Chapter 7 Ionic Compounds and Metals Mr Stripling










![Compound Formation & Charge �Ca: [Ar] 4 s 2 Needs to lose 2 electrons Compound Formation & Charge �Ca: [Ar] 4 s 2 Needs to lose 2 electrons](https://slidetodoc.com/presentation_image_h/158f52215b9bb2ddf4afb61859a22099/image-15.jpg)








- Slides: 31

Chapter 7: Ionic Compounds and Metals Mr. Stripling Pre-AP Chemistry Room 402

Ion Formation �Valence electrons are involved in the formation of chemical bonds between two atoms. �Chemical Bond – force that holds two atoms together �Chemical bonds form by: 1. Attraction between the positive nucleus of one atom and the negative electrons of another atom 2. The attraction between positive ions and negative ions

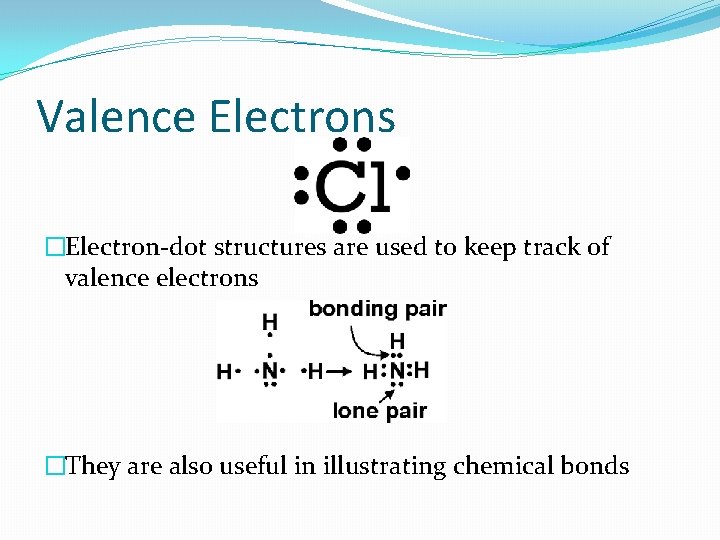
Valence Electrons �Electron-dot structures are used to keep track of valence electrons �They are also useful in illustrating chemical bonds

Review � Ionization Energy – how easily an atom loses an electron �Electron Affinity – how much attraction an atom has for electrons �Reactivity depends on the number of valence electrons �Elements tend to react in order to acquire the stable electron configurations of noble gases

Positive Ion Formation �Occurs when an atom loses one or more valence electrons �Positively charged ions are called cations

�Ne: 1 s 22 p 6 �Na: 1 s 22 p 63 s 1 �When Na loses its valence electron its electron configuration becomes identical to Ne �Na gains a stable electron configuration �Na DOES NOT BECOME Ne �Na is a sodium ion with a single positive charge

Metal Ions �Reactive because they lose valence electrons easily �Groups 1 and 2 metals are the most reactive metals �Some group 13 atoms also form ions

Transition Metal Ions �Have an outer energy level of ns 2 �Form 2+ ions when forming positive ions �d electrons can also be lost, making it possible for transition metals to form ions of 3+ or greater �Difficult to predict the number of electrons that will be lost �Fe forms both Fe 2+ and Fe 3+ ions

Pseudo-Noble Gas Configurations �Electron configurations other that the noble gas notation can provide stability �Elements in groups 11 -14 lose electrons to form an outer energy level containing full s, p, and d sublevels

Negative Ion Formation �Nonmetals, on the right side of the table, easily gain electrons to attain a stable outer electron configuration �Gaining electrons forms anions, negatively charged ions �To designate an anion, the ending –ide is added to the root name of the element �Chlorine atom -> chloride anion

Nonmetal Ions �Nonmetals gain valence electrons in order to have a total of 8 �Some nonmetals can lose OR gain electrons to form an octet �Phosphorus can lose five or gain three valence electrons to form an octet �In general, group 15 -17 elements gain valence electrons to form an octet


