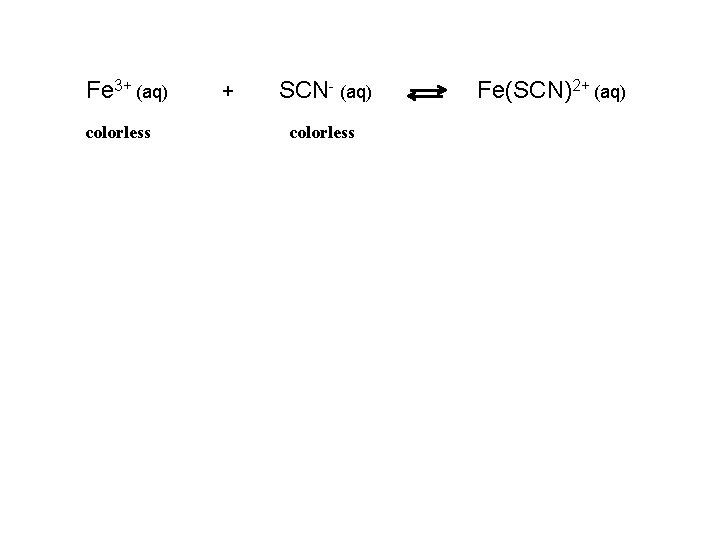
Fe 3 aq colorless SCN aq colorless FeSCN2

![Standard solutions Pg. 32 Standards [Fe(SCN)2+], M standard A 0. 00 standard B 4. Standard solutions Pg. 32 Standards [Fe(SCN)2+], M standard A 0. 00 standard B 4.](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-5.jpg)
![Standard solutions Pg. 32 Standards [Fe(SCN)2+], M absorbance standard A 0. 000 standard B Standard solutions Pg. 32 Standards [Fe(SCN)2+], M absorbance standard A 0. 000 standard B](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-6.jpg)


![Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2. Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2.](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-12.jpg)
![Pg. 34 units are M Experimental test solutions 1 [Fe(SCN)2+]equil 2. 96 x 10 Pg. 34 units are M Experimental test solutions 1 [Fe(SCN)2+]equil 2. 96 x 10](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-13.jpg)
![Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2. Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2.](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-14.jpg)

![Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2. Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2.](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-16.jpg)
![Pg. 34 units are M Experimental test solutions 1 2 [Fe(SCN)2+]equil 2. 96 x Pg. 34 units are M Experimental test solutions 1 2 [Fe(SCN)2+]equil 2. 96 x](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-17.jpg)

![Pg. 34 units are M Experimental test solutions 1 2 [Fe(SCN)2+]equil 2. 96 x Pg. 34 units are M Experimental test solutions 1 2 [Fe(SCN)2+]equil 2. 96 x](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-20.jpg)

![[ ], M Fe 3+ (aq) + SCN- (aq) Fe(SCN)2+ (aq) initial 1. 00 [ ], M Fe 3+ (aq) + SCN- (aq) Fe(SCN)2+ (aq) initial 1. 00](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-25.jpg)
![Equilibrium Concentrations for Trial 1 [Fe 3+]equil = [Fe 3+] initial – [Fe(SCN)2+]equil [Fe Equilibrium Concentrations for Trial 1 [Fe 3+]equil = [Fe 3+] initial – [Fe(SCN)2+]equil [Fe](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-26.jpg)
![Equilibrium Concentrations for Trial 1 [SCN ]equil = [SCN ] initial – [Fe(SCN)2+]equil [SCN Equilibrium Concentrations for Trial 1 [SCN ]equil = [SCN ] initial – [Fe(SCN)2+]equil [SCN](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-28.jpg)
- Slides: 31


