Ch 2 Atoms and Elements Nucleus Rutherfords experiment





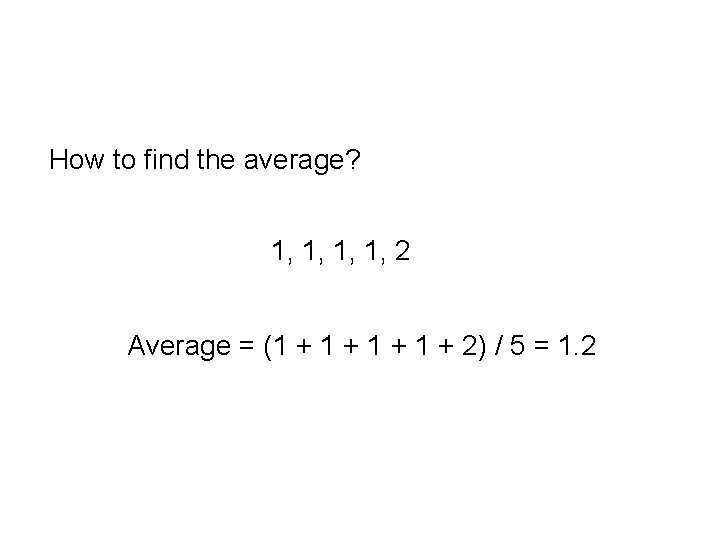




























- Slides: 94

Ch 2. Atoms and Elements

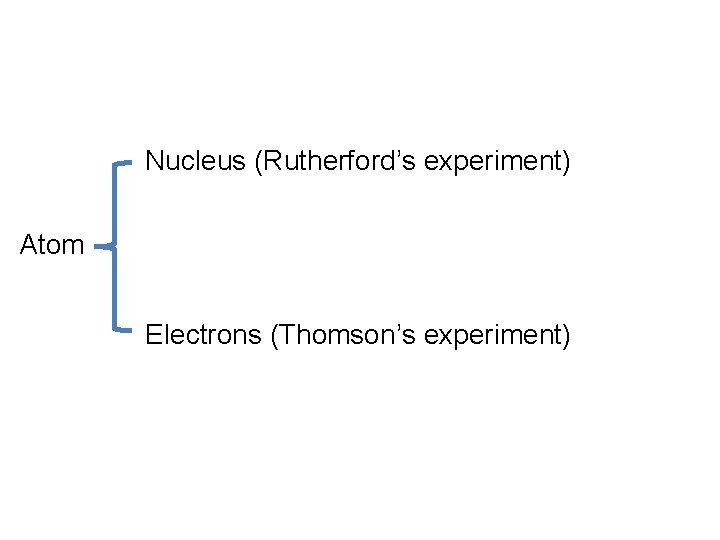
Nucleus (Rutherford’s experiment) Atom Electrons (Thomson’s experiment)


Atomic Nucleus

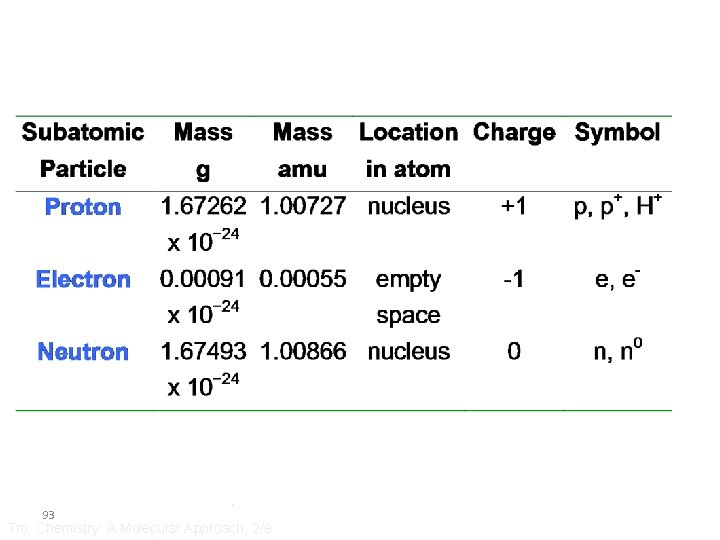
Nucleus carries almost all the mass of an atom Nucleus carries positive charges Each electron carries one negative charge

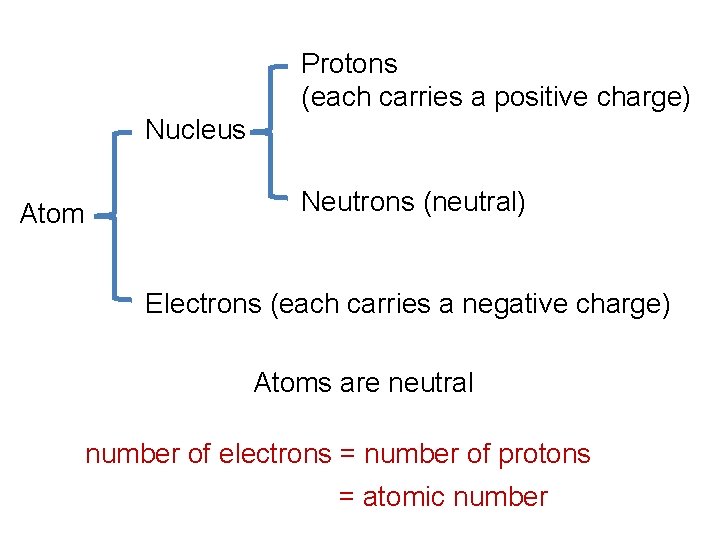
Protons (each carries a positive charge) Nucleus Atom Neutrons (neutral) Electrons (each carries a negative charge) Atoms are neutral number of electrons = number of protons = atomic number

Atoms that have the same number of protons belong to one kind of element.


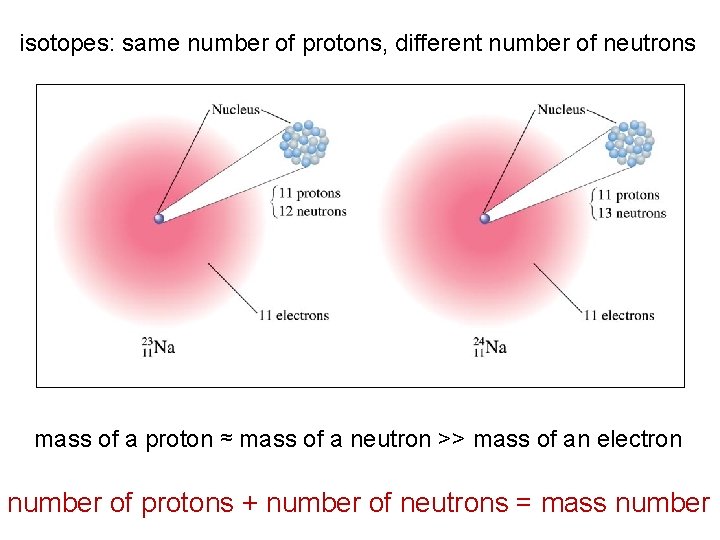
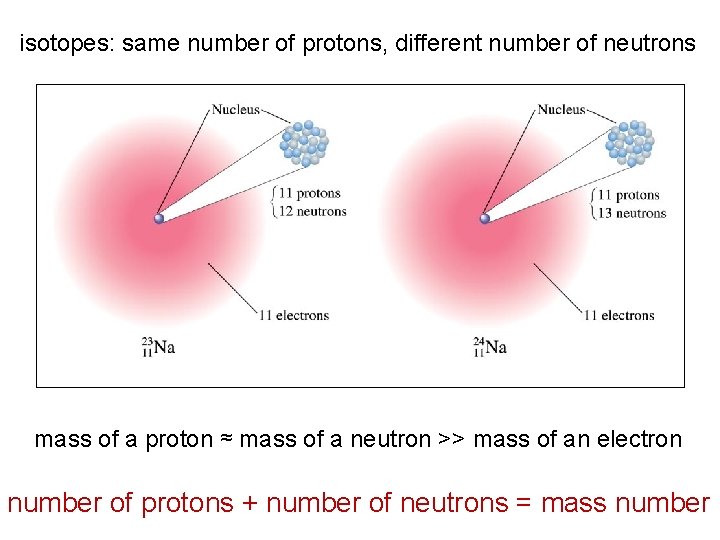
isotopes: same number of protons, different number of neutrons mass of a proton ≈ mass of a neutron >> mass of an electron number of protons + number of neutrons = mass number

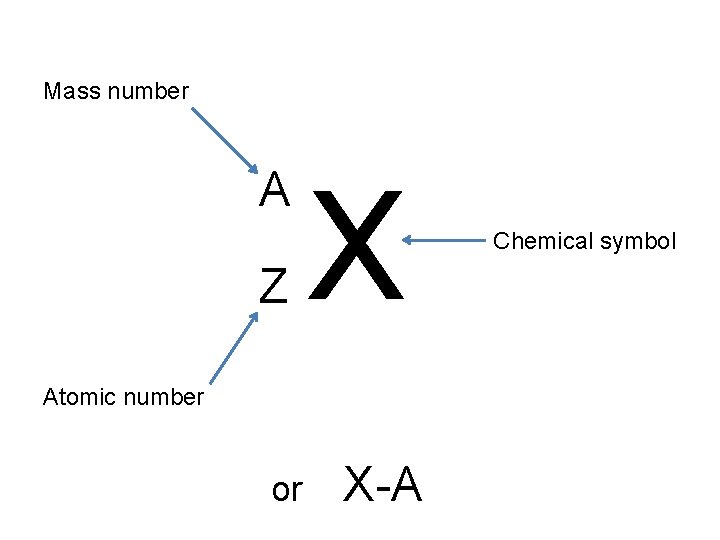
Mass number A Z X Atomic number or X-A Chemical symbol

