PHYSICAL PROPERTIES It is a colorless gas with















- Slides: 21

PHYSICAL PROPERTIES • It is a colorless gas with a faint sweet smell • It is fairly soluble in cold water but insoluble in hot/warm water and that is why it is collected over warm water. 11/27/2020 WANYERA C

An aqueous solution of the gas is neutral to litmus paper. It is slightly denser than air. (has a density of 1. 977 kg/m 3 while that of air is 1. 2929 kg/m 3) Has a melting point of -90. 8°C and boiling point of -88. 5°C 11/27/2020 WANYERA C

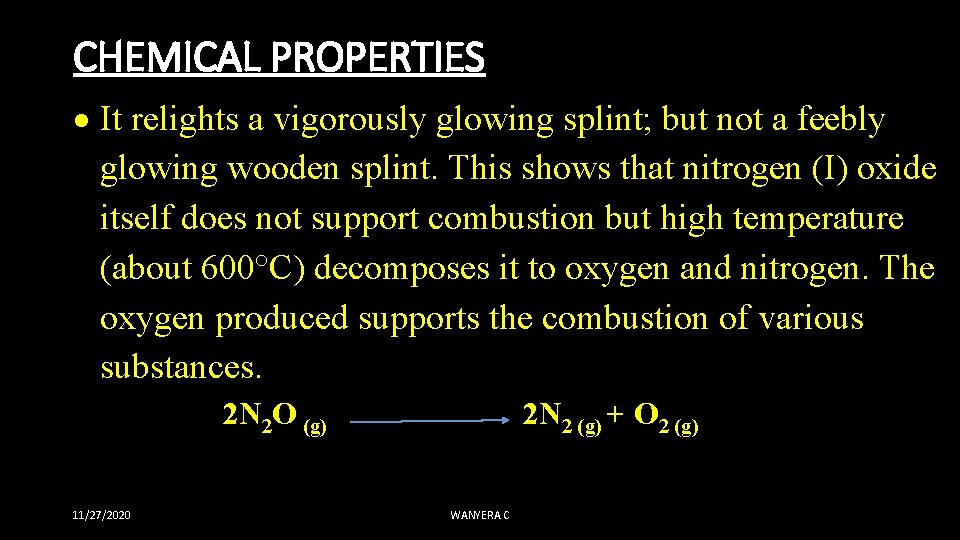
CHEMICAL PROPERTIES It relights a vigorously glowing splint; but not a feebly glowing wooden splint. This shows that nitrogen (I) oxide itself does not support combustion but high temperature (about 600°C) decomposes it to oxygen and nitrogen. The oxygen produced supports the combustion of various substances. 2 N 2 O (g) 2 N 2 (g) + O 2 (g) 11/27/2020 WANYERA C

It is thus distinguished from oxygen by its sweet smell. Burning metals and some non - metals continue to burn in the gas forming their corresponding oxides and nitrogen gas are formed. 11/27/2020 WANYERA C

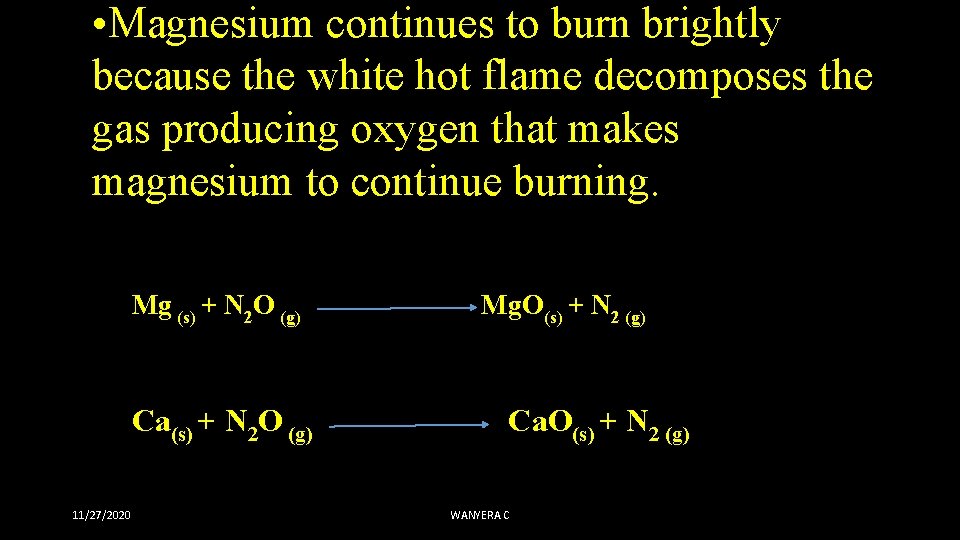
• Magnesium continues to burn brightly because the white hot flame decomposes the gas producing oxygen that makes magnesium to continue burning. Mg (s) + N 2 O (g) Mg. O(s) + N 2 (g) Ca(s) + N 2 O (g) Ca. O(s) + N 2 (g) 11/27/2020 WANYERA C

