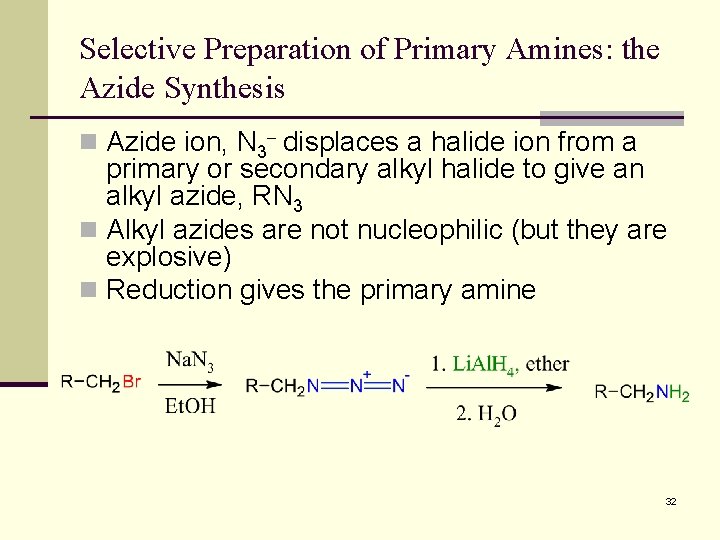
Selective Preparation of Primary Amines: the Azide Synthesis n Azide ion, N 3 displaces a halide ion from a primary or secondary alkyl halide to give an alkyl azide, RN 3 n Alkyl azides are not nucleophilic (but they are explosive) n Reduction gives the primary amine 32

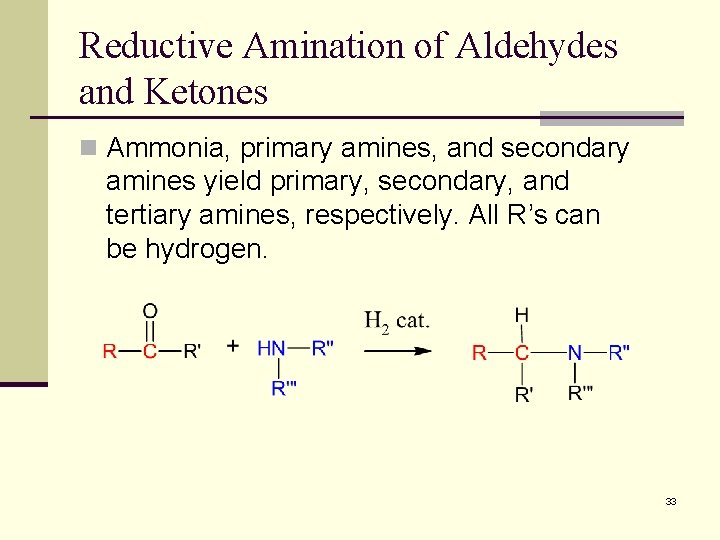
Reductive Amination of Aldehydes and Ketones n Ammonia, primary amines, and secondary amines yield primary, secondary, and tertiary amines, respectively. All R’s can be hydrogen. 33

34

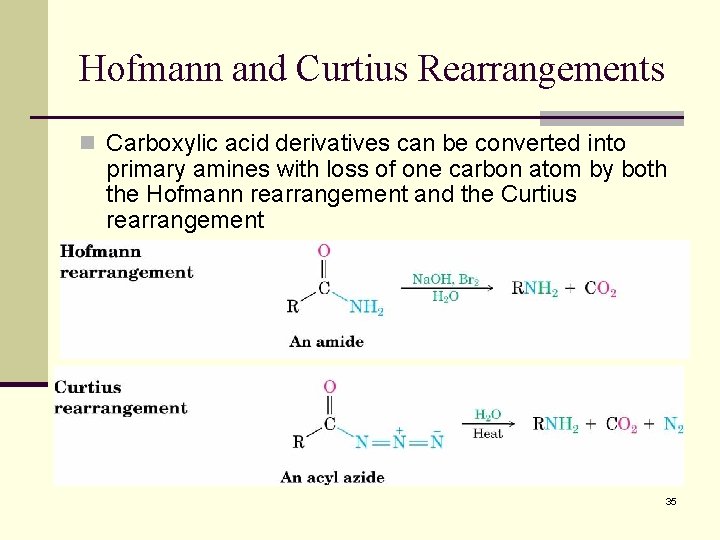
Hofmann and Curtius Rearrangements n Carboxylic acid derivatives can be converted into primary amines with loss of one carbon atom by both the Hofmann rearrangement and the Curtius rearrangement 35

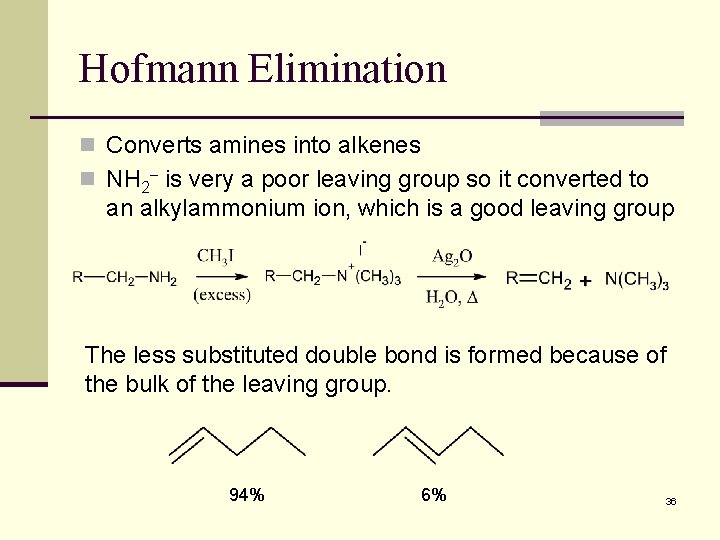
Hofmann Elimination n Converts amines into alkenes n NH 2 is very a poor leaving group so it converted to an alkylammonium ion, which is a good leaving group The less substituted double bond is formed because of the bulk of the leaving group. 94% 6% 36

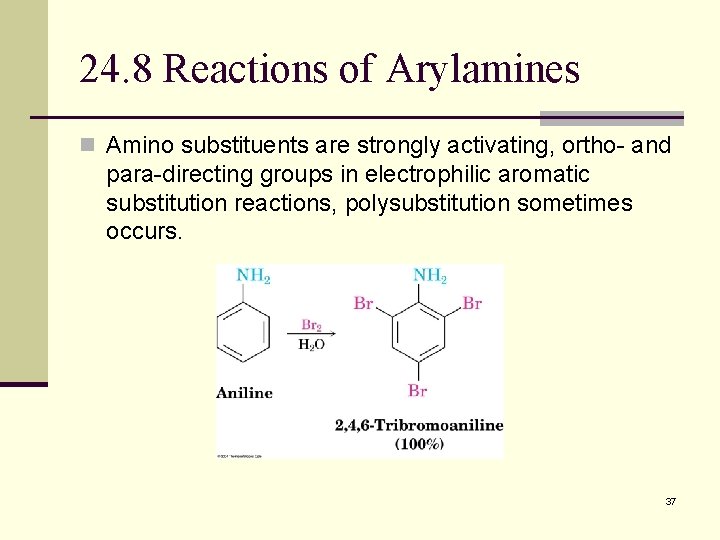
24. 8 Reactions of Arylamines n Amino substituents are strongly activating, ortho- and para-directing groups in electrophilic aromatic substitution reactions, polysubstitution sometimes occurs. 37