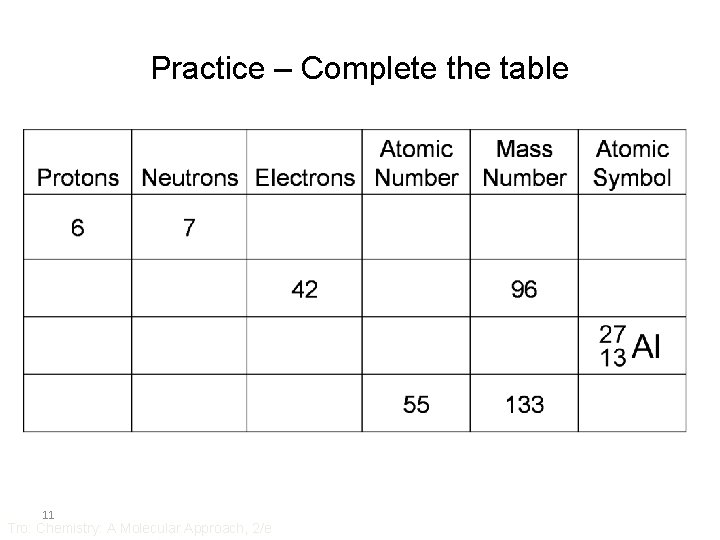
Practice – Complete the table 11 Tro: Chemistry: A Molecular Approach, 2/e

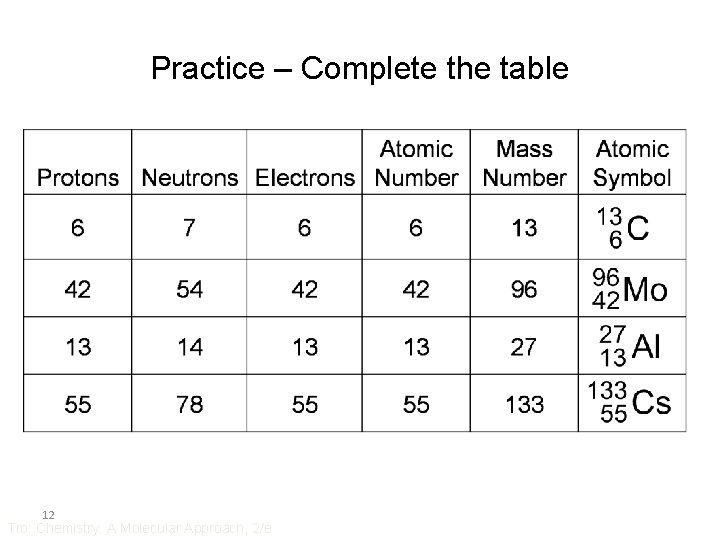
Practice – Complete the table 12 Tro: Chemistry: A Molecular Approach, 2/e

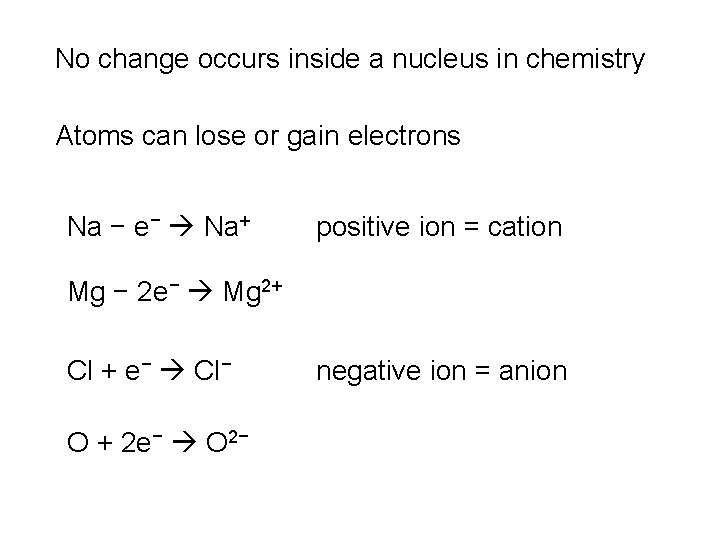
No change occurs inside a nucleus in chemistry Atoms can lose or gain electrons Na − e− Na+ positive ion = cation Mg − 2 e− Mg 2+ Cl + e− Cl− O + 2 e− O 2− negative ion = anion

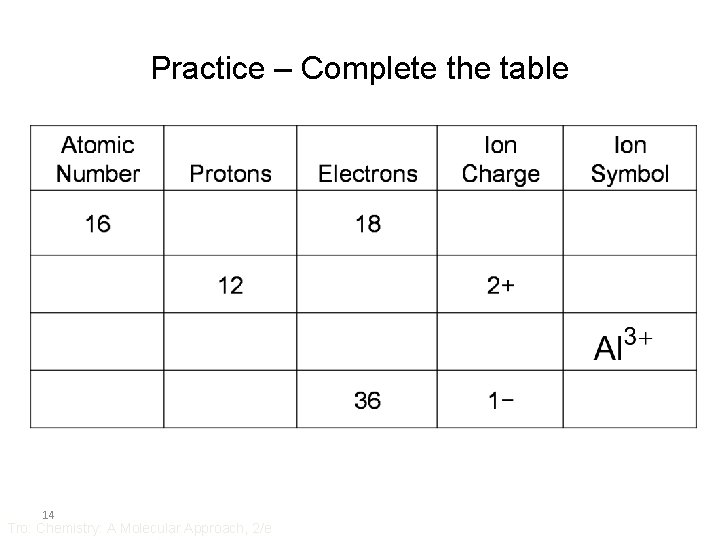
Practice – Complete the table 14 Tro: Chemistry: A Molecular Approach, 2/e

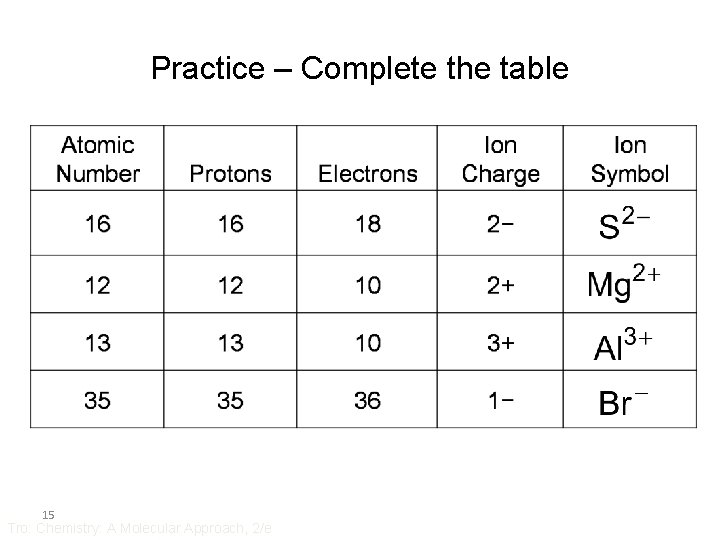
Practice – Complete the table 15 Tro: Chemistry: A Molecular Approach, 2/e

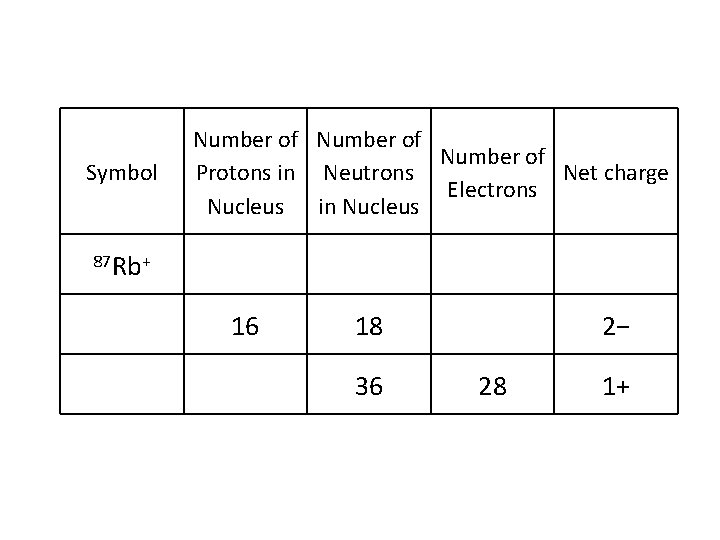
Symbol Number of Protons in Neutrons Net charge Electrons Nucleus in Nucleus 87 Rb+ 16 18 36 2− 28 1+

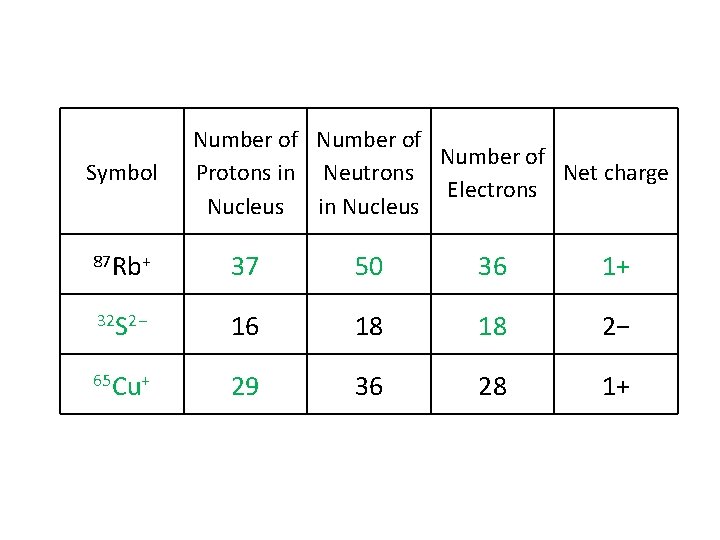
Symbol Number of Protons in Neutrons Net charge Electrons Nucleus in Nucleus 87 Rb+ 37 50 36 1+ 32 S 2− 16 18 18 2− 65 Cu+ 29 36 28 1+

isotopes: same number of protons, different number of neutrons mass of a proton ≈ mass of a neutron >> mass of an electron number of protons + number of neutrons = mass number