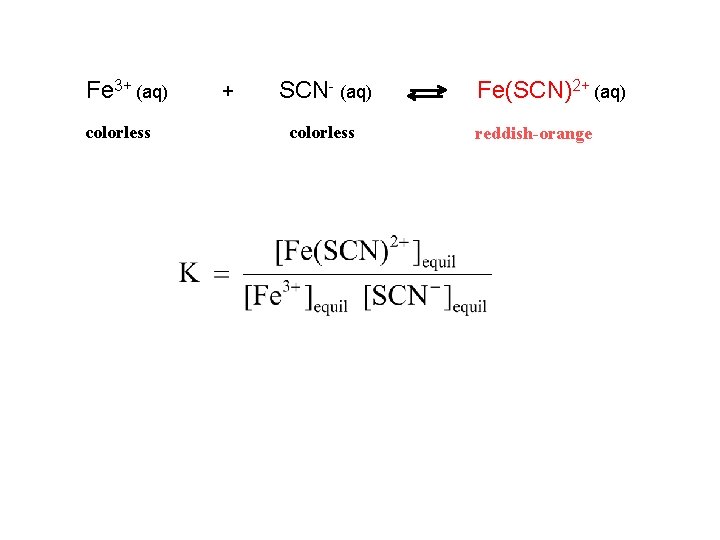
Fe 3+ (aq) colorless + SCN- (aq) colorless Fe(SCN)2+ (aq)

Fe 3+ (aq) colorless + SCN- (aq) colorless Fe(SCN)2+ (aq) reddish orange

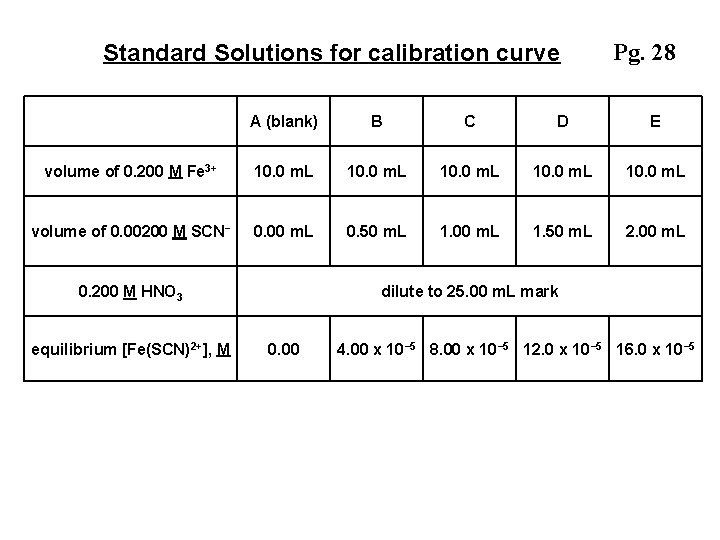
Standard Solutions for calibration curve Pg. 28 A (blank) B C D E volume of 0. 200 M Fe 3+ 10. 0 m. L volume of 0. 00200 M SCN- 0. 00 m. L 0. 50 m. L 1. 00 m. L 1. 50 m. L 2. 00 m. L 0. 200 M HNO 3 equilibrium [Fe(SCN)2+], M dilute to 25. 00 m. L mark 0. 00 4. 00 x 10 -5 8. 00 x 10 -5 12. 0 x 10 -5 16. 0 x 10 -5
![Standard solutions Pg 32 Standards FeSCN2 M standard A 0 00 standard B 4 Standard solutions Pg. 32 Standards [Fe(SCN)2+], M standard A 0. 00 standard B 4.](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-5.jpg)
Standard solutions Pg. 32 Standards [Fe(SCN)2+], M standard A 0. 00 standard B 4. 00 x 10 -5 standard C 8. 00 x 10 -5 standard D 12. 0 x 10 -5 standard E 16. 0 x 10 -5 absorbance
![Standard solutions Pg 32 Standards FeSCN2 M absorbance standard A 0 000 standard B Standard solutions Pg. 32 Standards [Fe(SCN)2+], M absorbance standard A 0. 000 standard B](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-6.jpg)
Standard solutions Pg. 32 Standards [Fe(SCN)2+], M absorbance standard A 0. 000 standard B 4. 00 x 10 -5 0. 196 standard C 8. 00 x 10 -5 0. 371 standard D 12. 0 x 10 -5 0. 557 standard E 16. 0 x 10 -5 0. 759