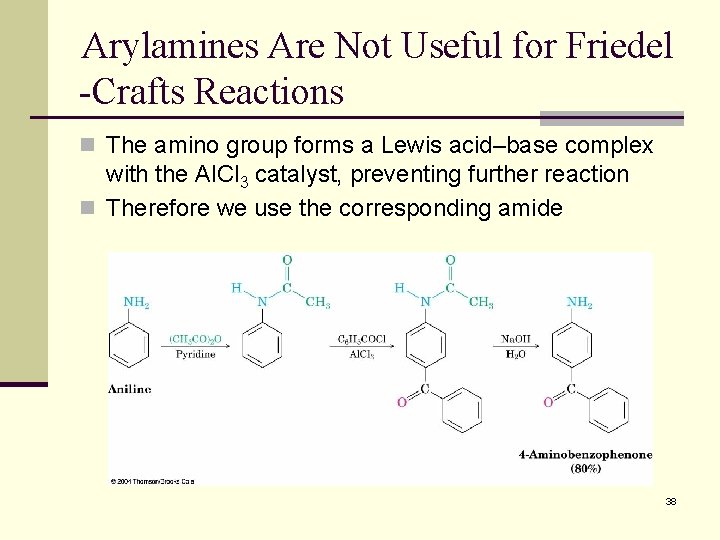
Arylamines Are Not Useful for Friedel -Crafts Reactions n The amino group forms a Lewis acid–base complex with the Al. Cl 3 catalyst, preventing further reaction n Therefore we use the corresponding amide 38

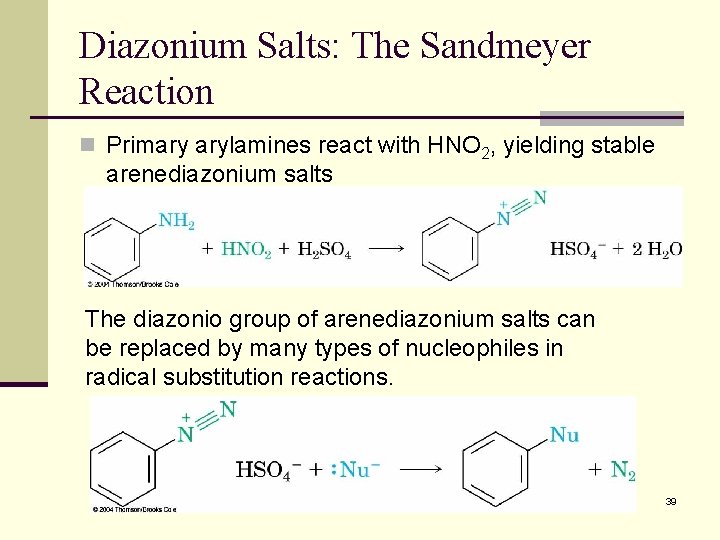
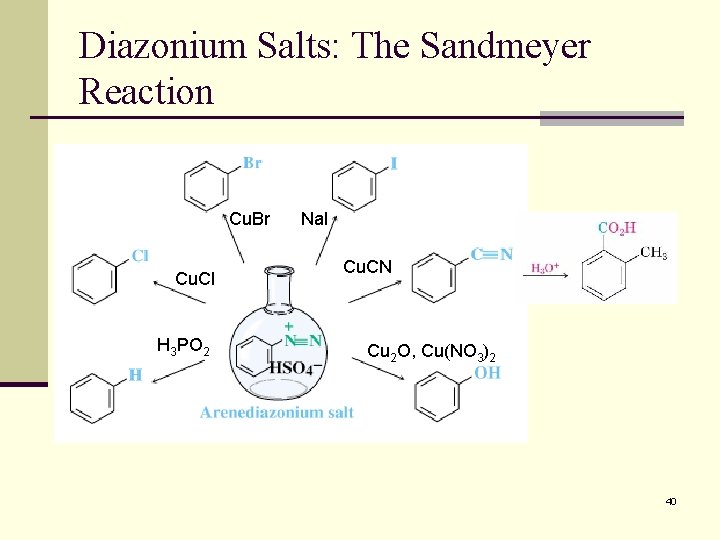
Diazonium Salts: The Sandmeyer Reaction n Primary arylamines react with HNO 2, yielding stable arenediazonium salts The diazonio group of arenediazonium salts can be replaced by many types of nucleophiles in radical substitution reactions. 39

Diazonium Salts: The Sandmeyer Reaction Cu. Br Cu. Cl H 3 PO 2 Na. I Cu. CN Cu 2 O, Cu(NO 3)2 40

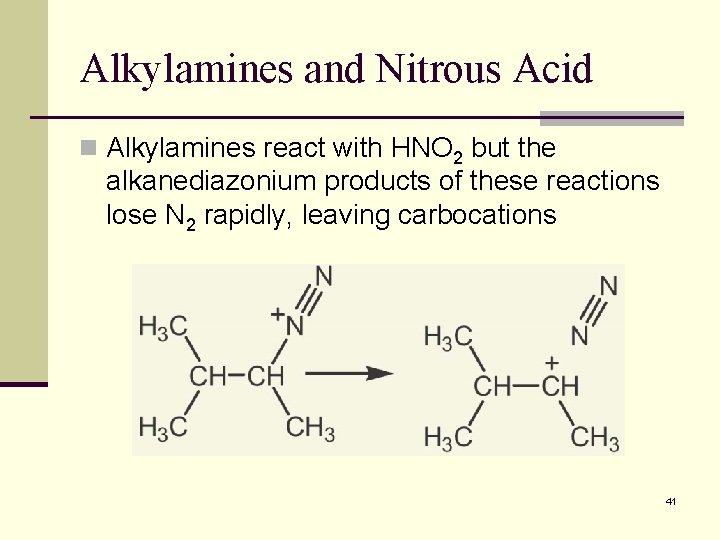
Alkylamines and Nitrous Acid n Alkylamines react with HNO 2 but the alkanediazonium products of these reactions lose N 2 rapidly, leaving carbocations 41

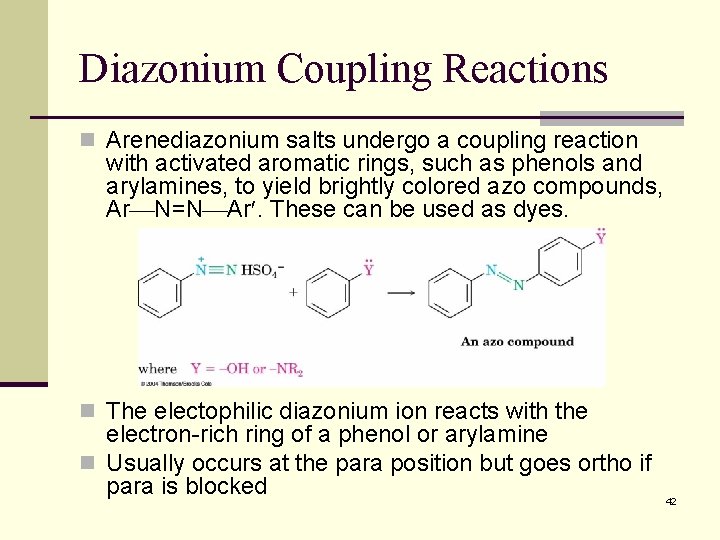
Diazonium Coupling Reactions n Arenediazonium salts undergo a coupling reaction with activated aromatic rings, such as phenols and arylamines, to yield brightly colored azo compounds, Ar N=N Ar. These can be used as dyes. n The electophilic diazonium ion reacts with the electron-rich ring of a phenol or arylamine n Usually occurs at the para position but goes ortho if para is blocked 42