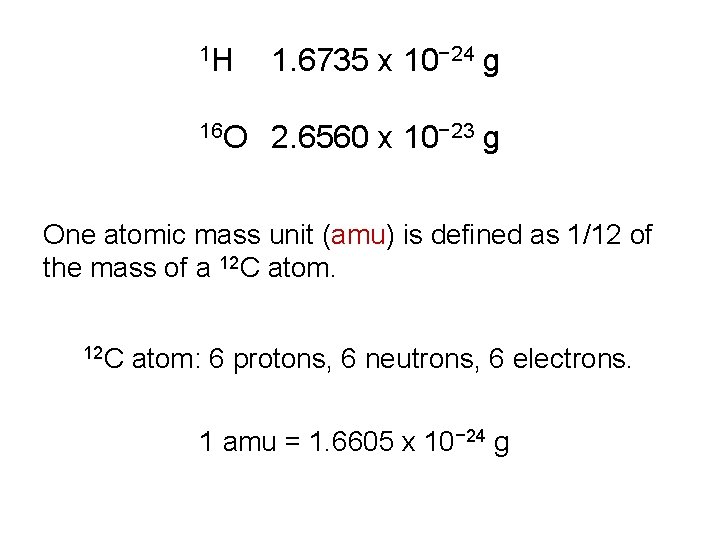
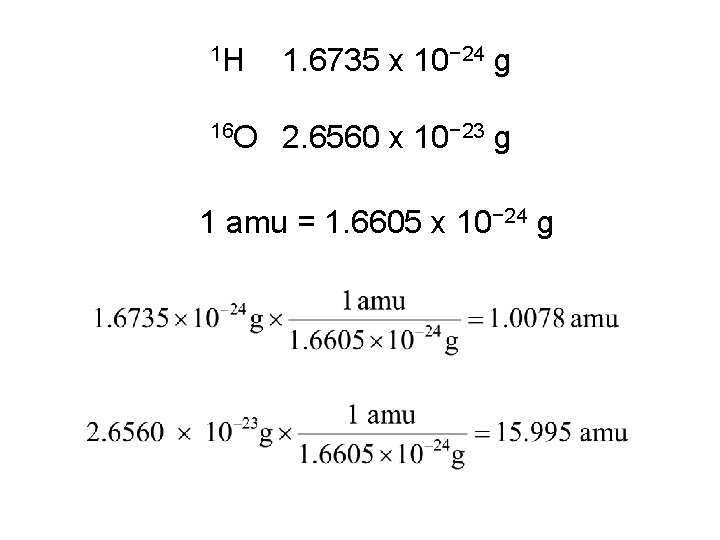
1 H 1. 6735 x 10− 24 g 16 O 2. 6560 x 10− 23 g One atomic mass unit (amu) is defined as 1/12 of the mass of a 12 C atom: 6 protons, 6 neutrons, 6 electrons. 1 amu = 1. 6605 x 10− 24 g

1 H 1. 6735 x 10− 24 g 16 O 2. 6560 x 10− 23 g 1 amu = 1. 6605 x 10− 24 g

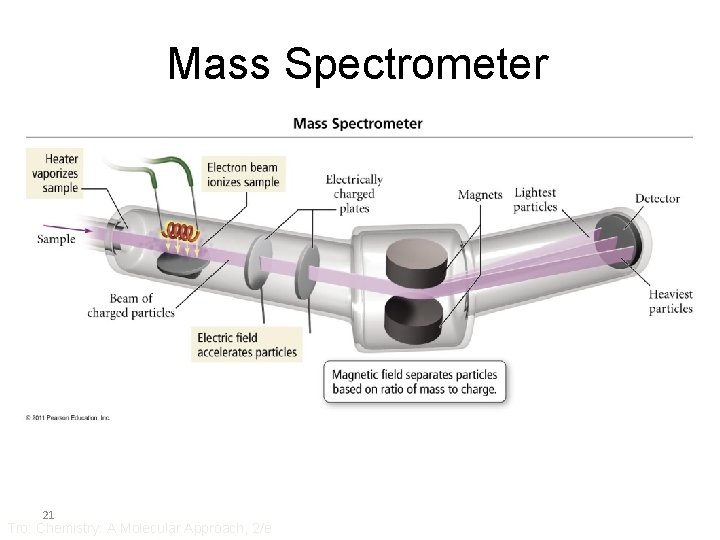
Mass Spectrometer 21 Tro: Chemistry: A Molecular Approach, 2/e

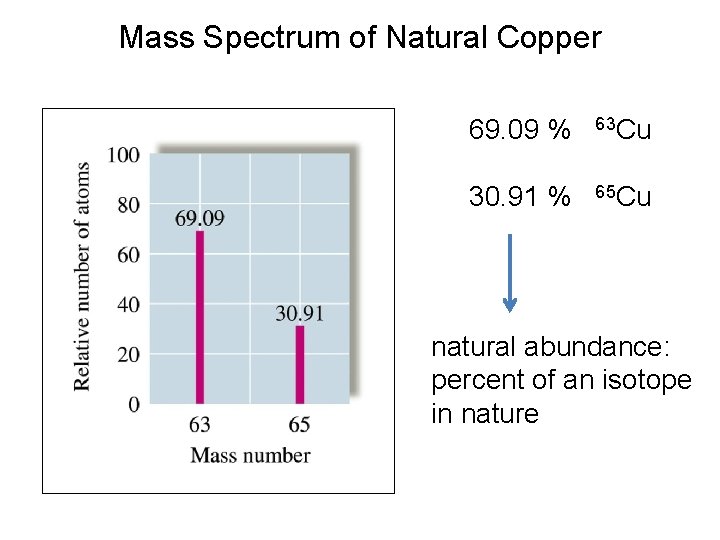
Mass Spectrum of Natural Copper 69. 09 % 63 Cu 30. 91 % 65 Cu natural abundance: percent of an isotope in nature

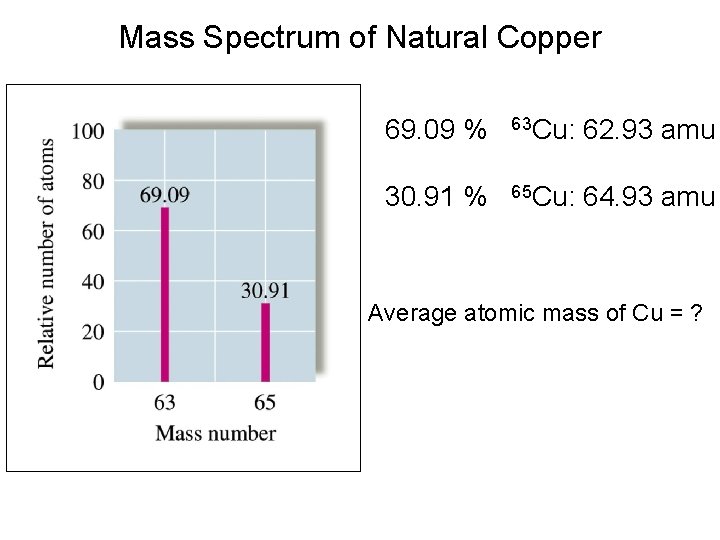
Mass Spectrum of Natural Copper 69. 09 % 63 Cu: 62. 93 amu 30. 91 % 65 Cu: 64. 93 amu Average atomic mass of Cu = ?
How to find the average? 1, 1, 2 Average = (1 + 1 + 2) / 5 = 1. 2


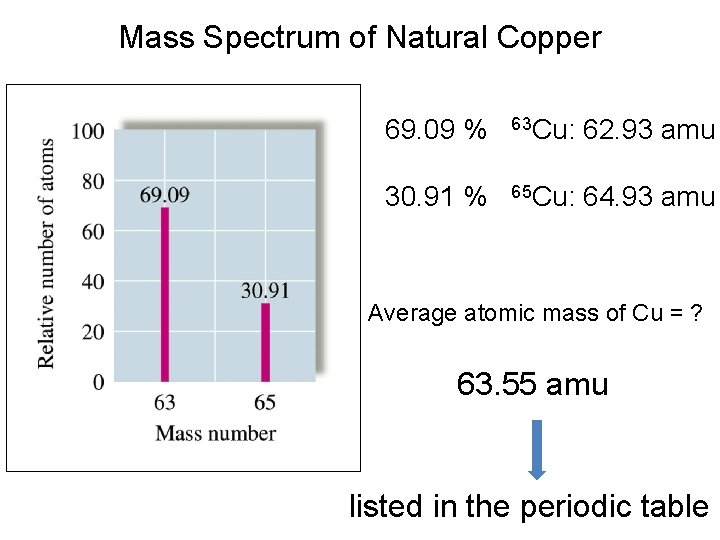
Mass Spectrum of Natural Copper 69. 09 % 63 Cu: 62. 93 amu 30. 91 % 65 Cu: 64. 93 amu Average atomic mass of Cu = ? 63. 55 amu listed in the periodic table

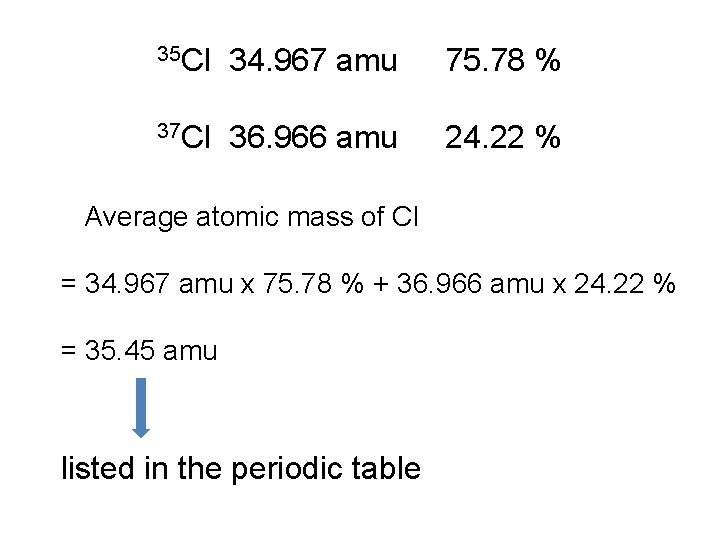
35 Cl 34. 967 amu 75. 78 % 37 Cl 36. 966 amu 24. 22 % Average atomic mass of Cl = 34. 967 amu x 75. 78 % + 36. 966 amu x 24. 22 % = 35. 45 amu listed in the periodic table

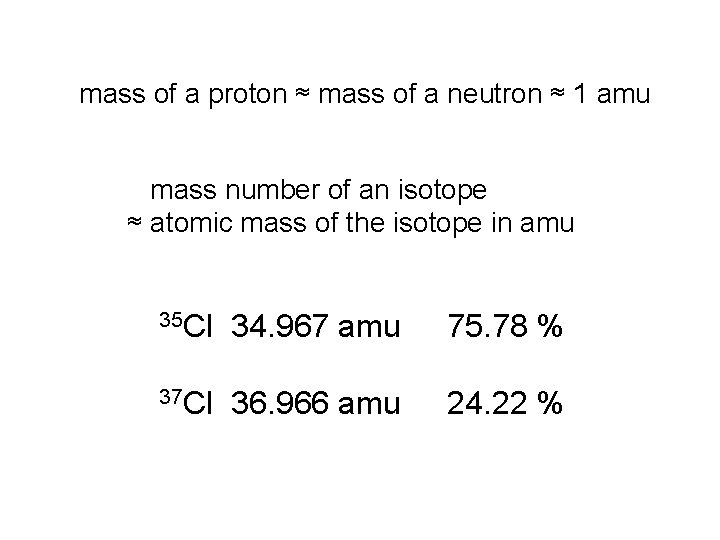
mass of a proton ≈ mass of a neutron ≈ 1 amu mass number of an isotope ≈ atomic mass of the isotope in amu 35 Cl 34. 967 amu 75. 78 % 37 Cl 36. 966 amu 24. 22 %