Formation of an Ionic Bond �Ionic Bond – the electrostatic force that holds oppositely charged particles together in an ionic compound �Ionic compounds – compounds that contain ionic bonds. �Ionic bonds that occur between metals and oxygen form an oxide ion

Binary Ionic Compounds �Compounds that ONLY contain TWO different elements �Part metallic cation, part nonmetallic anion �Examples: �Sodium chloride (Na. Cl) �Magnesium oxide (Mg. O)
![Compound Formation Charge Ca Ar 4 s 2 Needs to lose 2 electrons Compound Formation & Charge �Ca: [Ar] 4 s 2 Needs to lose 2 electrons](https://slidetodoc.com/presentation_image_h/158f52215b9bb2ddf4afb61859a22099/image-15.jpg)
Compound Formation & Charge �Ca: [Ar] 4 s 2 Needs to lose 2 electrons to be stable �F: [He] 2 s 22 p 5 Must gain 1 electron for stability �Number of electrons lost and gained must be equal � 2 F atoms need to accept the 2 electrons from the Ca atom �Yields calcium fluoride (Ca. F 2)

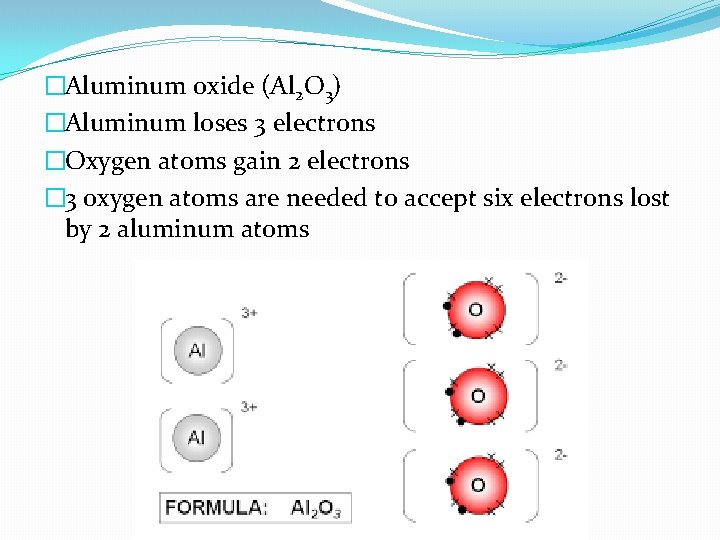
�Aluminum oxide (Al 2 O 3) �Aluminum loses 3 electrons �Oxygen atoms gain 2 electrons � 3 oxygen atoms are needed to accept six electrons lost by 2 aluminum atoms

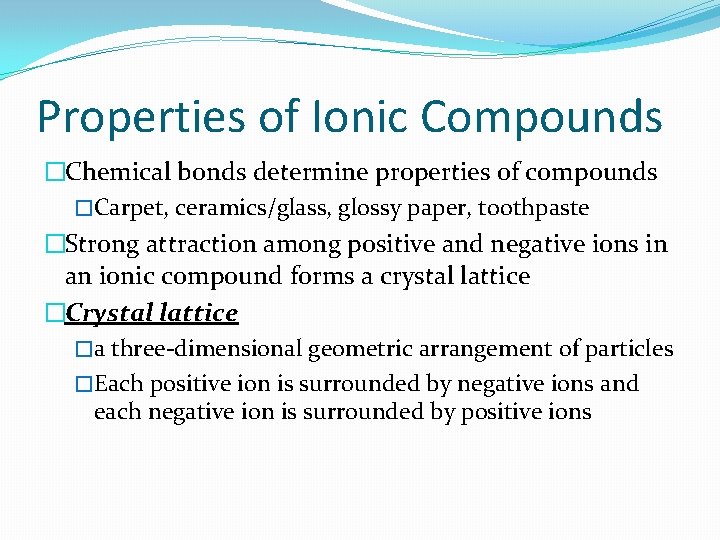
Properties of Ionic Compounds �Chemical bonds determine properties of compounds �Carpet, ceramics/glass, glossy paper, toothpaste �Strong attraction among positive and negative ions in an ionic compound forms a crystal lattice �Crystal lattice �a three-dimensional geometric arrangement of particles �Each positive ion is surrounded by negative ions and each negative ion is surrounded by positive ions