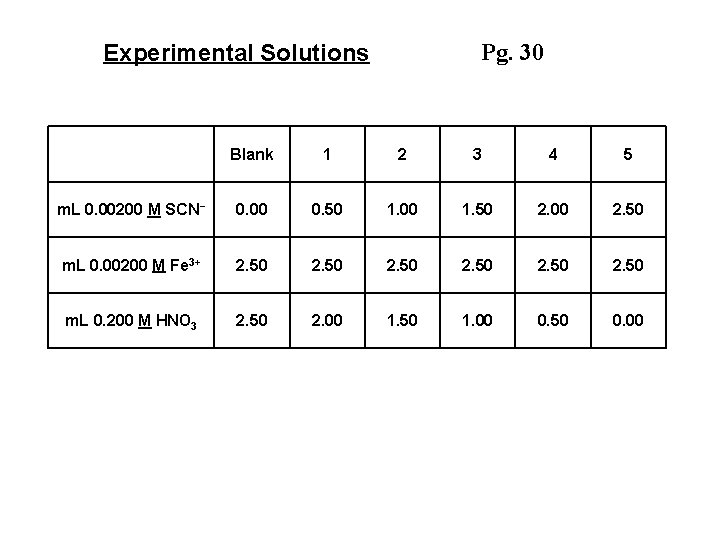
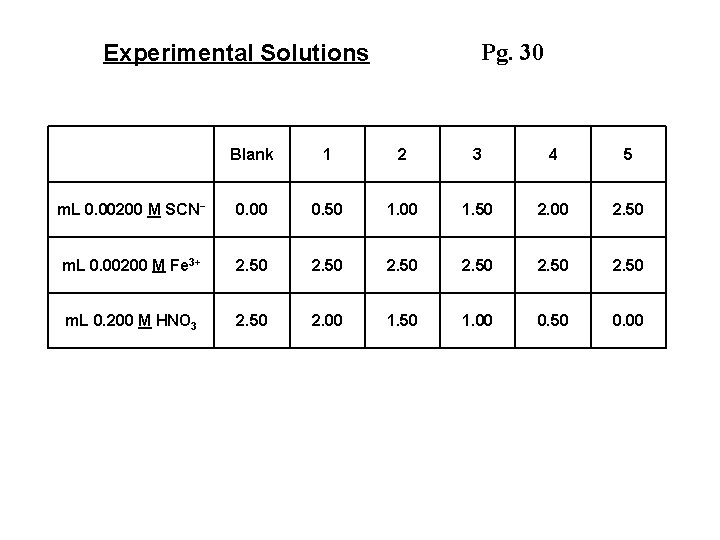
Pg. 30 Experimental Solutions Blank 1 2 3 4 5 m. L 0. 00200 M SCN- 0. 00 0. 50 1. 00 1. 50 2. 00 2. 50 m. L 0. 00200 M Fe 3+ 2. 50 m. L 0. 200 M HNO 3 2. 50 2. 00 1. 50 1. 00 0. 50 0. 00