• When the gas is passed over heated copper metal, nitrogen gas and copper (II) oxide are formed. Cu(s) + N 2 O (g) Cu. O(s) + N 2 (g) Diagram KLB Pg 140 fig 4. 5 11/27/2020 WANYERA C

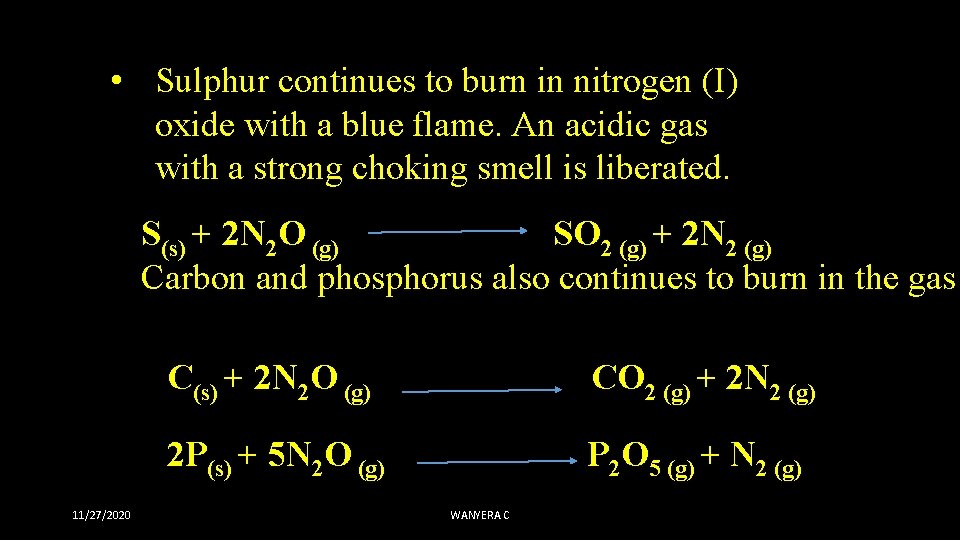
• Sulphur continues to burn in nitrogen (I) oxide with a blue flame. An acidic gas with a strong choking smell is liberated. S(s) + 2 N 2 O (g) SO 2 (g) + 2 N 2 (g) Carbon and phosphorus also continues to burn in the gas C(s) + 2 N 2 O (g) CO 2 (g) + 2 N 2 (g) 2 P(s) + 5 N 2 O (g) P 2 O 5 (g) + N 2 (g) 11/27/2020 WANYERA C

TESTS FOR NITROGEN (I) OXIDE Because of its ability to re-light a glowing splint and to allow various other substances to burn in it, the gas can be confused with oxygen. The two can be distinguished by the following properties 11/27/2020 WANYERA C

Property oxygen Nitrogen (I) Oxide Smell No smell Sweet smell Solubility in water Reaction with N 2 O Reaction with hot copper Almost insoluble in cold water Forms brown fumes of NO 2 Forms black copper (II) oxide only Fairly soluble in cold water No reaction 11/27/2020 WANYERA C Forms copper (II) oxide and nitrogen gas

USES OF NITROGEN (I) OXIDE Was used as a mild anesthetic during minor surgical operations when used with oxygen. But patients recovering from such surgery laugh hysterically hence commonly called laughing gas. Is used in rocket motors as an oxidizer. 11/27/2020 WANYERA C