Physical Properties �MP, BP, and hardness are physical properties that depend on how strong particles that make compounds are attracted to one another �Electrical conduction depends on availability of free moving charged particles �Ionic solids don’t conduct electricity �Ionic liquids do �An ionic compound whose aqueous solution conducts an electric current is an electrolyte

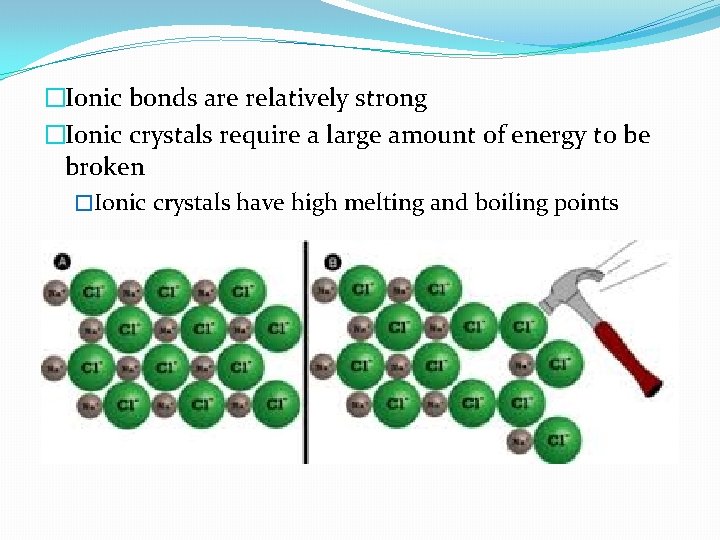
�Ionic bonds are relatively strong �Ionic crystals require a large amount of energy to be broken �Ionic crystals have high melting and boiling points

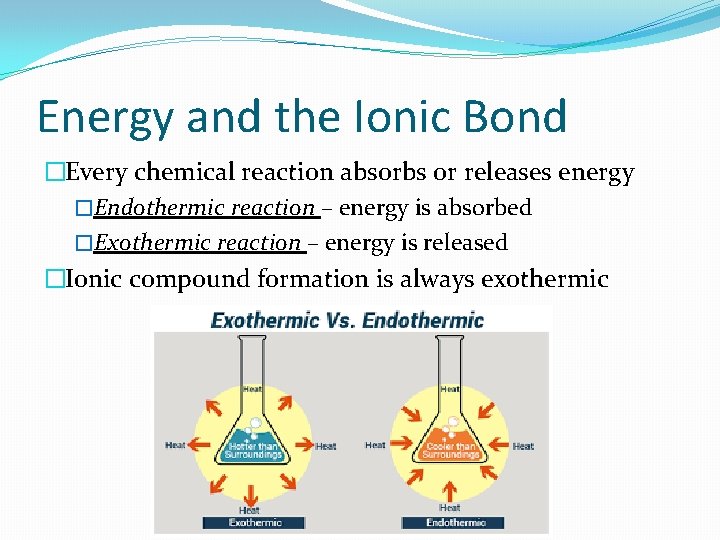
Energy and the Ionic Bond �Every chemical reaction absorbs or releases energy �Endothermic reaction – energy is absorbed �Exothermic reaction – energy is released �Ionic compound formation is always exothermic

Lattice Energy �Energy required to separate 1 mole of ions of an ionic compound �Strength of the forces holding ions in place �Directly related to the size of the ions bonded