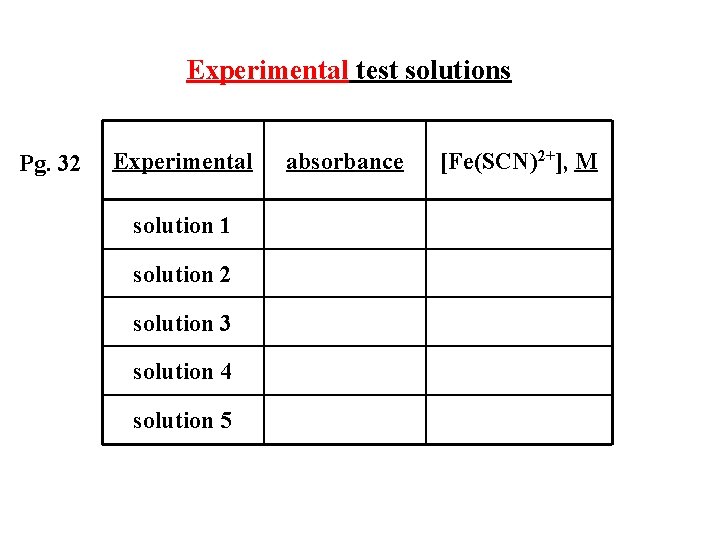
Experimental test solutions Pg. 32 Experimental solution 1 solution 2 solution 3 solution 4 solution 5 absorbance [Fe(SCN)2+], M

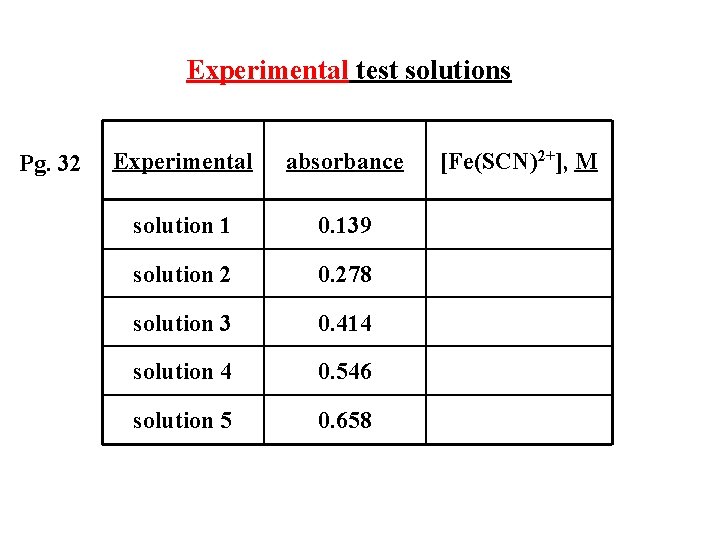
Experimental test solutions Pg. 32 Experimental absorbance solution 1 0. 139 solution 2 0. 278 solution 3 0. 414 solution 4 0. 546 solution 5 0. 658 [Fe(SCN)2+], M

![Experimental test solutions Pg 32 Experimental absorbance FeSCN2 M solution 1 0 139 2 Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2.](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-12.jpg)
Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2. 96 x 10 -5 solution 2 0. 278 from graph solution 3 0. 414 from graph solution 4 0. 546 from graph solution 5 0. 658 from graph
![Pg 34 units are M Experimental test solutions 1 FeSCN2equil 2 96 x 10 Pg. 34 units are M Experimental test solutions 1 [Fe(SCN)2+]equil 2. 96 x 10](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-13.jpg)
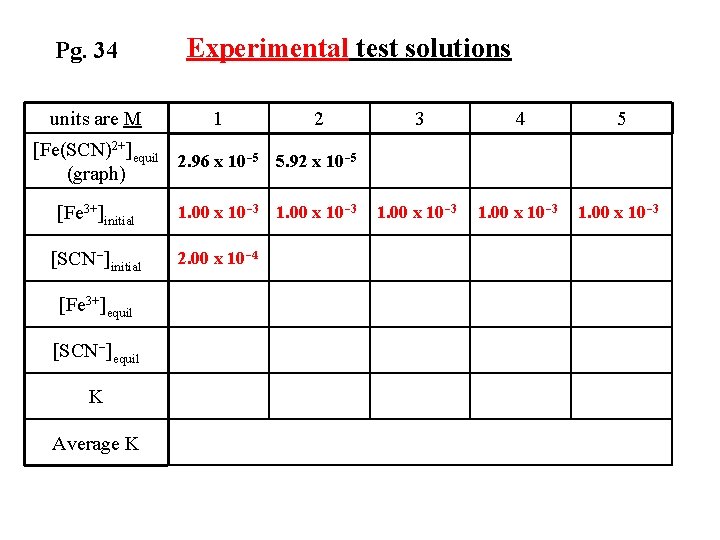
Pg. 34 units are M Experimental test solutions 1 [Fe(SCN)2+]equil 2. 96 x 10 -5 (graph) [Fe 3+]initial [SCN-]initial [Fe 3+]equil [SCN-]equil K Average K 2 3 4 5
![Experimental test solutions Pg 32 Experimental absorbance FeSCN2 M solution 1 0 139 2 Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2.](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-14.jpg)
Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2. 96 x 10 -5 solution 2 0. 278 from graph solution 3 0. 414 from graph solution 4 0. 546 from graph solution 5 0. 658 from graph