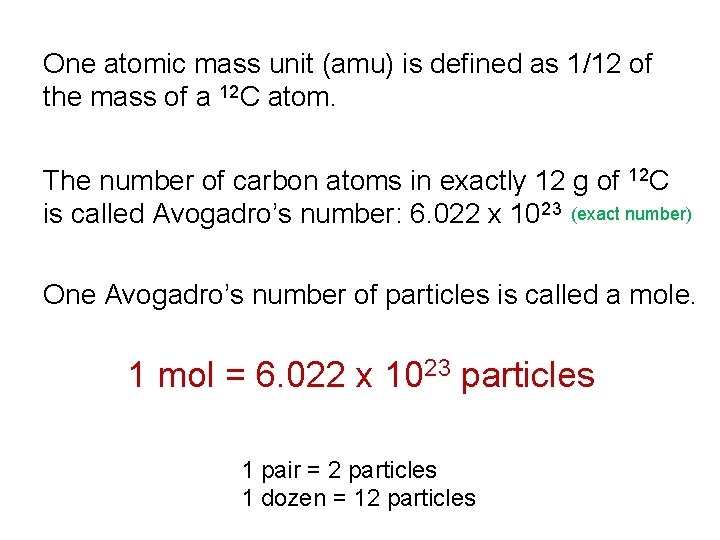
One atomic mass unit (amu) is defined as 1/12 of the mass of a 12 C atom. The number of carbon atoms in exactly 12 g of 12 C is called Avogadro’s number: 6. 022 x 1023 (exact number) One Avogadro’s number of particles is called a mole. 1 mol = 6. 022 x 1023 particles 1 pair = 2 particles 1 dozen = 12 particles

1 mol = 6. 022 x 1023 particles Example 2. 6, page 67 Calculate the number of atoms in 2. 45 mol of Cu.

1 mol = 6. 022 x 1023 particles Practice 2. 6, page 67 A pure Ag ring contains 2. 80 x 1022 Ag atoms. How many moles of Ag does it contain?

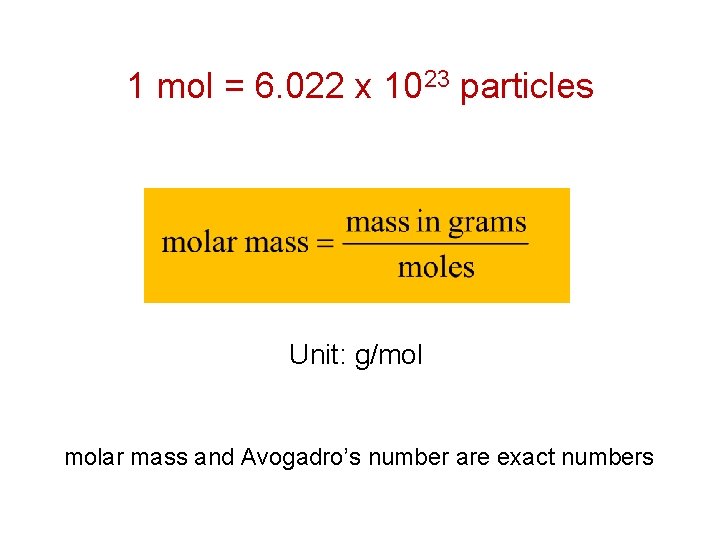
For an element X, its atomic mass is x amu. What is the mass of 1 mol of X in grams? The mass of 1 mol of X is x g. The molar mass of an element is the mass in grams per mole of the element. Unit: g/mol

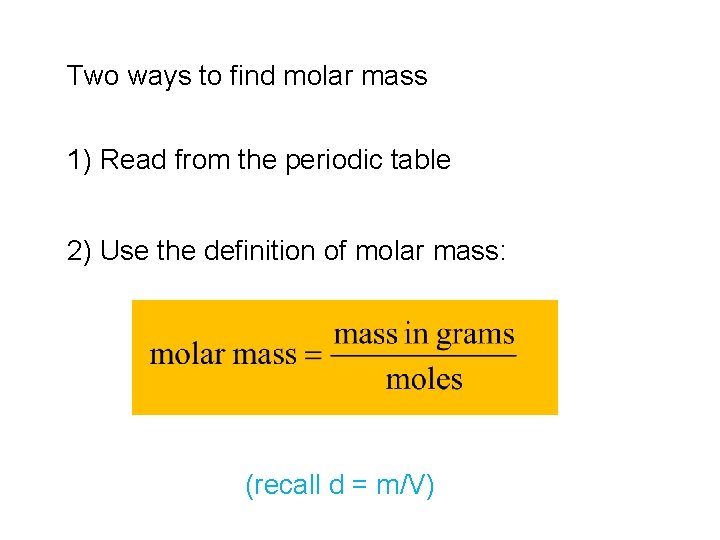
Two ways to find molar mass 1) Read from the periodic table 2) Use the definition of molar mass: (recall d = m/V)

1 mol = 6. 022 x 1023 particles Unit: g/mol molar mass and Avogadro’s number are exact numbers

A piece of Cu has a mass of 200. g. How many copper atoms are present? 1 mol = 6. 022 x 1023 particles

A silicon chip has a mass of 5. 68 mg. How many silicon atoms are present in the chip? 1 mol = 6. 022 x 1023 particles

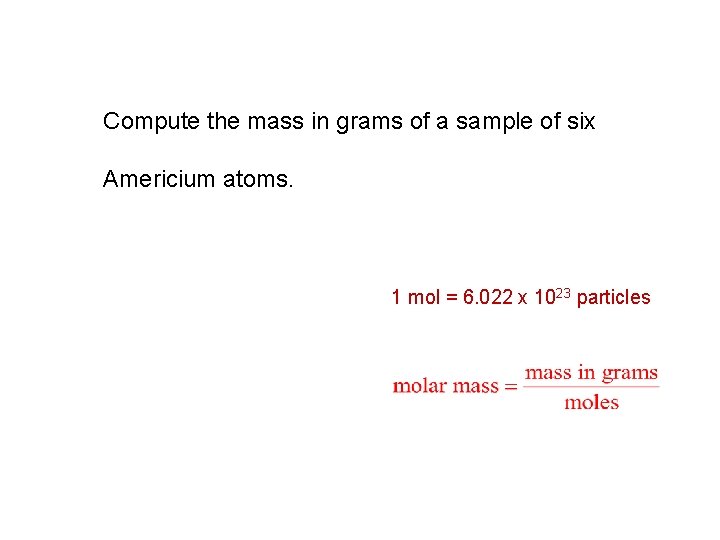
Compute the mass in grams of a sample of six Americium atoms. 1 mol = 6. 022 x 1023 particles

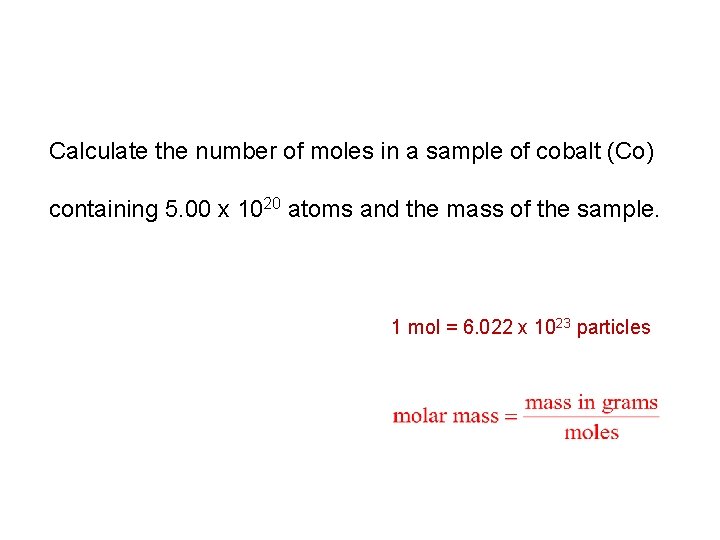
Calculate the number of moles in a sample of cobalt (Co) containing 5. 00 x 1020 atoms and the mass of the sample. 1 mol = 6. 022 x 1023 particles


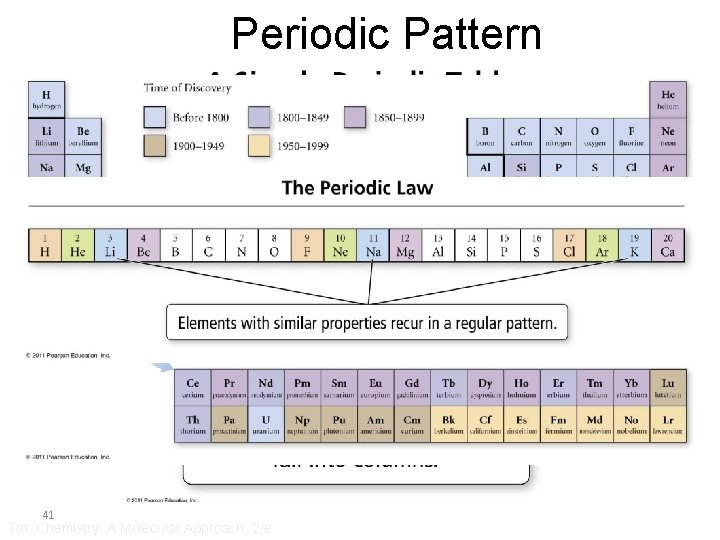
Mendeleev • Ordered elements by atomic mass • Saw a repeating pattern of properties • Periodic Law – when the elements are arranged in order of increasing atomic mass, certain sets of properties recur periodically • Put elements with similar properties in the same column • Used pattern to predict properties of undiscovered elements • Where atomic mass order did not fit other properties, he re-ordered by other properties – Te & I 40 Tro: Chemistry: A Molecular Approach, 2/e