Classwork �Problems 7 -11 on page 212 �Problems 1 & 2 (Chapter 7) on page 980 �Only write the formula units

Formulas for Ionic Compounds �Formula Unit – the chemical formula for an ionic compound represent the simplest ratio of the ions involved �Mg. Cl 2 – magnesium and chloride ions exist in a 1: 2 ratio �Contains one Mg 2+ ion and two Cl- ions for a total charge of zero �Mg 2+ and Cl- are monatomic ions – one-atom ions

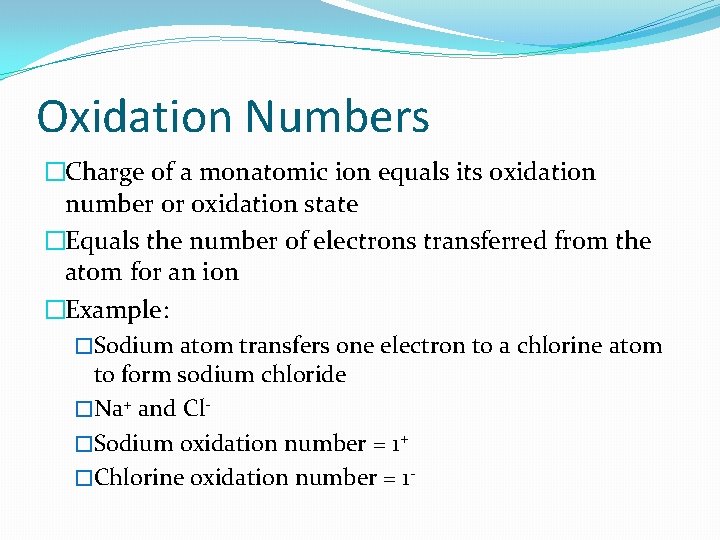
Oxidation Numbers �Charge of a monatomic ion equals its oxidation number or oxidation state �Equals the number of electrons transferred from the atom for an ion �Example: �Sodium atom transfers one electron to a chlorine atom to form sodium chloride �Na+ and Cl�Sodium oxidation number = 1+ �Chlorine oxidation number = 1 -

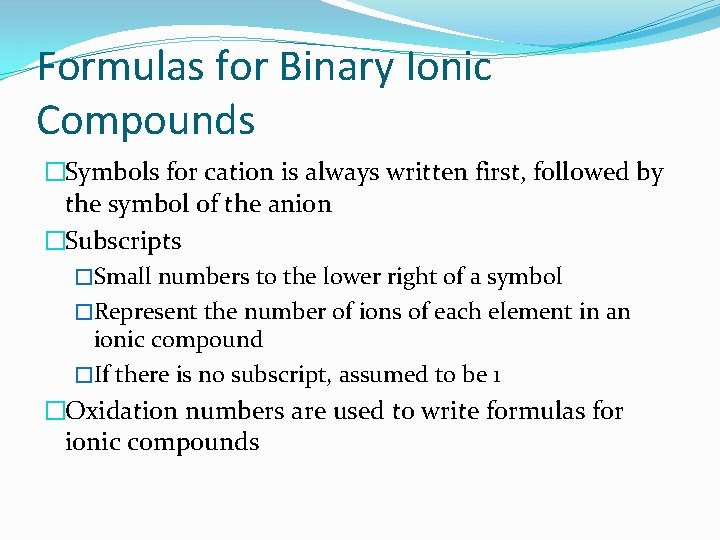
Formulas for Binary Ionic Compounds �Symbols for cation is always written first, followed by the symbol of the anion �Subscripts �Small numbers to the lower right of a symbol �Represent the number of ions of each element in an ionic compound �If there is no subscript, assumed to be 1 �Oxidation numbers are used to write formulas for ionic compounds