![Experimental test solutions Pg 32 Experimental absorbance FeSCN2 M solution 1 0 139 2 Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2.](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-16.jpg)
Experimental test solutions Pg. 32 Experimental absorbance [Fe(SCN)2+], M solution 1 0. 139 2. 96 x 10 -5 solution 2 0. 278 5. 92 x 10 -5 solution 3 0. 414 from graph solution 4 0. 546 from graph solution 5 0. 658 from graph
![Pg 34 units are M Experimental test solutions 1 2 FeSCN2equil 2 96 x Pg. 34 units are M Experimental test solutions 1 2 [Fe(SCN)2+]equil 2. 96 x](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-17.jpg)
Pg. 34 units are M Experimental test solutions 1 2 [Fe(SCN)2+]equil 2. 96 x 10 -5 5. 92 x 10 -5 (graph) [Fe 3+]initial [SCN-]initial [Fe 3+]equil [SCN-]equil K Average K 3 4 5

Pg. 30 Experimental Solutions Blank 1 2 3 4 5 m. L 0. 00200 M SCN- 0. 00 0. 50 1. 00 1. 50 2. 00 2. 50 m. L 0. 00200 M Fe 3+ 2. 50 m. L 0. 200 M HNO 3 2. 50 2. 00 1. 50 1. 00 0. 50 0. 00

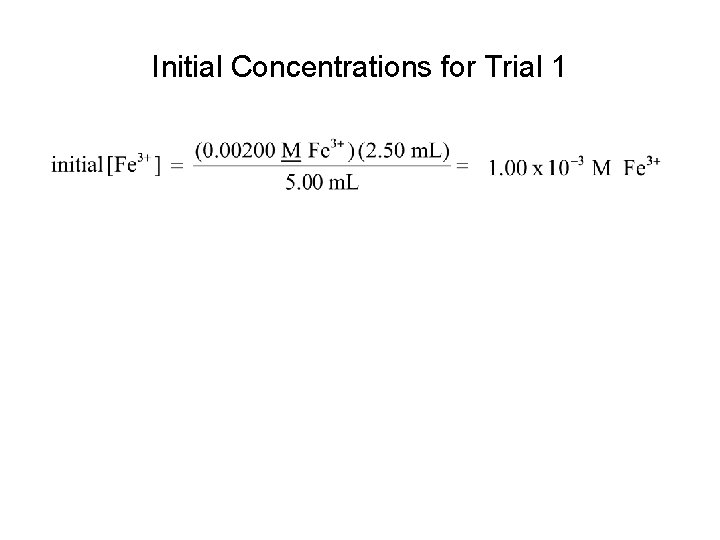
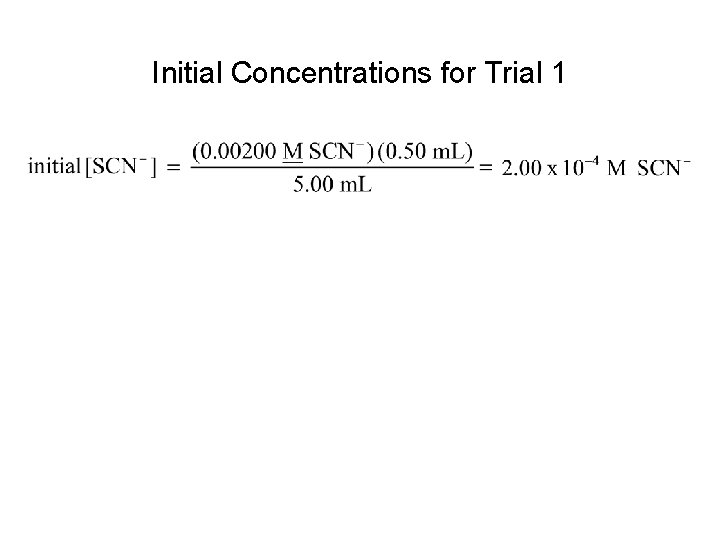
Initial Concentrations for Trial 1
![Pg 34 units are M Experimental test solutions 1 2 FeSCN2equil 2 96 x Pg. 34 units are M Experimental test solutions 1 2 [Fe(SCN)2+]equil 2. 96 x](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-20.jpg)
Pg. 34 units are M Experimental test solutions 1 2 [Fe(SCN)2+]equil 2. 96 x 10 -5 5. 92 x 10 -5 (graph) [Fe 3+]initial [SCN-]initial [Fe 3+]equil [SCN-]equil K Average K 1. 00 x 10 -3 3 4 5