Periodic Pattern 41 Tro: Chemistry: A Molecular Approach, 2/e

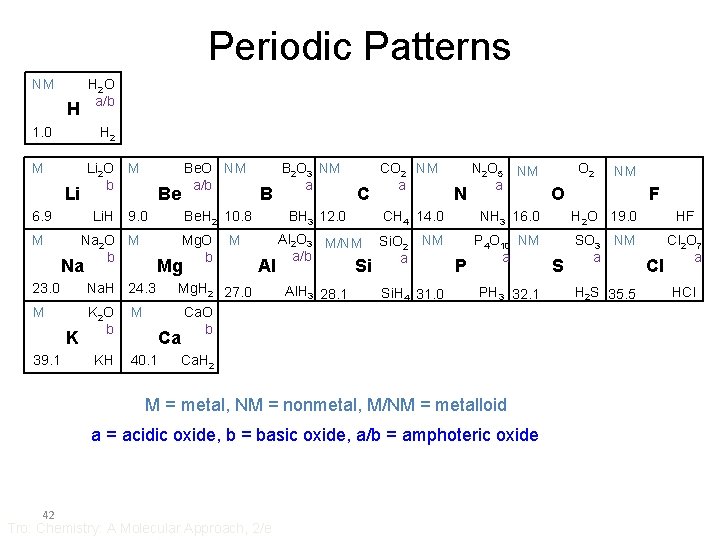
Periodic Patterns NM H 2 O a/b H 1. 0 H 2 M Li 6. 9 M Li 2 O b M Li. H 9. 0 Be Na 2 O M b Na. H 24. 3 M K 2 O b 39. 1 KH M Mg 23. 0 B Be. H 2 10. 8 Mg. O b Na K Be. O NM a/b M Al Mg. H 2 27. 0 Ca. O b B 2 O 3 NM a C CO 2 NM a N N 2 O 5 NM a F NH 3 16. 0 H 2 O 19. 0 HF Al 2 O 3 M/NM a/b Si. O 2 NM a P 4 O 10 NM a SO 3 NM a Cl 2 O 7 a Al. H 3 28. 1 Si. H 4 31. 0 Si P PH 3 32. 1 Ca. H 2 a = acidic oxide, b = basic oxide, a/b = amphoteric oxide Tro: Chemistry: A Molecular Approach, 2/e O CH 4 14. 0 M = metal, NM = nonmetal, M/NM = metalloid 42 NM BH 3 12. 0 Ca 40. 1 O 2 S H 2 S 35. 5 Cl HCl

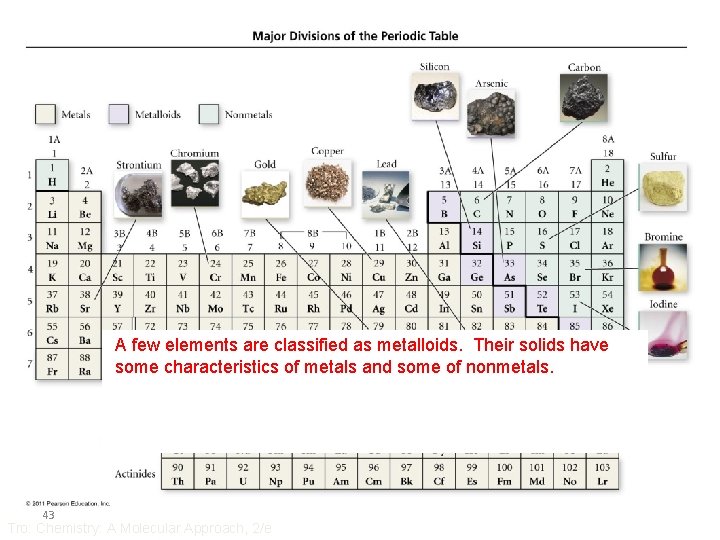
Most About A fewofelements ¾ the of remaining the elements are classified asare metalloids. classified as metals. Their as nonmetals. They solidshavea Their reflective some solids characteristics surface, have a conduct non-reflective of metals heatand surface, some electricity of dononmetals. not better conduct thanheat other and elements, electricity andwell, are malleable and are brittle. and ductile 43 Tro: Chemistry: A Molecular Approach, 2/e

Metals • Solids at room temperature, except Hg • Reflective surface – shiny • Conduct heat • Conduct electricity • Malleable – can be shaped • Ductile – can be drawn or pulled into wires • Lose electrons and form cations in reactions • About 75% of the elements are metals • 44 Lower left on the table Tro: Chemistry: A Molecular Approach, 2/e

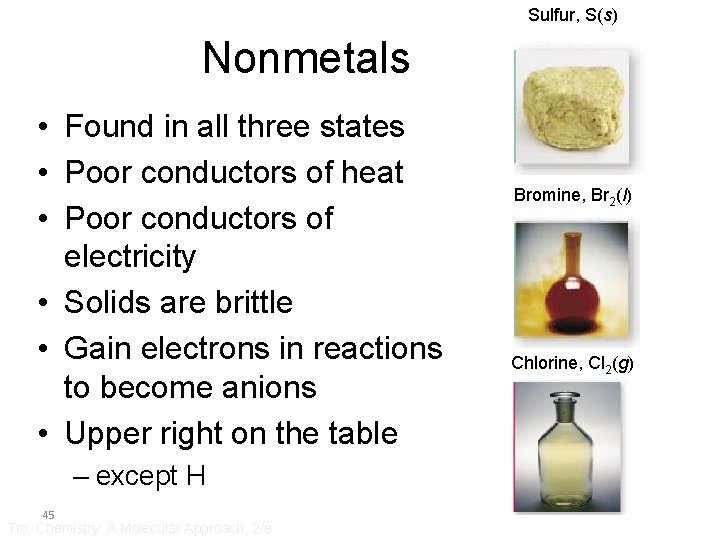
Sulfur, S(s) Nonmetals • Found in all three states • Poor conductors of heat • Poor conductors of electricity • Solids are brittle • Gain electrons in reactions to become anions • Upper right on the table – except H 45 Tro: Chemistry: A Molecular Approach, 2/e Bromine, Br 2(l) Chlorine, Cl 2(g)

Metalloids • Show some properties of metals and some of nonmetals • Also known as semiconductors 46 Tro: Chemistry: A Molecular Approach, 2/e Properties of Silicon shiny conducts electricity does not conduct heat well brittle

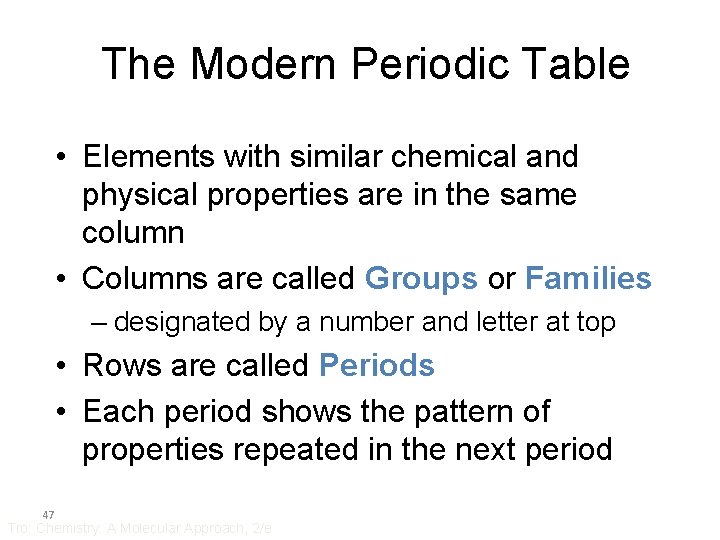
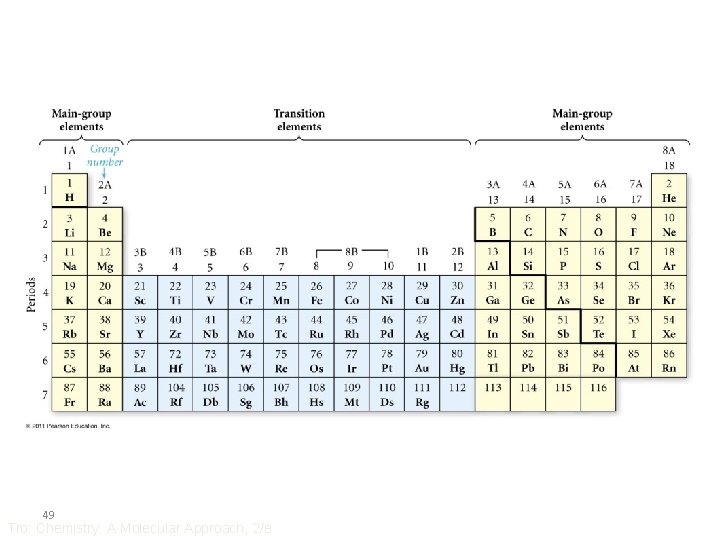
The Modern Periodic Table • Elements with similar chemical and physical properties are in the same column • Columns are called Groups or Families – designated by a number and letter at top • Rows are called Periods • Each period shows the pattern of properties repeated in the next period 47 Tro: Chemistry: A Molecular Approach, 2/e

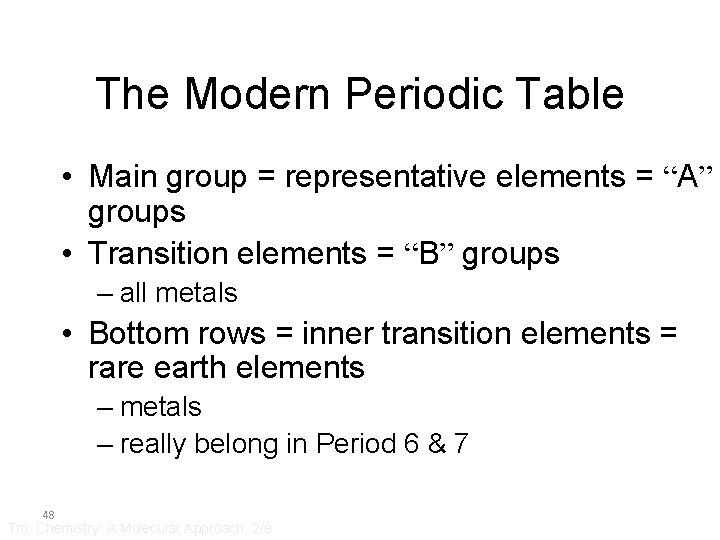
The Modern Periodic Table • Main group = representative elements = “A” groups • Transition elements = “B” groups – all metals • Bottom rows = inner transition elements = rare earth elements – metals – really belong in Period 6 & 7 48 Tro: Chemistry: A Molecular Approach, 2/e