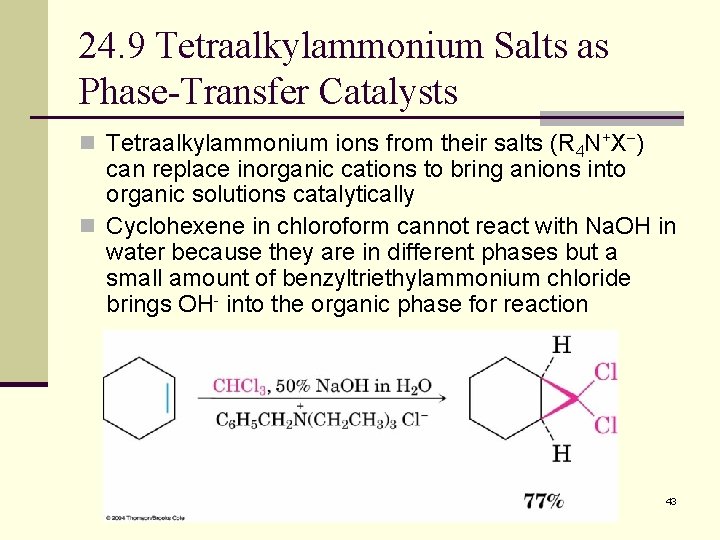
24. 9 Tetraalkylammonium Salts as Phase-Transfer Catalysts n Tetraalkylammonium ions from their salts (R 4 N+X ) can replace inorganic cations to bring anions into organic solutions catalytically n Cyclohexene in chloroform cannot react with Na. OH in water because they are in different phases but a small amount of benzyltriethylammonium chloride brings OH- into the organic phase for reaction 43

Definition of Phase Transfer Catalysis n The transfer of an inorganic ion such as OH from one phase to another is called phase transfer, and the tetraalkylammonium salt is a phase-transfer catalyst n Inorganic nucleophiles can be transferred from an aqueous phase to an organic phase, where they are much more reactive in substitution reactions 44

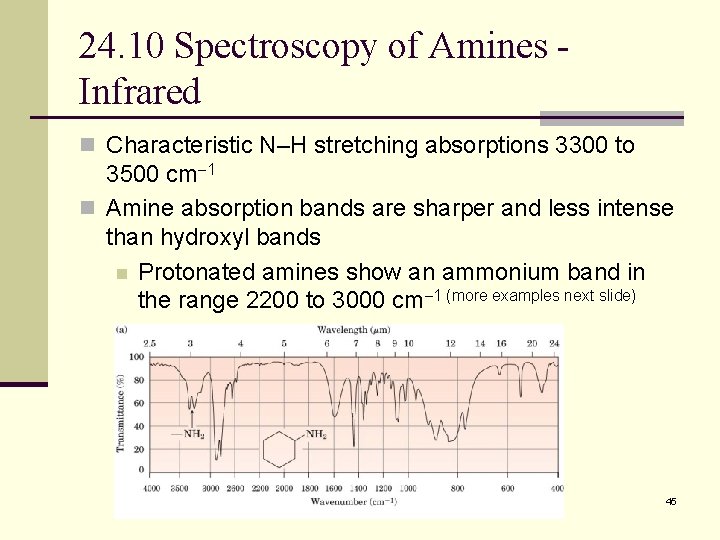
24. 10 Spectroscopy of Amines Infrared n Characteristic N–H stretching absorptions 3300 to 3500 cm 1 n Amine absorption bands are sharper and less intense than hydroxyl bands n Protonated amines show an ammonium band in the range 2200 to 3000 cm 1 (more examples next slide) 45

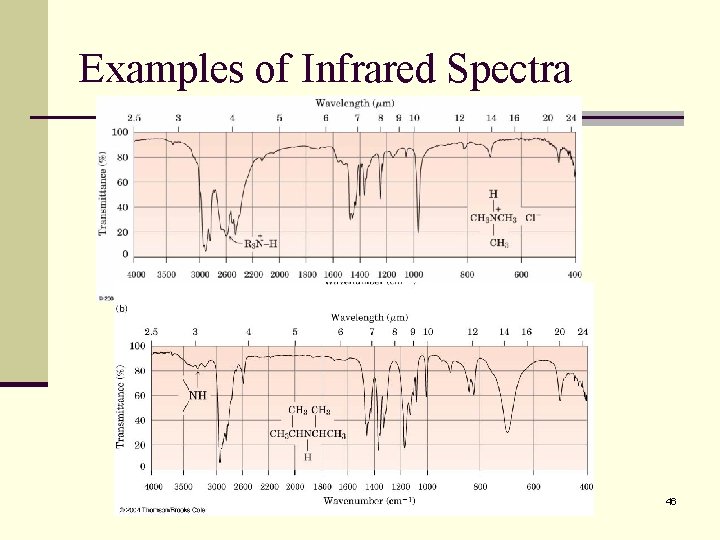
Examples of Infrared Spectra 46

Nuclear Magnetic Resonance Spectroscopy n N–H hydrogens appear as broad signals without clear -cut coupling to neighboring C–H hydrogens n In D 2 O exchange of N–D for N–H occurs, and the N– H signal disappears 47

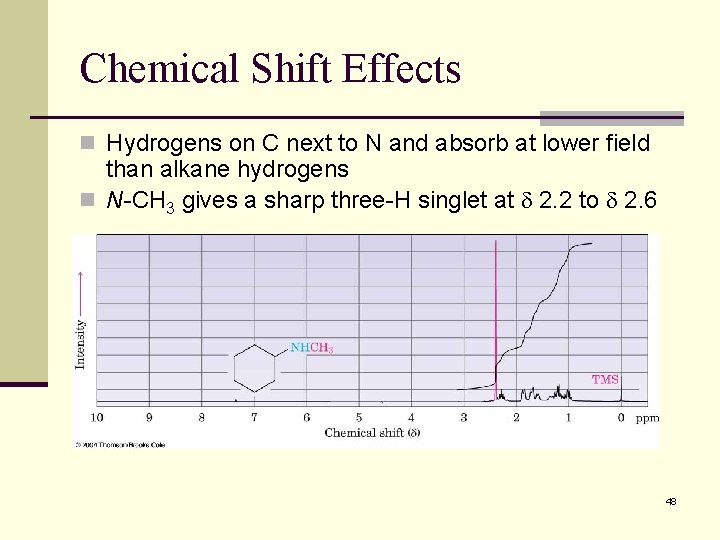
Chemical Shift Effects n Hydrogens on C next to N and absorb at lower field than alkane hydrogens n N-CH 3 gives a sharp three-H singlet at 2. 2 to 2. 6 48

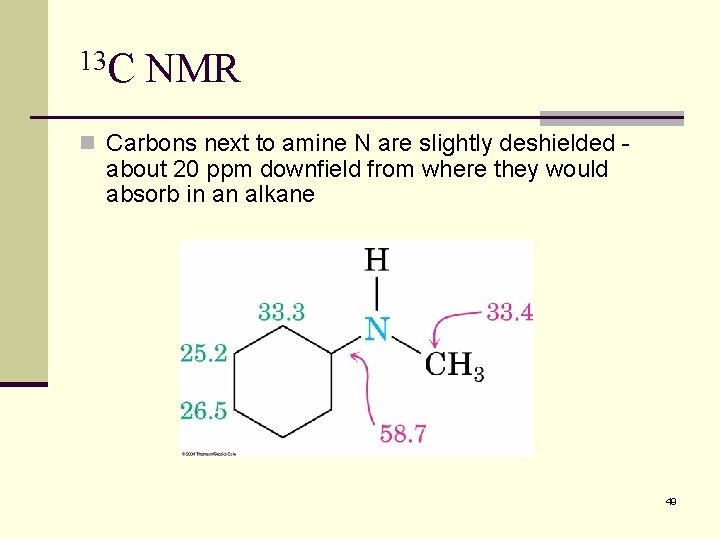
13 C NMR n Carbons next to amine N are slightly deshielded - about 20 ppm downfield from where they would absorb in an alkane 49