Pg. 34 units are M Experimental test solutions 1 2 3 4 5 1. 00 x 10 -3 [Fe(SCN)2+]equil 2. 96 x 10 -5 5. 92 x 10 -5 (graph) [Fe 3+]initial [SCN-]initial [Fe 3+]equil [SCN-]equil K Average K 1. 00 x 10 -3

Pg. 30 Experimental Solutions Blank 1 2 3 4 5 m. L 0. 00200 M SCN- 0. 00 0. 50 1. 00 1. 50 2. 00 2. 50 m. L 0. 00200 M Fe 3+ 2. 50 m. L 0. 200 M HNO 3 2. 50 2. 00 1. 50 1. 00 0. 50 0. 00

Initial Concentrations for Trial 1

Pg. 34 units are M Experimental test solutions 1 2 3 4 5 1. 00 x 10 -3 [Fe(SCN)2+]equil 2. 96 x 10 -5 5. 92 x 10 -5 (graph) [Fe 3+]initial [SCN-]initial [Fe 3+]equil [SCN-]equil K Average K 1. 00 x 10 -3 2. 00 x 10 -4
![M Fe 3 aq SCN aq FeSCN2 aq initial 1 00 [ ], M Fe 3+ (aq) + SCN- (aq) Fe(SCN)2+ (aq) initial 1. 00](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-25.jpg)
[ ], M Fe 3+ (aq) + SCN- (aq) Fe(SCN)2+ (aq) initial 1. 00 x 10 -3 2. 00 x 10 -4 0 change x x +x equilibrium 1. 00 x 10 -3 x 2. 00 x 10 -4 x x x = [Fe(SCN)2+]equil (obtained from graph)
![Equilibrium Concentrations for Trial 1 Fe 3equil Fe 3 initial FeSCN2equil Fe Equilibrium Concentrations for Trial 1 [Fe 3+]equil = [Fe 3+] initial – [Fe(SCN)2+]equil [Fe](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-26.jpg)
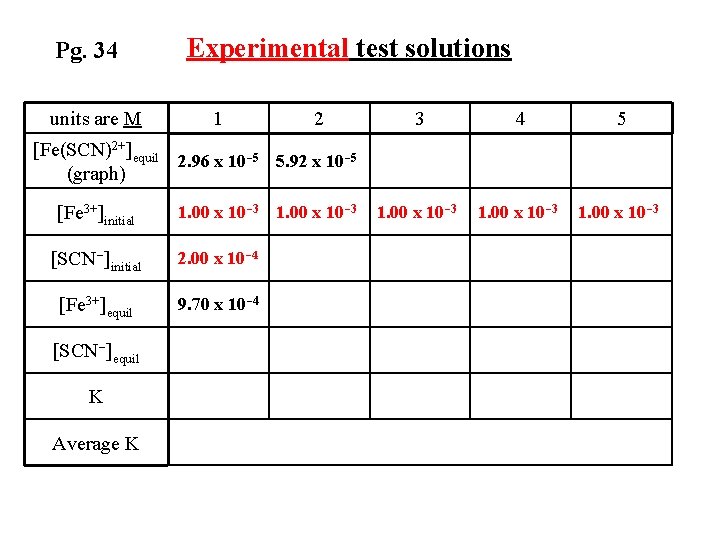
Equilibrium Concentrations for Trial 1 [Fe 3+]equil = [Fe 3+] initial – [Fe(SCN)2+]equil [Fe 3+]equil = 1. 00 x 10 -3 M – 2. 96 x 10 -5 M = 9. 70 x 10 -4 M