49 Tro: Chemistry: A Molecular Approach, 2/e

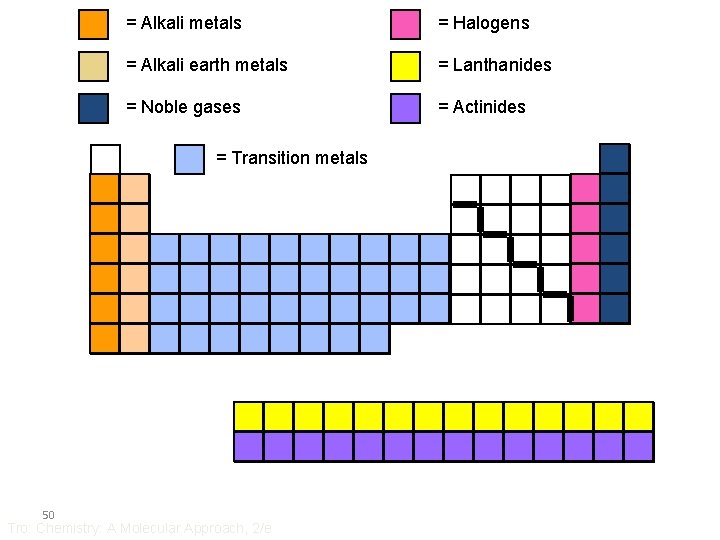
= Alkali metals = Halogens = Alkali earth metals = Lanthanides = Noble gases = Actinides = Transition metals 50 Tro: Chemistry: A Molecular Approach, 2/e

Important Groups - Hydrogen • Nonmetal • Colorless, diatomic gas – very low melting point and density • Reacts with nonmetals to form molecular compounds – HCl is acidic gas – H 2 O is a liquid • Reacts with metals to form hydrides – metal hydrides react with water to form H 2 • HX dissolves in water to form acids 51 Tro: Chemistry: A Molecular Approach, 2/e

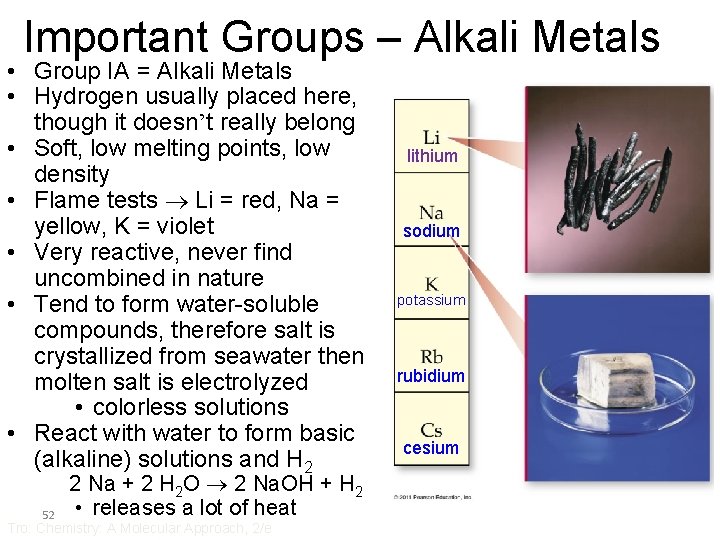
Important Groups – Alkali Metals • Group IA = Alkali Metals • Hydrogen usually placed here, though it doesn’t really belong • Soft, low melting points, low density • Flame tests Li = red, Na = yellow, K = violet • Very reactive, never find uncombined in nature • Tend to form water-soluble compounds, therefore salt is crystallized from seawater then molten salt is electrolyzed • colorless solutions • React with water to form basic (alkaline) solutions and H 2 52 2 Na + 2 H 2 O 2 Na. OH + H 2 • releases a lot of heat Tro: Chemistry: A Molecular Approach, 2/e lithium sodium potassium rubidium cesium

Important Groups – Alkali Earth Metals • Group IIA = Alkali earth metals • Harder, higher melting, and denser than alkali metals – Mg alloys used as structural materials • Flame tests Ca = red, Sr = red, Ba = green • Reactive, but less than corresponding alkali metal • Form stable, insoluble oxides from which they are normally extracted • Oxides are basic = alkaline earth • Reactivity with water to form H 2 – Be = none; Mg = steam; Ca, Sr, Ba = cold water 53 Tro: Chemistry: A Molecular Approach, 2/e

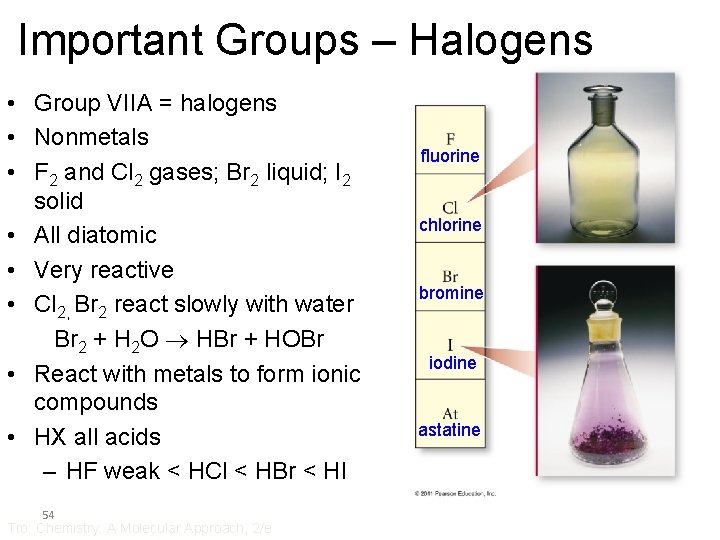
Important Groups – Halogens • Group VIIA = halogens • Nonmetals • F 2 and Cl 2 gases; Br 2 liquid; I 2 solid • All diatomic • Very reactive • Cl 2, Br 2 react slowly with water Br 2 + H 2 O HBr + HOBr • React with metals to form ionic compounds • HX all acids – HF weak < HCl < HBr < HI 54 Tro: Chemistry: A Molecular Approach, 2/e fluorine chlorine bromine iodine astatine

Important Groups – Noble Gases • Group VIIIA = Noble Gases • All gases at room temperature – very low melting and boiling points • Very unreactive, practically inert • Very hard to remove electron from or give electron to 55 Tro: Chemistry: A Molecular Approach, 2/e

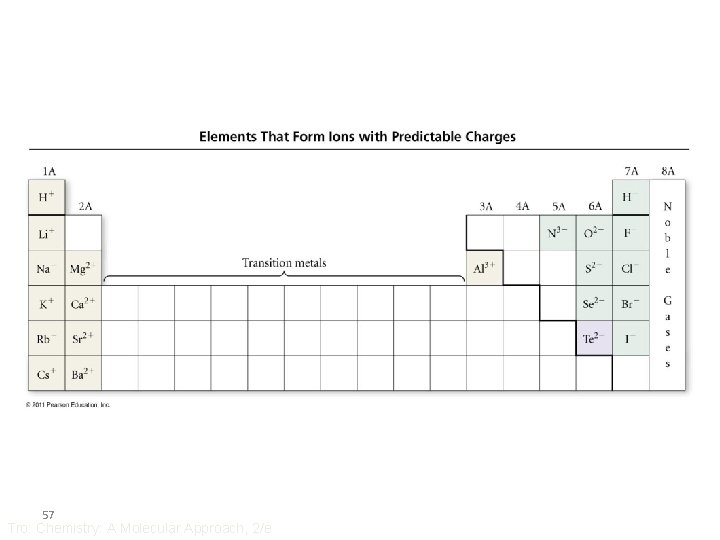
Ion Charge and the Periodic Table • The charge on an ion can often be determined from an element’s position on the Periodic Table • Metals always form positively charged cations • For many main group metals, the charge = the group number • Nonmetals form negatively charged anions • For nonmetals, the charge = the group number − 8 56 Tro: Chemistry: A Molecular Approach, 2/e

57 Tro: Chemistry: A Molecular Approach, 2/e

Practice – What is the charge on each of the following ions? • • • 58 potassium cation sulfide anion calcium cation bromide anion aluminum cation Tro: Chemistry: A Molecular Approach, 2/e K+ S 2− Ca 2+ Br− Al 3+

Law of Conservation of Mass • In a chemical reaction, • matter is neither created nor destroyed Total mass of the materials you have before the reaction must equal the total mass of the materials you have at the end ü total mass of reactants = total mass of products 59 Tro: Chemistry: A Molecular Approach, 2/e Antoine Lavoisier 1743 -1794

Reaction of Sodium with Chlorine to Make Sodium Chloride The mass of sodium and chlorine used is determined by the number of atoms • that combine Because only whole atoms combine and atoms are not changed or destroyed in the process, the mass of sodium chloride made must equal the total mass of sodium and chlorine atoms that combine together • 7. 7 g Na 60 + 11. 9 g Cl 2 Tro: Chemistry: A Molecular Approach, 2/e 19. 6 g Na. Cl

Law of Definite Proportions Joseph Proust 1754 -1826 • All samples of a given compound, regardless of their source or how they were prepared, have the same proportions of their constituent elements 61 Tro: Chemistry: A Molecular Approach, 2/e

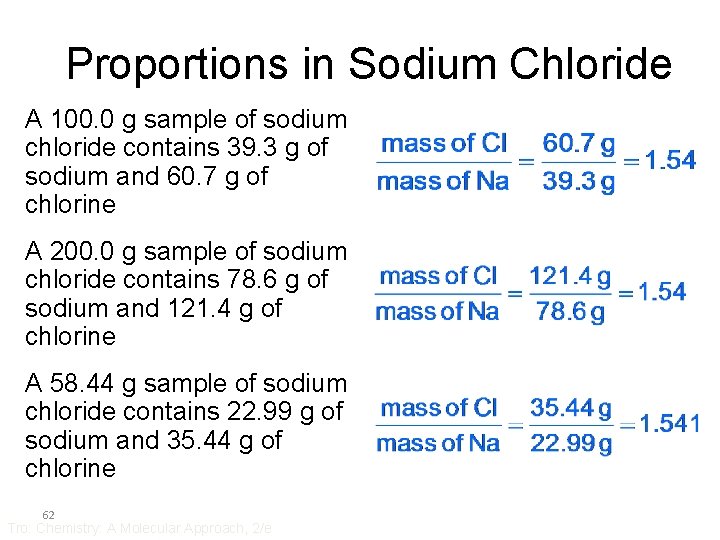
Proportions in Sodium Chloride A 100. 0 g sample of sodium chloride contains 39. 3 g of sodium and 60. 7 g of chlorine A 200. 0 g sample of sodium chloride contains 78. 6 g of sodium and 121. 4 g of chlorine A 58. 44 g sample of sodium chloride contains 22. 99 g of sodium and 35. 44 g of chlorine 62 Tro: Chemistry: A Molecular Approach, 2/e