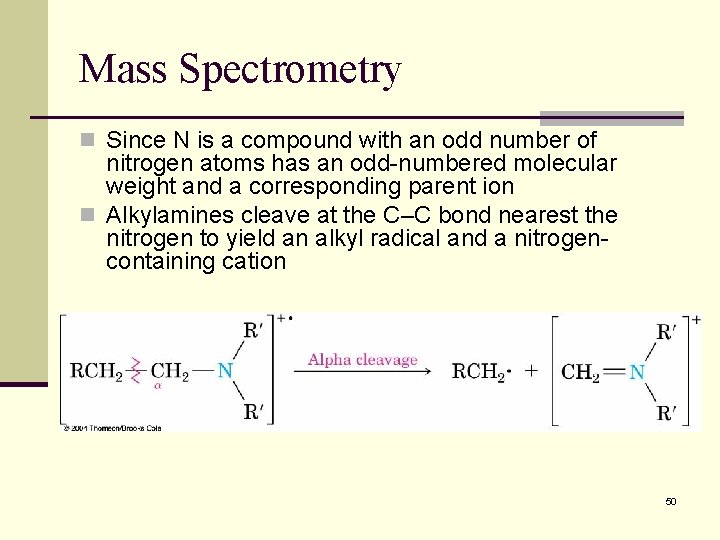
Mass Spectrometry n Since N is a compound with an odd number of nitrogen atoms has an odd-numbered molecular weight and a corresponding parent ion n Alkylamines cleave at the C–C bond nearest the nitrogen to yield an alkyl radical and a nitrogencontaining cation 50

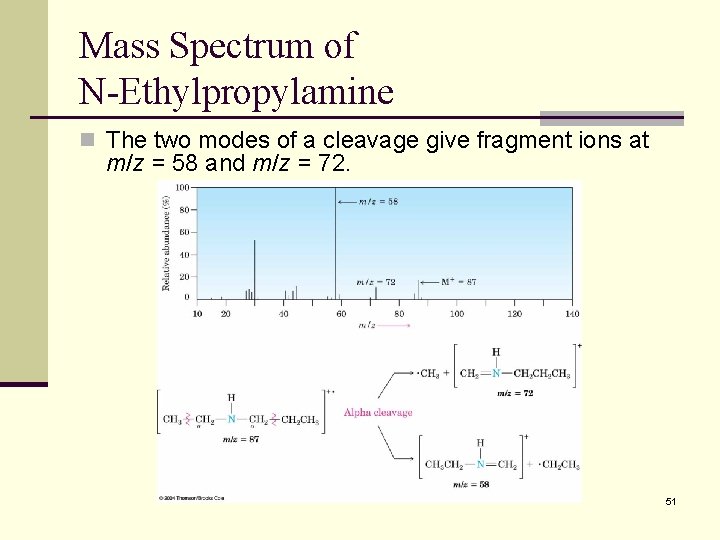
Mass Spectrum of N-Ethylpropylamine n The two modes of a cleavage give fragment ions at m/z = 58 and m/z = 72. 51

Chapter 26: Biomolecules: Amino Acids, Peptides, and Proteins

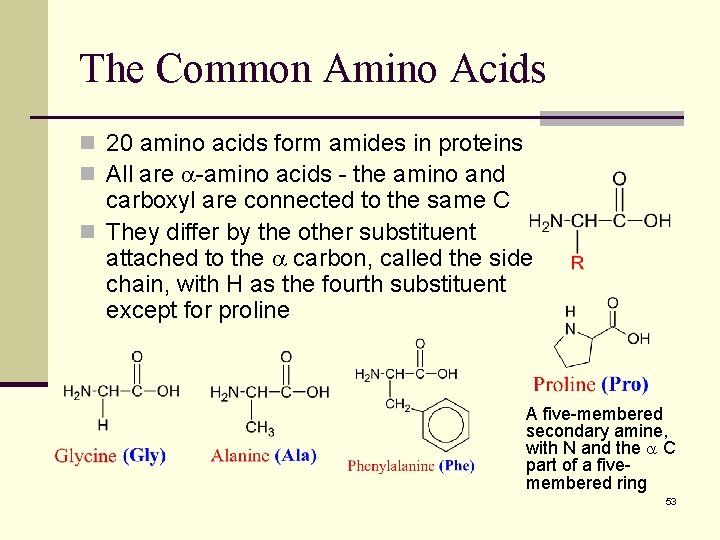
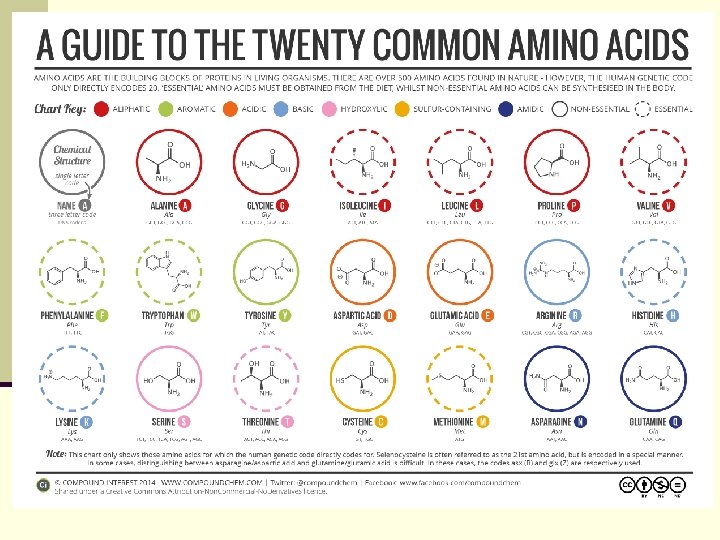
The Common Amino Acids n 20 amino acids form amides in proteins n All are -amino acids - the amino and carboxyl are connected to the same C n They differ by the other substituent attached to the carbon, called the side chain, with H as the fourth substituent except for proline A five-membered secondary amine, with N and the C part of a fivemembered ring 53

Abbreviations and Codes Alanine A, Ala Arginine R, Arg Asparagine N, Asn Aspartic acid D, Asp Cysteine C, Cys Glutamine Q, Gln Glutamic Acid E, Glu Glycine G, Gly Histidine H, His Isoleucine I, Ile Leucine L, Leu Lysine K, Lys Methionine M, Met Phenylalanine F, Phe Proline P, Pro Serine S, Ser Threonine T, Thr Tryptophan W, Trp Tyrosine Y, Tyr Valine V, Val 54

Essential Amino Acids n All 20 of the amino acids are necessary for protein synthesis n Humans can synthesize only 10 of the 20 n The other 10 must be obtained from food 55

56

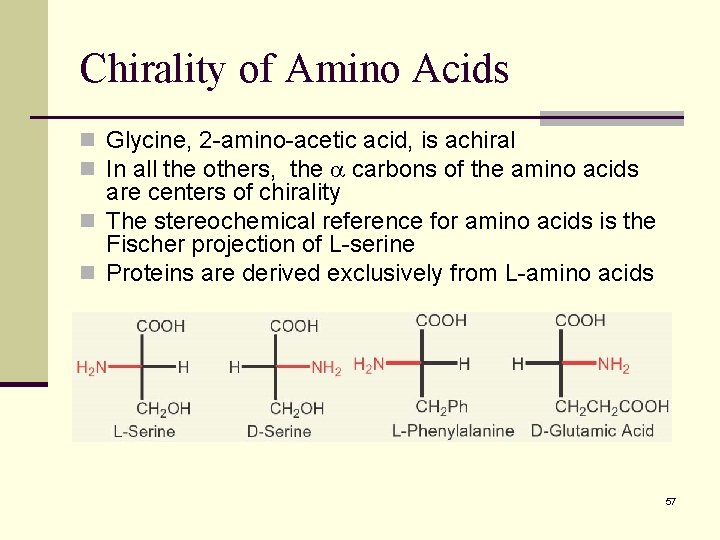
Chirality of Amino Acids n Glycine, 2 -amino-acetic acid, is achiral n In all the others, the carbons of the amino acids are centers of chirality n The stereochemical reference for amino acids is the Fischer projection of L-serine n Proteins are derived exclusively from L-amino acids 57