Pg. 34 units are M Experimental test solutions 1 2 3 4 5 1. 00 x 10 -3 [Fe(SCN)2+]equil 2. 96 x 10 -5 5. 92 x 10 -5 (graph) [Fe 3+]initial 1. 00 x 10 -3 [SCN-]initial 2. 00 x 10 -4 [Fe 3+]equil 9. 70 x 10 -4 [SCN-]equil K Average K
![Equilibrium Concentrations for Trial 1 SCN equil SCN initial FeSCN2equil SCN Equilibrium Concentrations for Trial 1 [SCN ]equil = [SCN ] initial – [Fe(SCN)2+]equil [SCN](https://slidetodoc.com/presentation_image_h2/7a04f5d3161cd38ec964ac30f3aca7fb/image-28.jpg)
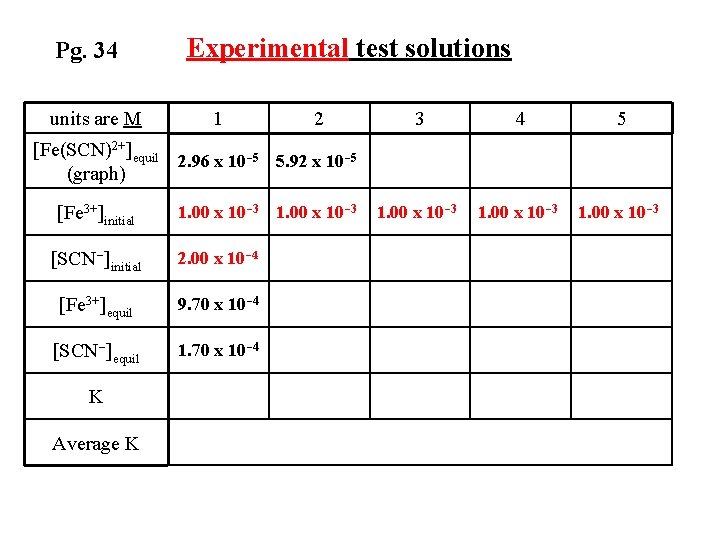
Equilibrium Concentrations for Trial 1 [SCN ]equil = [SCN ] initial – [Fe(SCN)2+]equil [SCN ]equil = 2. 00 x 10 -4 M – 2. 96 x 10 -5 M = 1. 70 x 10 -4 M

Pg. 34 units are M Experimental test solutions 1 2 3 4 5 1. 00 x 10 -3 [Fe(SCN)2+]equil 2. 96 x 10 -5 5. 92 x 10 -5 (graph) [Fe 3+]initial 1. 00 x 10 -3 [SCN-]initial 2. 00 x 10 -4 [Fe 3+]equil 9. 70 x 10 -4 [SCN-]equil 1. 70 x 10 -4 K Average K