Is used in internal combustion engine of racing cars to cause engine to burn more fuel by providing more oxygen for powerful combustion for more energy. 11/27/2020 WANYERA C

Is used as a food additive in which as an inert gas is used to displace oxygen to inhibit bacteria growth when packaging of snacks. Used to produce flames for analytical work. 11/27/2020 WANYERA C

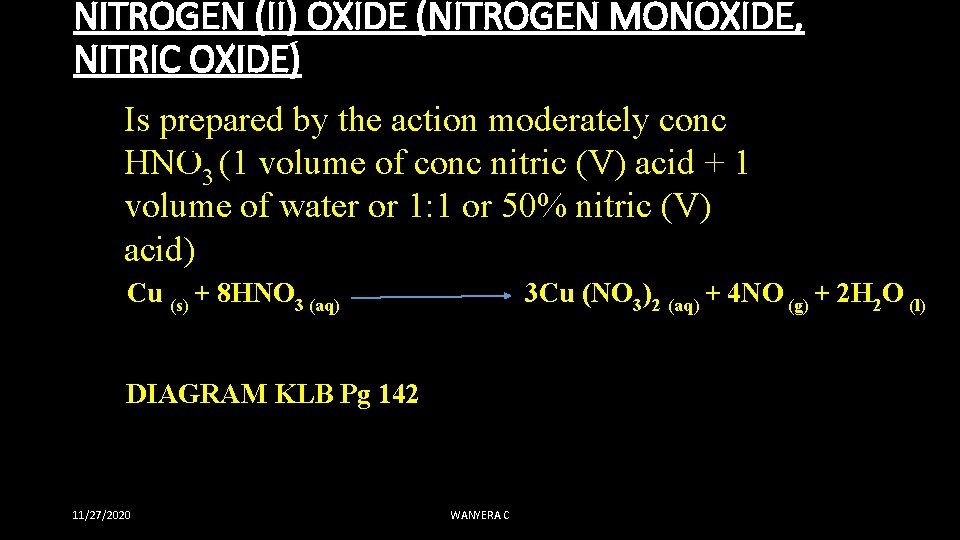
NITROGEN (II) OXIDE (NITROGEN MONOXIDE, NITRIC OXIDE) Is prepared by the action moderately conc HNO 3 (1 volume of conc nitric (V) acid + 1 volume of water or 1: 1 or 50% nitric (V) acid) Cu (s) + 8 HNO 3 (aq) 3 Cu (NO 3)2 (aq) + 4 NO (g) + 2 H 2 O (l) DIAGRAM KLB Pg 142 11/27/2020 WANYERA C

A vigorous reaction occurs and brown fumes of nitrogen (IV) oxide are produced due to presence of oxygen in the apparatus. This gas dissolves in water. The remaining gas is nitrogen (II) oxide. 11/27/2020 WANYERA C

Physical properties of NO Colorless Slightly denser than air. Is slightly soluble in water. Forms a neutral solution. Has a melting point and boiling point of -164ºC and 152ºC respectively. NB its smell is unknown because it quickly gets oxidized to nitrogen (IV) oxide when exposed to air. 11/27/2020 WANYERA C

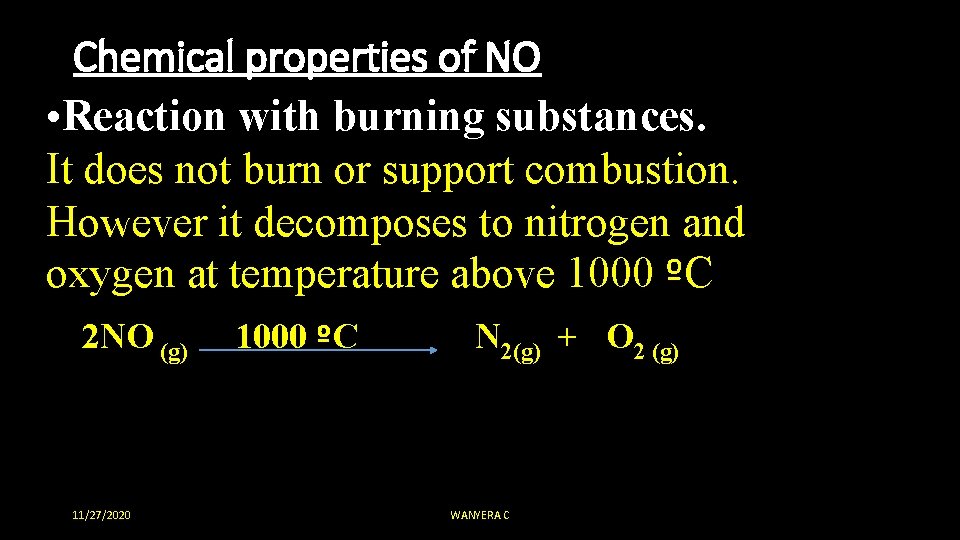
Chemical properties of NO • Reaction with burning substances. It does not burn or support combustion. However it decomposes to nitrogen and oxygen at temperature above 1000 ºC 2 NO (g) 1000 ºC N 2(g) + O 2 (g) 11/27/2020 WANYERA C