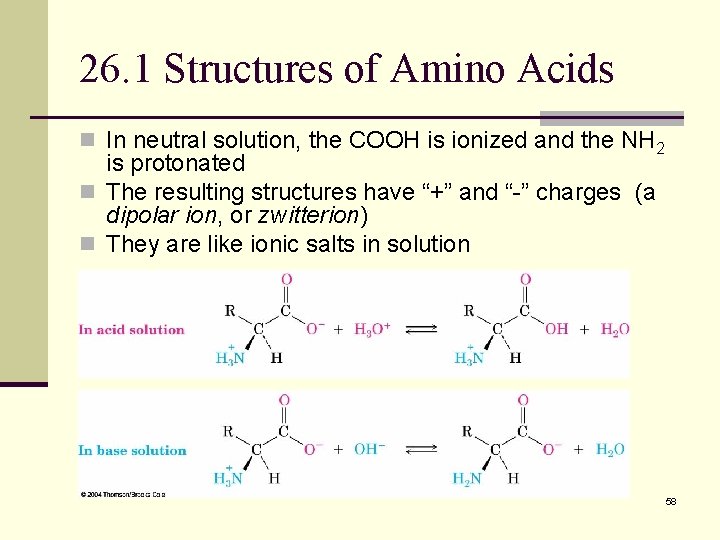
26. 1 Structures of Amino Acids n In neutral solution, the COOH is ionized and the NH 2 is protonated n The resulting structures have “+” and “-” charges (a dipolar ion, or zwitterion) n They are like ionic salts in solution 58

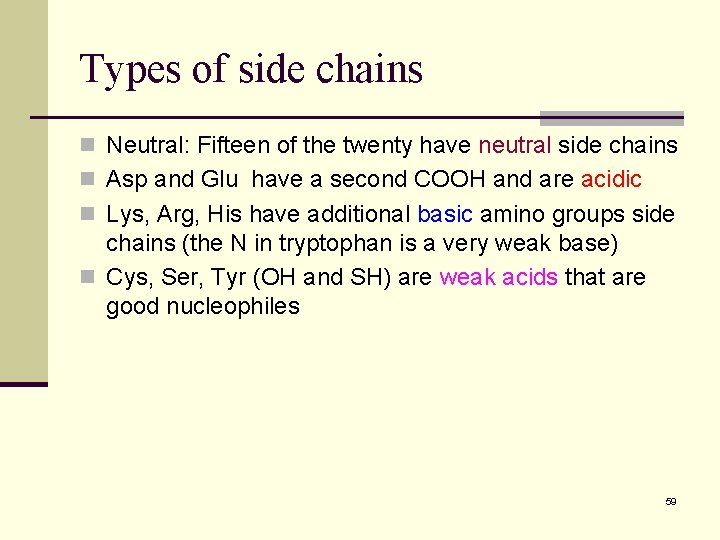
Types of side chains n Neutral: Fifteen of the twenty have neutral side chains n Asp and Glu have a second COOH and are acidic n Lys, Arg, His have additional basic amino groups side chains (the N in tryptophan is a very weak base) n Cys, Ser, Tyr (OH and SH) are weak acids that are good nucleophiles 59

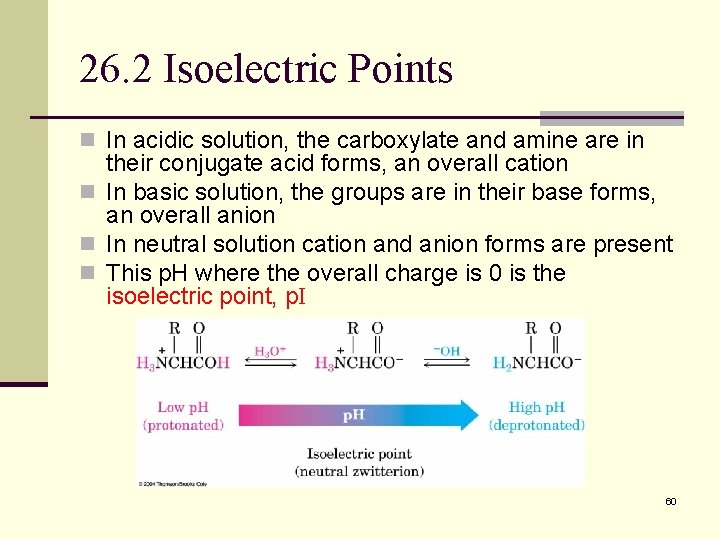
26. 2 Isoelectric Points n In acidic solution, the carboxylate and amine are in their conjugate acid forms, an overall cation n In basic solution, the groups are in their base forms, an overall anion n In neutral solution cation and anion forms are present n This p. H where the overall charge is 0 is the isoelectric point, p. I 60

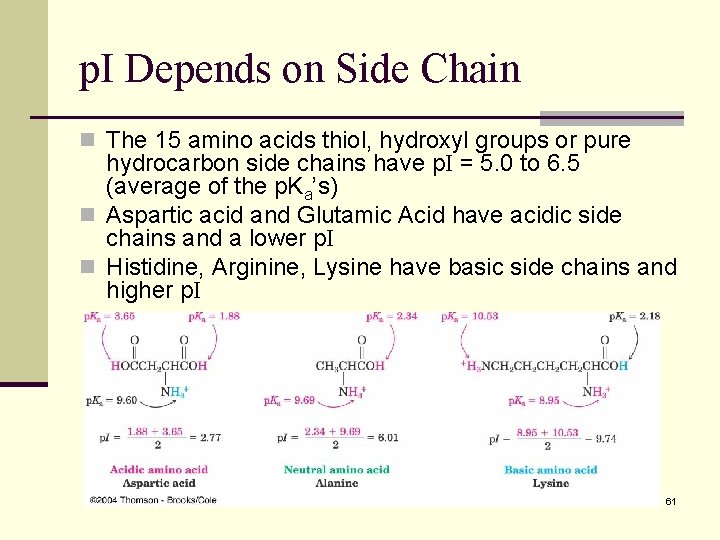
p. I Depends on Side Chain n The 15 amino acids thiol, hydroxyl groups or pure hydrocarbon side chains have p. I = 5. 0 to 6. 5 (average of the p. Ka’s) n Aspartic acid and Glutamic Acid have acidic side chains and a lower p. I n Histidine, Arginine, Lysine have basic side chains and higher p. I 61

Electrophoresis n Proteins have an overall p. I that depends on the net acidity/basicity of the side chains n The differences in p. I can be used for separating proteins on a solid phase permeated with liquid n Different amino acids migrate at different rates, depending on their isoelectric points and on the p. H of the aqueous buffer Protonated Species Isoelectric point above p. H of buffer Deprotonated Species 62 Isoelectric point below p. H buffer

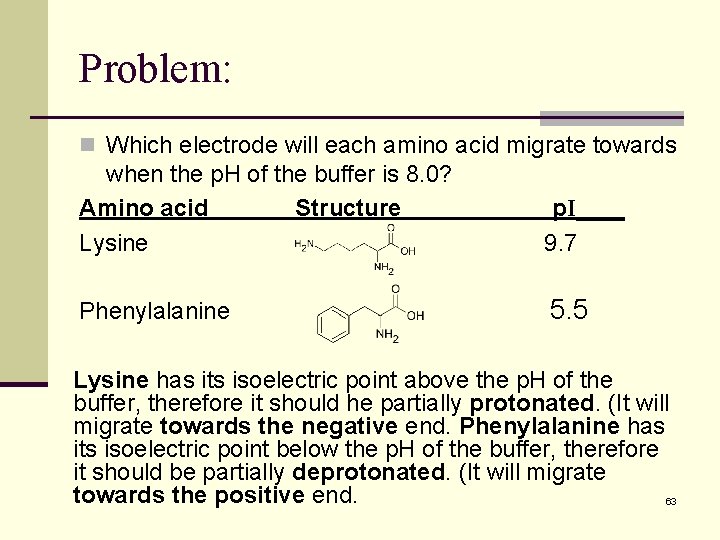
Problem: n Which electrode will each amino acid migrate towards when the p. H of the buffer is 8. 0? Amino acid Structure Lysine p. I____ 9. 7 Phenylalanine 5. 5 Lysine has its isoelectric point above the p. H of the buffer, therefore it should he partially protonated. (It will migrate towards the negative end. Phenylalanine has its isoelectric point below the p. H of the buffer, therefore it should be partially deprotonated. (It will migrate towards the positive end. 63