Law of Multiple Proportions John Dalton 1766 -1844 • When two elements (call them A and B) form two different compounds, the masses of B that combine with 1 g of A can be expressed as a ratio of small, whole numbers 63 Tro: Chemistry: A Molecular Approach, 2/e

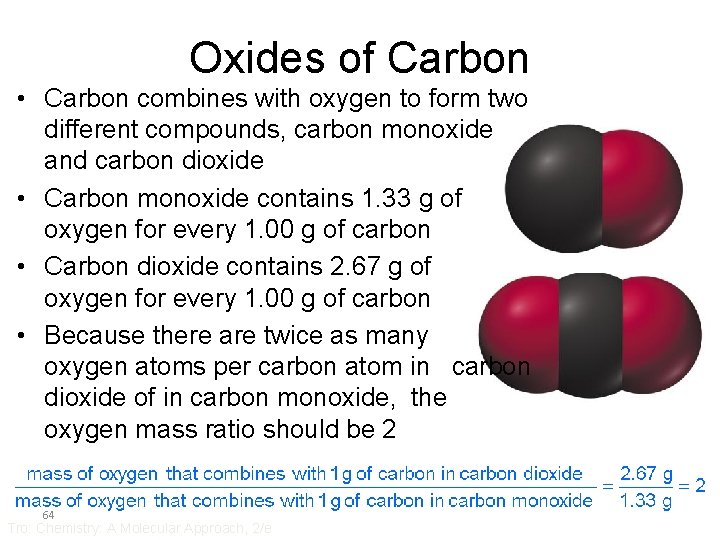
Oxides of Carbon • Carbon combines with oxygen to form two different compounds, carbon monoxide and carbon dioxide • Carbon monoxide contains 1. 33 g of oxygen for every 1. 00 g of carbon • Carbon dioxide contains 2. 67 g of oxygen for every 1. 00 g of carbon • Because there are twice as many oxygen atoms per carbon atom in carbon dioxide of in carbon monoxide, the oxygen mass ratio should be 2 64 Tro: Chemistry: A Molecular Approach, 2/e

Dalton’s Atomic Theory • 1. 2. 3. 4. Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements Atoms combine in simple, whole-number ratios to form molecules of compounds In a chemical reaction, atoms of one element cannot change into atoms of another element ü 65 they simply rearrange the way they are attached Tro: Chemistry: A Molecular Approach, 2/e

Practice – Decide if each statement is correct according to Dalton’s model of the atom • Copper atoms can combine with zinc atoms to make gold atoms • Water is composed of many identical molecules that have one oxygen atom and two hydrogen atoms 66 Tro: Chemistry: A Molecular Approach, 2/e

Practice – Decide if each statement is correct according to Dalton’s model of the atom • Copper atoms can combine with zinc atoms to make gold atoms – incorrect; according to Dalton, atoms of one element cannot turn into atoms of another element by a chemical reaction. He knew this because if atoms could change it would change the total mass and violate the Law of Conservation of Mass. • Water is composed of many identical molecules that have one oxygen atom and two hydrogen atoms – correct; according to Dalton, atoms combine together in compounds in small whole-number ratios, so that you could describe a compound by describing the number of atoms of each element in a molecule. He used this idea to explain why compounds obey the Law of Definite Proportions. 67 Tro: Chemistry: A Molecular Approach, 2/e

Practice – Decide if each statement is correct according to Dalton’s Model of the Atom • Some carbon atoms weigh more than other carbon atoms • Because the mass ratio of Fe: O in wüsite is 1. 5 times larger than the Fe: O ratio in hematite, there must be 1. 5 Fe atoms in a unit of wüsite and 1 Fe atom in a unit of hematite 68 Tro: Chemistry: A Molecular Approach, 2/e

Practice – Decide if each statement is correct according to Dalton’s model of the atom • Some carbon atoms weigh more than other carbon atoms – incorrect; according to Dalton, all atoms of an element are identical. • Because the mass ratio of Fe: O in wüsite is 1. 5 times larger than the Fe: O ratio in hematite, there must be 1. 5 Fe atoms in a unit of wüsite and 1 Fe atom in a unit of hematite – incorrect; according to Dalton, atoms must combine in small whole-number ratios. If you could combine fractions of atoms, that would mean the atom is breakable and Dalton’s first premise would be incorrect. You can get the Fe: Fe mass ratio to be 1. 5 if the formula for wüsite is Fe. O and the formula for hematite is Fe 2 O 3. 69 Tro: Chemistry: A Molecular Approach, 2/e

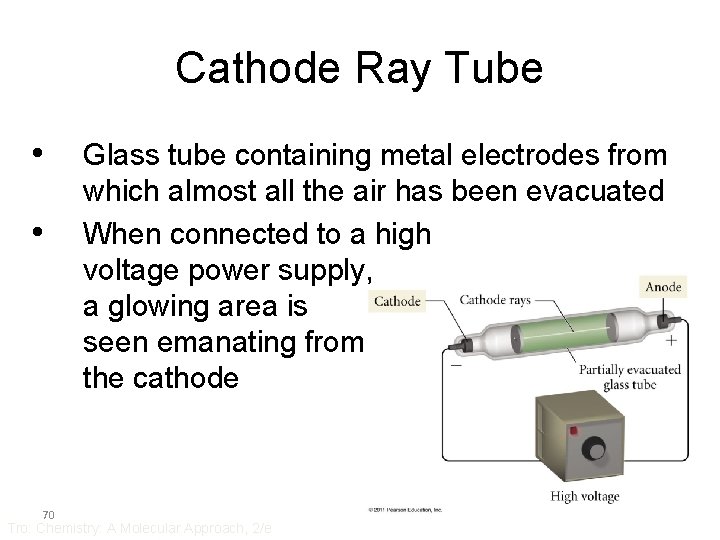
Cathode Ray Tube • • 70 Glass tube containing metal electrodes from which almost all the air has been evacuated When connected to a high voltage power supply, a glowing area is seen emanating from the cathode Tro: Chemistry: A Molecular Approach, 2/e

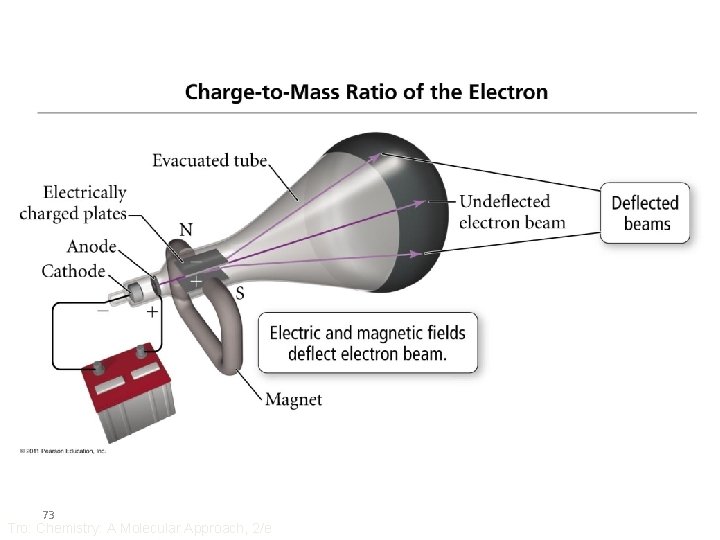
J. J. Thomson • Believed that the cathode ray was composed of tiny particles with an electrical charge • Designed an experiment to demonstrate that there were particles by measuring the amount of force it takes to deflect their path a given amount – like measuring the amount of force it takes to make a car turn 71 Tro: Chemistry: A Molecular Approach, 2/e

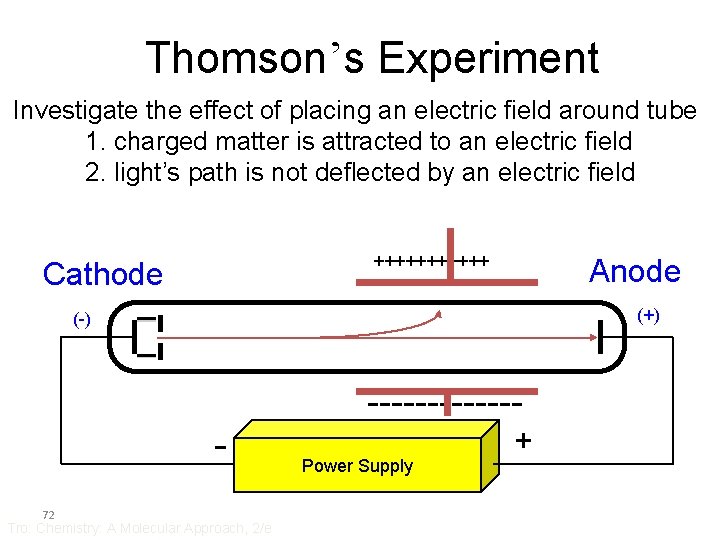
Thomson’s Experiment Investigate the effect of placing an electric field around tube 1. charged matter is attracted to an electric field 2. light’s path is not deflected by an electric field ++++++ Cathode Anode (+) (-) ------- 72 Tro: Chemistry: A Molecular Approach, 2/e Power Supply +

73 Tro: Chemistry: A Molecular Approach, 2/e

Thomson’s Results • The cathode rays are made of tiny particles • These particles have a negative charge – because the beam always deflected toward the + plate • The amount of deflection was related to two factors, the charge and mass of the particles • Every material tested contained these same particles • The charge: mass ratio of these particles was − 1. 76 x 108 C/g – the charge/mass of the hydrogen ion is +9. 58 x 104 C/g 74 Tro: Chemistry: A Molecular Approach, 2/e