The oxygen produced will support the combustion of hot burning substances. The substances are oxidized. 2 Mg (s) + 2 NO (g) 2 Mg. O (s) + N 2 (g) P 4 (s) + 10 NO (g) 2 P 2 O 5(s) + 5 N 2 (g) 2 Cu(s) + 2 NO (g) 2 Cu. O(s) + N 2 (g) The heat energy produced by a burning splint is so weak that it cannot decompose NO and thus it is extinguished. 11/27/2020 WANYERA C

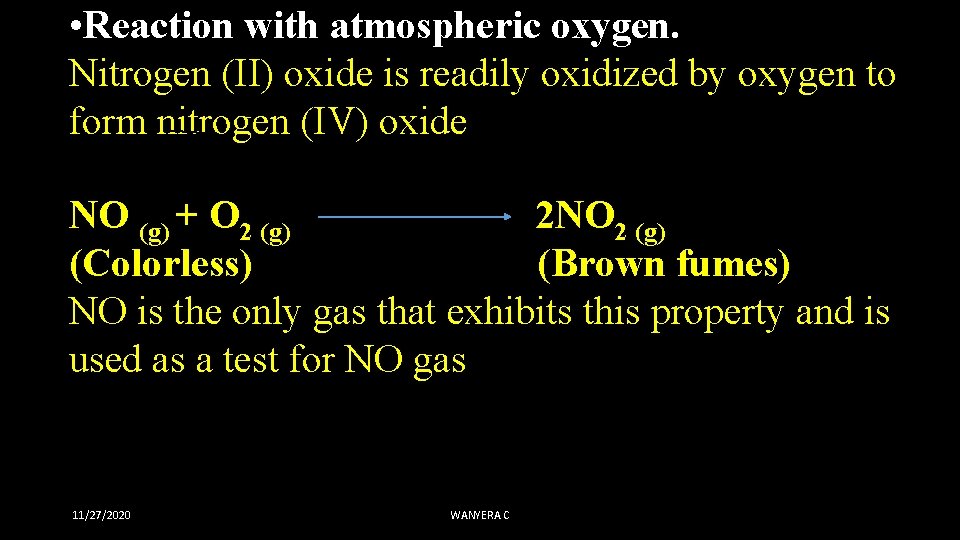
• Reaction with atmospheric oxygen. Nitrogen (II) oxide is readily oxidized by oxygen to form nitrogen (IV) oxide NO (g) + O 2 (g) 2 NO 2 (g) (Colorless) (Brown fumes) NO is the only gas that exhibits this property and is used as a test for NO gas 11/27/2020 WANYERA C

• Reaction with iron (II) sulphate solution. It dissolves in cold iron (II) sulphate turning it from a pale green solution to dark brown solution, iron (II) sulphate – nitrogen (II) oxide complex, Fe. SO 4 • NO. (nitroso-iron (II) sulphate) Fe. SO 4 (aq) + NO(g) Fe. SO 4 • NO (aq) This is the confirmatory test for NO gas 11/27/2020 WANYERA C

USES OF NITROGEN (II OXIDE Is used in manufacture of nitric (V) acid Is given to premature babies. Inhaling the gas mixed with oxygen reduces complications related to premature babies like breathing problems. 11/27/2020 WANYERA C

NITROGEN (IV) OXIDE (NITROGEN DIOXIDE) 11/27/2020 WANYERA C