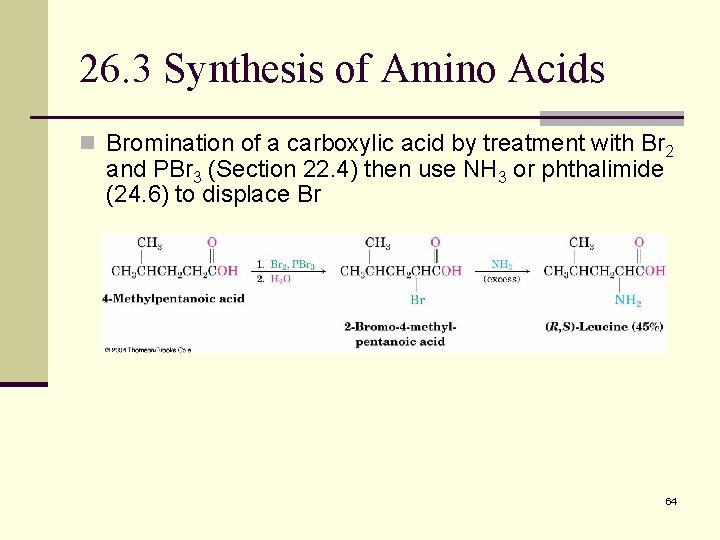
26. 3 Synthesis of Amino Acids n Bromination of a carboxylic acid by treatment with Br 2 and PBr 3 (Section 22. 4) then use NH 3 or phthalimide (24. 6) to displace Br 64

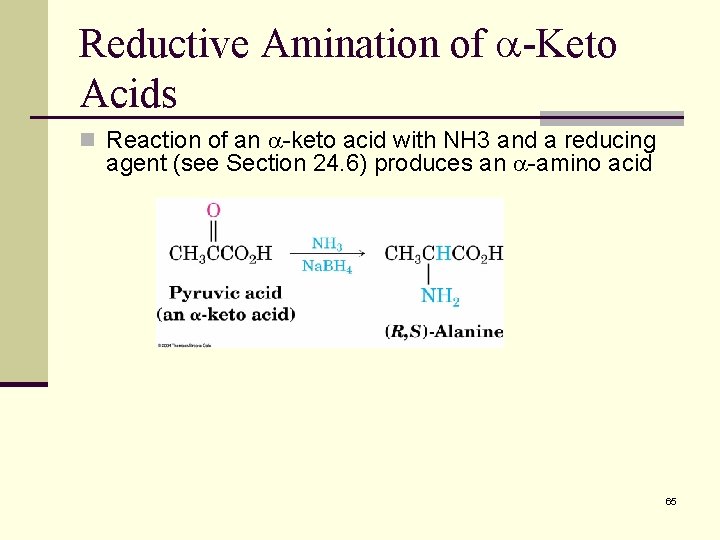
Reductive Amination of -Keto Acids n Reaction of an -keto acid with NH 3 and a reducing agent (see Section 24. 6) produces an -amino acid 65

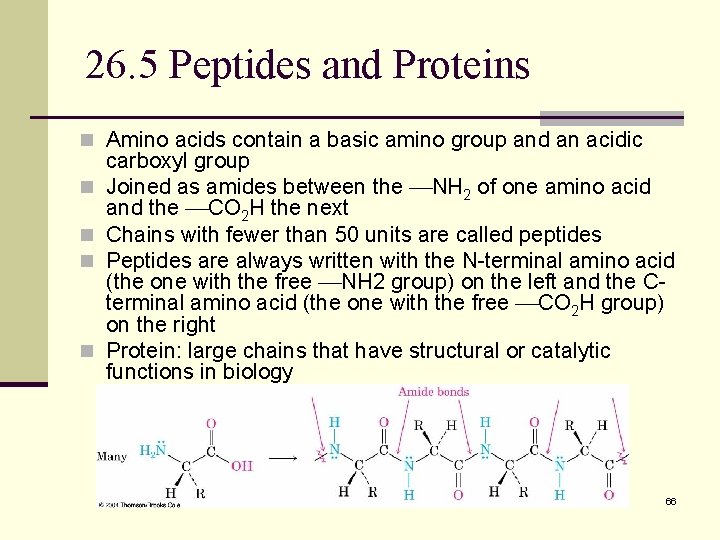
26. 5 Peptides and Proteins n Amino acids contain a basic amino group and an acidic n n carboxyl group Joined as amides between the NH 2 of one amino acid and the CO 2 H the next Chains with fewer than 50 units are called peptides Peptides are always written with the N-terminal amino acid (the one with the free NH 2 group) on the left and the Cterminal amino acid (the one with the free CO 2 H group) on the right Protein: large chains that have structural or catalytic functions in biology 66

Peptides Are classified according to the number of amino acids that are linked together. n n Dipeptides: Two amino acids Tripeptides: Three amino acids Polypeptides: Generally four or more amino acids Protein: Over eight amino acids 67

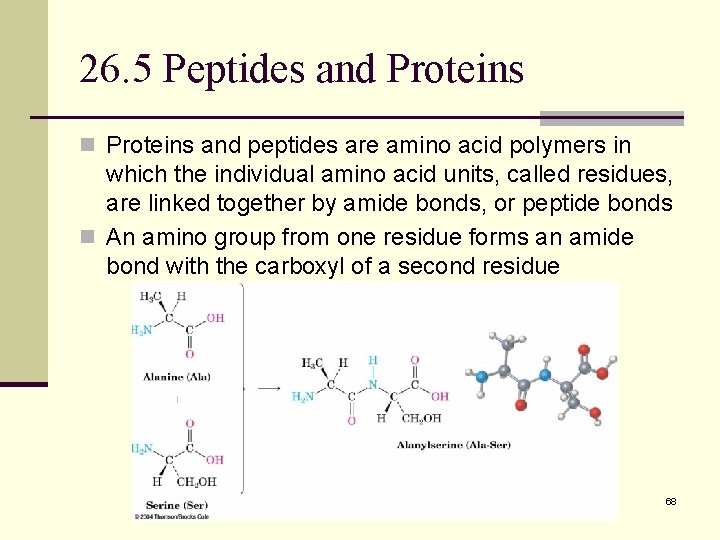
26. 5 Peptides and Proteins n Proteins and peptides are amino acid polymers in which the individual amino acid units, called residues, are linked together by amide bonds, or peptide bonds n An amino group from one residue forms an amide bond with the carboxyl of a second residue 68