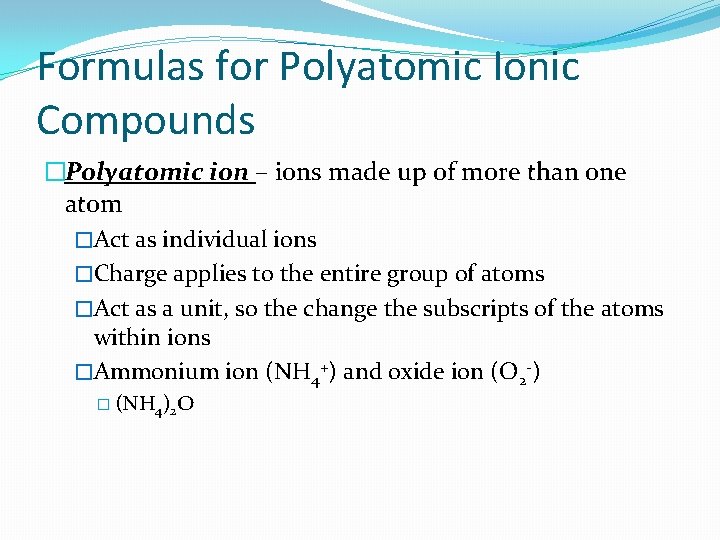
Formulas for Polyatomic Ionic Compounds �Polyatomic ion – ions made up of more than one atom �Act as individual ions �Charge applies to the entire group of atoms �Act as a unit, so the change the subscripts of the atoms within ions �Ammonium ion (NH 4+) and oxide ion (O 2 -) � (NH 4)2 O

Naming an Oxyanion �Oxyanion – a polyatomic ion composed of an element (usually nonmetal) bonded to one or more oxygen atoms

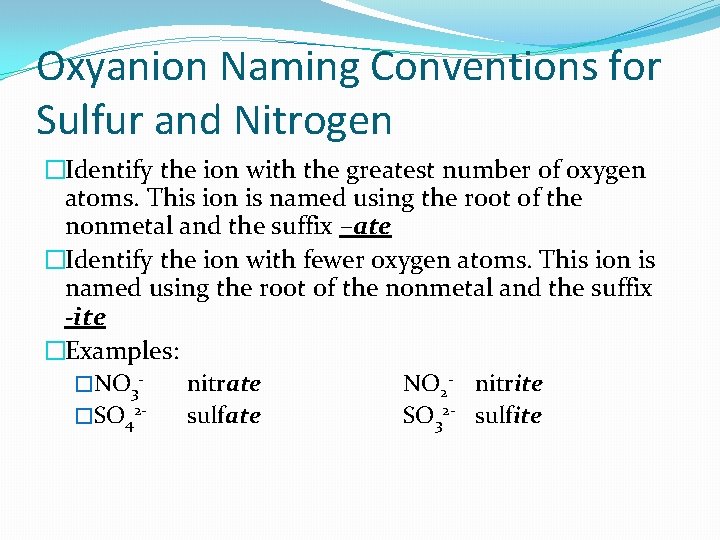
Oxyanion Naming Conventions for Sulfur and Nitrogen �Identify the ion with the greatest number of oxygen atoms. This ion is named using the root of the nonmetal and the suffix –ate �Identify the ion with fewer oxygen atoms. This ion is named using the root of the nonmetal and the suffix -ite �Examples: �NO 3 nitrate NO 2 - nitrite �SO 42 sulfate SO 32 - sulfite

Oxyanion Naming Convention for Chlorine �The oxyanion with the greatest number of oxygen atoms is named using the prefix per-, the root of the nonmetal and the suffix –ate �The oxyanion with one fewer oxygen atom is named using the root of the nonmetal and the suffix –ate �The oxyanion with two fewer oxygen atoms is named using the root of the nonmetal and the suffix –ite �The oxyanion with three fewer oxygen atoms is named using the prefix hypo-, the root of the nonmetal, and the suffix -ite

Classwork �Problems 19 -22 on page 221 �Problems 24 -26 on page 222 �Problems 28 -32 on page 223

Read Page 226