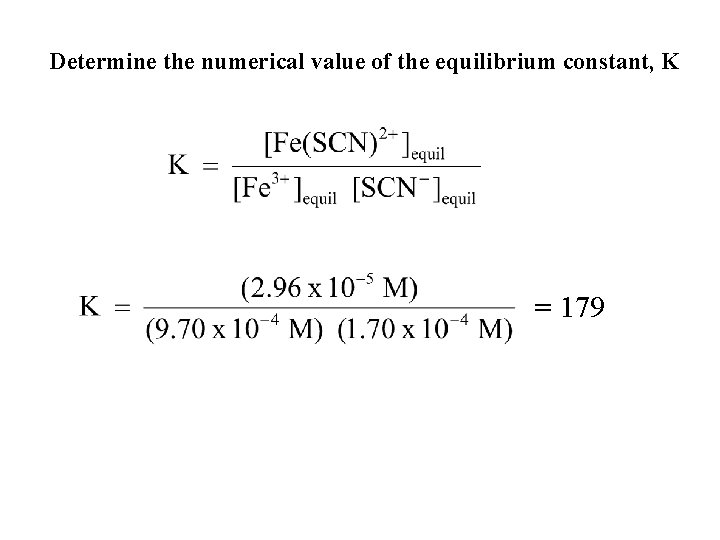
Determine the numerical value of the equilibrium constant, K = 179

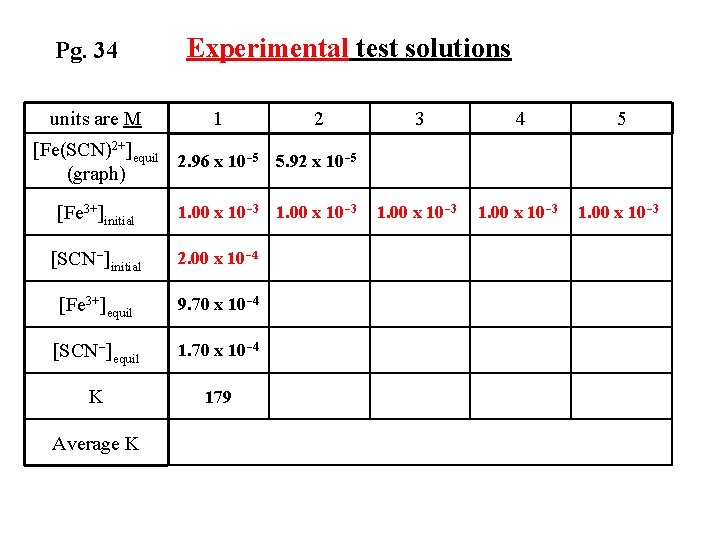
Pg. 34 units are M Experimental test solutions 1 2 3 4 5 1. 00 x 10 -3 [Fe(SCN)2+]equil 2. 96 x 10 -5 5. 92 x 10 -5 (graph) [Fe 3+]initial 1. 00 x 10 -3 [SCN-]initial 2. 00 x 10 -4 [Fe 3+]equil 9. 70 x 10 -4 [SCN-]equil 1. 70 x 10 -4 K 179 Average K
 Equilibrium
Equilibrium Thames valley strategic clinical network
Thames valley strategic clinical network Perła boraksowa
Perła boraksowa Simple bonding theory
Simple bonding theory Oracle replication
Oracle replication Scn1- lewis structure
Scn1- lewis structure Retinohypothalamic tract
Retinohypothalamic tract Colorless green ideas sleep furiously
Colorless green ideas sleep furiously Colorless gas
Colorless gas The dead colorless tissue attached
The dead colorless tissue attached Nitrogen is a colorless gas physical or chemical
Nitrogen is a colorless gas physical or chemical Clearway steam inhaler
Clearway steam inhaler Beware of pat expressions
Beware of pat expressions Is pure water colorless
Is pure water colorless Overgeneralization
Overgeneralization Tom swifties examples
Tom swifties examples Eosinophil histology
Eosinophil histology