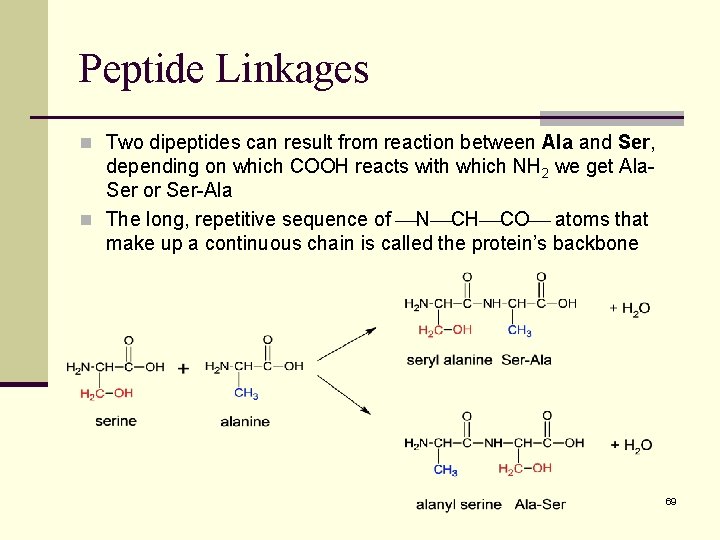
Peptide Linkages n Two dipeptides can result from reaction between Ala and Ser, depending on which COOH reacts with which NH 2 we get Ala. Ser or Ser-Ala n The long, repetitive sequence of N CH CO atoms that make up a continuous chain is called the protein’s backbone 69

Problem: n What are the peptides can be made from 3 different amino acids? Use A, B, C. A- B- C B- A- C C- A- B A- C- B B- C- A C- B- A 70

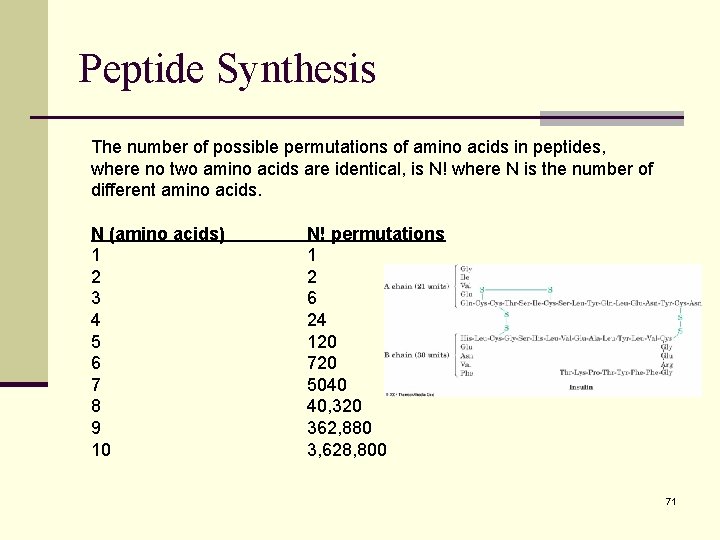
Peptide Synthesis The number of possible permutations of amino acids in peptides, where no two amino acids are identical, is N! where N is the number of different amino acids. N (amino acids) 1 2 3 4 5 6 7 8 9 10 N! permutations 1 2 6 24 120 720 5040 40, 320 362, 880 3, 628, 800 71

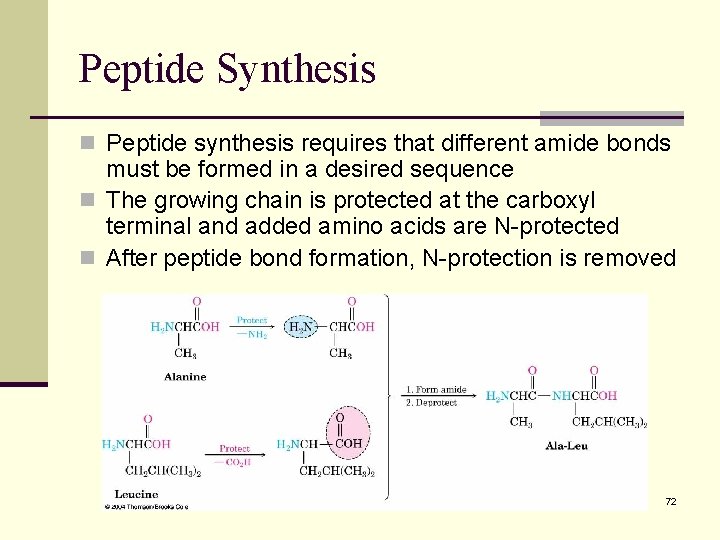
Peptide Synthesis n Peptide synthesis requires that different amide bonds must be formed in a desired sequence n The growing chain is protected at the carboxyl terminal and added amino acids are N-protected n After peptide bond formation, N-protection is removed 72

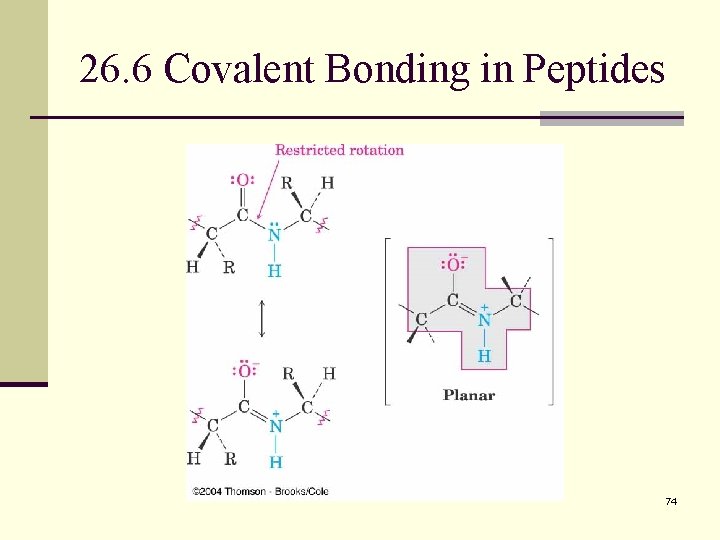
26. 6 Covalent Bonding in Peptides n The amide bond that links different amino acids together in peptides is no different from any other amide bond (see Section 24. 4). n Amide nitrogens are nonbasic because their unshared electron pair is delocalized by interaction with the carbonyl group. This overlap of the nitrogen p orbital with the π orbitals of the carbonyl group imparts a certain amount of double-bond character to the C–N bond and restricts rotation around it. n The amide bond is therefore planar, and the N–H is oriented 180° to the C=O. 73

26. 6 Covalent Bonding in Peptides 74

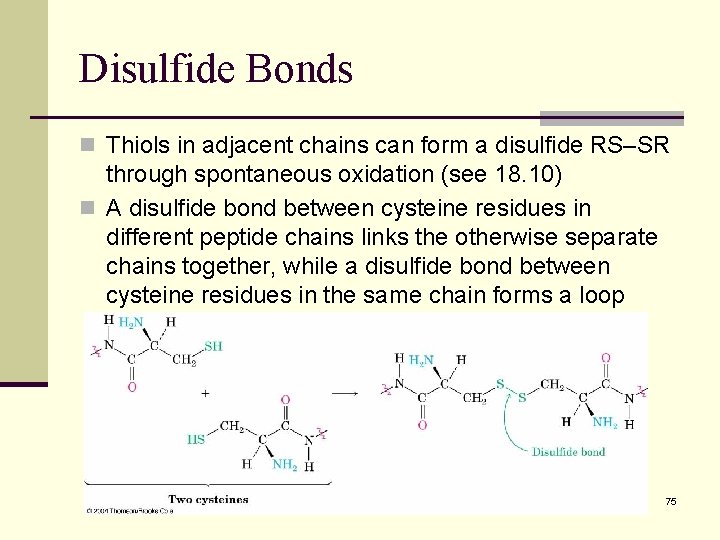
Disulfide Bonds n Thiols in adjacent chains can form a disulfide RS–SR through spontaneous oxidation (see 18. 10) n A disulfide bond between cysteine residues in different peptide chains links the otherwise separate chains together, while a disulfide bond between cysteine residues in the same chain forms a loop 75

76

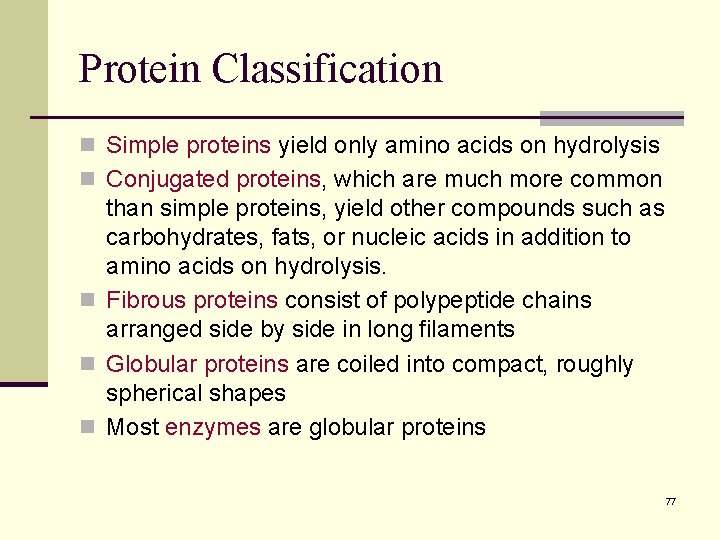
Protein Classification n Simple proteins yield only amino acids on hydrolysis n Conjugated proteins, which are much more common than simple proteins, yield other compounds such as carbohydrates, fats, or nucleic acids in addition to amino acids on hydrolysis. n Fibrous proteins consist of polypeptide chains arranged side by side in long filaments n Globular proteins are coiled into compact, roughly spherical shapes n Most enzymes are globular proteins 77

Some Common Fibrous and Globular Proteins 78

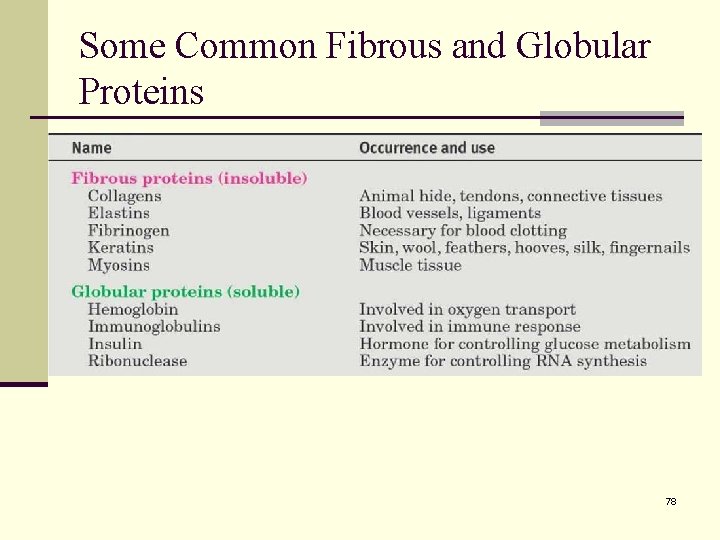
26. 13 Protein Structure n The primary structure of a protein is simply the amino acid sequence. n The secondary structure of a protein describes how segments of the peptide backbone orient into a regular pattern. There are three major types of protein secondary structure, the alpha helix, beta pleated sheet, and random. n The tertiary structure describes how the entire protein molecule coils into an overall threedimensional shape. Disulfide bonds, hydrogen bonds, salt bridges, and hydrophobic interactions are tertiary features. 79

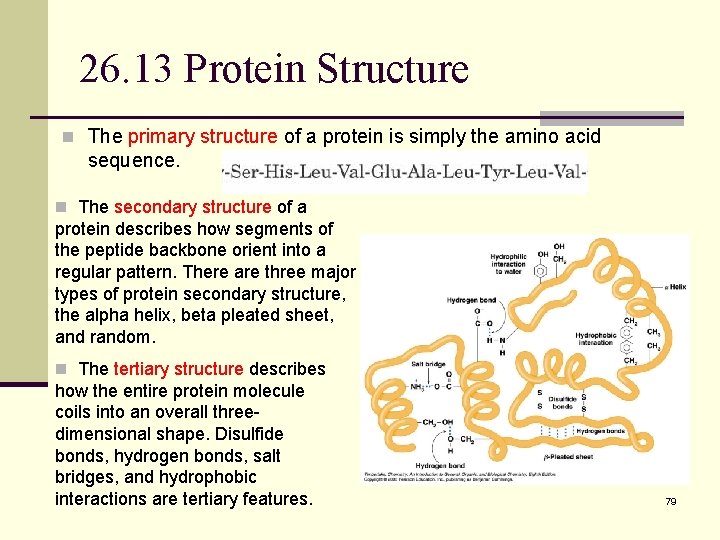
26. 13 Protein Structure The quaternary structure describes how different protein molecules come together to yield large aggregate structures. Fibrous and Globular are tertiary structure features. Hemoglobin (globular) 80

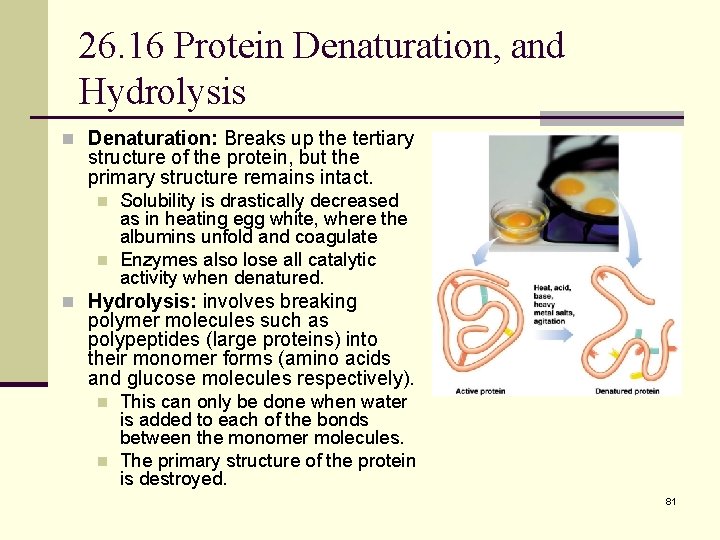
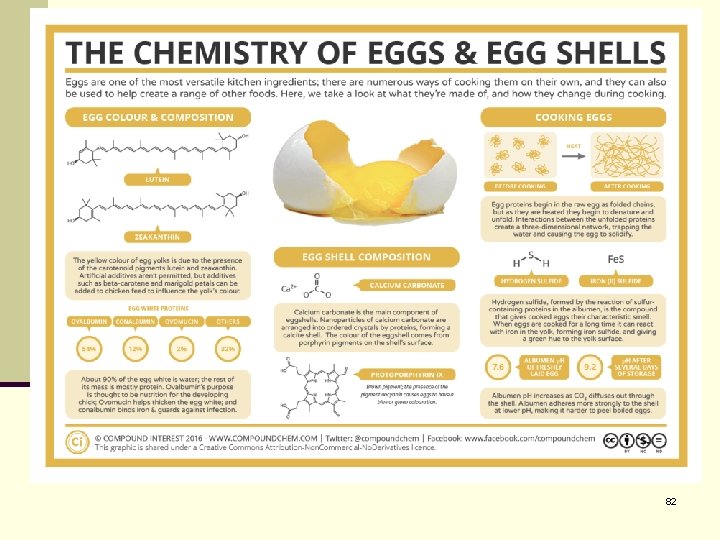
26. 16 Protein Denaturation, and Hydrolysis n Denaturation: Breaks up the tertiary structure of the protein, but the primary structure remains intact. n n Solubility is drastically decreased as in heating egg white, where the albumins unfold and coagulate Enzymes also lose all catalytic activity when denatured. n Hydrolysis: involves breaking polymer molecules such as polypeptides (large proteins) into their monomer forms (amino acids and glucose molecules respectively). n n This can only be done when water is added to each of the bonds between the monomer molecules. The primary structure of the protein is destroyed. 81

82

YOU ARE NOW FINISHED WITH CHEM 212 & 213 Lectures!! 83