Thomson’s Conclusions • If the particle has the same amount of charge as a hydrogen ion, then it must have a mass almost 2000 x smaller than hydrogen atoms! – later experiments by Millikan showed that the particle did have the same amount of charge as the hydrogen ion • The only way for this to be true is if these particles were pieces of atoms – apparently, the atom is not unbreakable 75 Tro: Chemistry: A Molecular Approach, 2/e

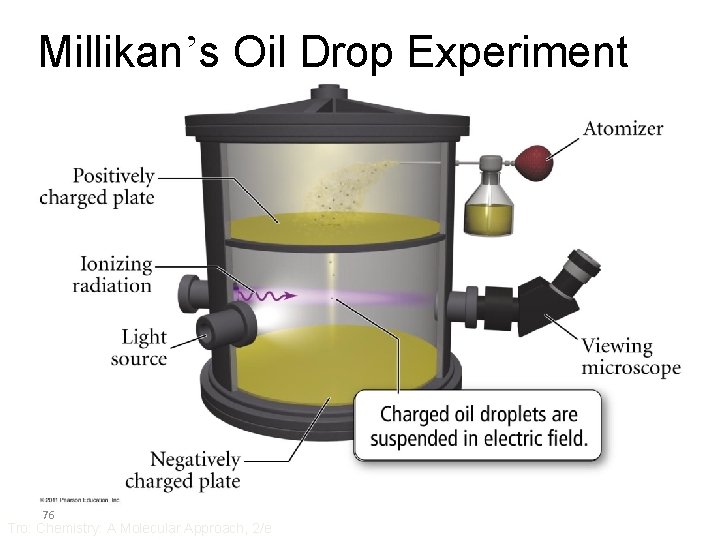
Millikan’s Oil Drop Experiment 76 Tro: Chemistry: A Molecular Approach, 2/e

Thomson’s Conclusions, cont’d • Thomson believed that these particles were therefore the ultimate building blocks of matter – “We have in the cathode rays matter in a new state, a state in which the subdivision of matter is carried very much further. . . a state in which all matter. . . is of one and the same kind; this matter being the substance from which all the chemical elements are built up. ” • These cathode ray particles became known as electrons Tro: Chemistry: A Molecular Approach, 2/e 77

Electrons • Electrons are tiny, negatively charged particles found in all atoms • Cathode rays are made of streams of electrons • The electron has a charge of − 1. 60 x 1019 C • The electron has a mass of 9. 1 x 10− 28 g 78 Tro: Chemistry: A Molecular Approach, 2/e

A New Theory of the Atom • Because the atom is no longer indivisible, Thomson must propose a new model of the atom to replace the first statement in Dalton’s Atomic Theory – rest of Dalton’s theory still valid at this point • Thomson proposes that instead of being a hard, marble-like unbreakable sphere, the way Dalton described it, the atom actually had an inner structure 79 Tro: Chemistry: A Molecular Approach, 2/e

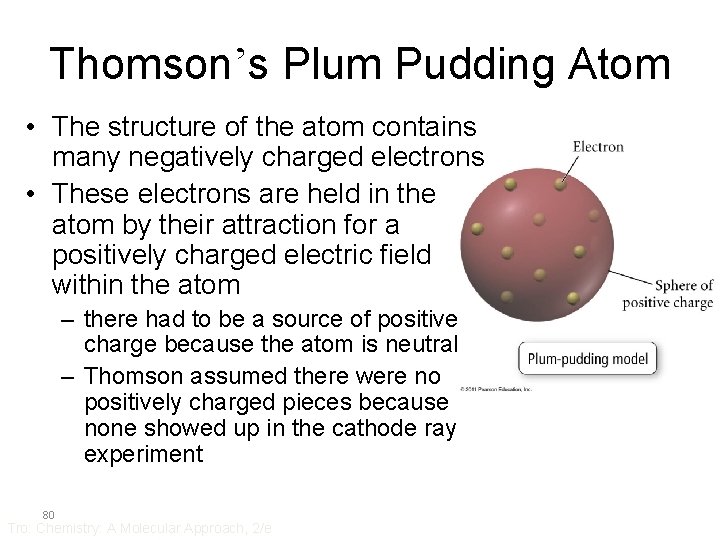
Thomson’s Plum Pudding Atom • The structure of the atom contains many negatively charged electrons • These electrons are held in the atom by their attraction for a positively charged electric field within the atom – there had to be a source of positive charge because the atom is neutral – Thomson assumed there were no positively charged pieces because none showed up in the cathode ray experiment 80 Tro: Chemistry: A Molecular Approach, 2/e

Predictions of the Plum Pudding Atom • The mass of the atom is due to the mass of the electrons within it – electrons are the only particles in Plum Pudding atoms, therefore the only source of mass • The atom is mostly empty space – should not have a bunch of negatively charged particles near each other as they would repel 81 Tro: Chemistry: A Molecular Approach, 2/e

Radioactivity • In the late 1800 s, Henri Becquerel and Marie Curie discovered that certain elements would constantly emit small, energetic particles and rays • These energetic particles could penetrate matter • Ernest Rutherford discovered that there were three different kinds of emissions – alpha, a, rays made of particles with a mass 4 x H atom and + charge – beta, b, rays made of particles with a mass ~1/2000 th H atom and – charge – gamma, g, rays that are energy rays, not particles 82 Tro: Chemistry: A Molecular Approach, 2/e Marie Curie 1867 -1934

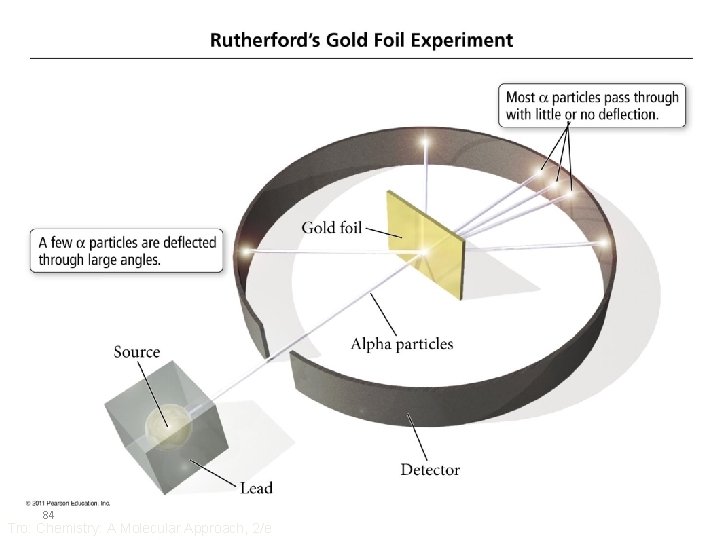
Rutherford’s Experiment • How can you prove something is empty space? • Put something through it! – use large target atoms • use very thin sheets of target so it will not absorb “bullet” – use very small particle as bullet with very high energy • but not so small that electrons will affect it • Bullet = alpha particles, target atoms = gold foil 83 – a particles have a mass of 4 amu & charge of +2 Tro: Chemistry: A Molecular Approach, 2/e

84 Tro: Chemistry: A Molecular Approach, 2/e

Rutherford’s Results • Over 98% of the a particles went straight through • About 2% of the a particles went through but were deflected by large angles • About 0. 005% of the a particles bounced off the gold foil – “. . . as if you fired a 15” cannon shell at a piece of tissue paper and it came back and hit you. ” 85 Tro: Chemistry: A Molecular Approach, 2/e

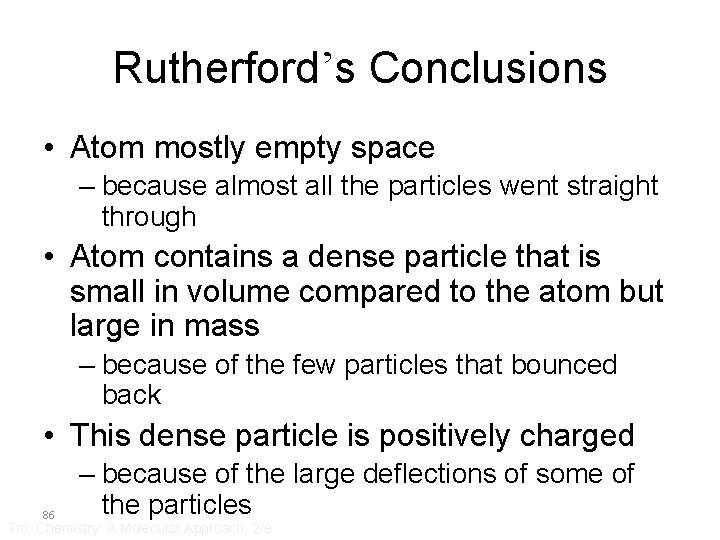
Rutherford’s Conclusions • Atom mostly empty space – because almost all the particles went straight through • Atom contains a dense particle that is small in volume compared to the atom but large in mass – because of the few particles that bounced back • This dense particle is positively charged 86 – because of the large deflections of some of the particles Tro: Chemistry: A Molecular Approach, 2/e

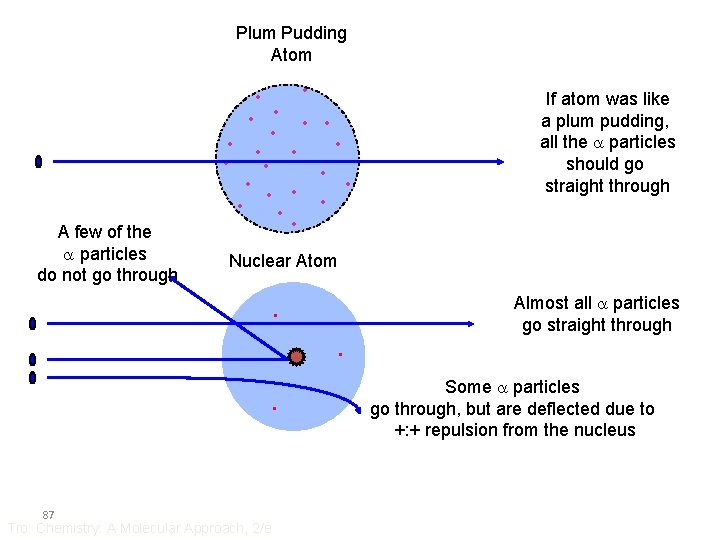
Plum Pudding Atom • • • A few of the a particles do not go through • • • Nuclear Atom . . . 87 If atom was like a plum pudding, all the a particles should go straight through Tro: Chemistry: A Molecular Approach, 2/e Almost all a particles go straight through Some a particles go through, but are deflected due to +: + repulsion from the nucleus

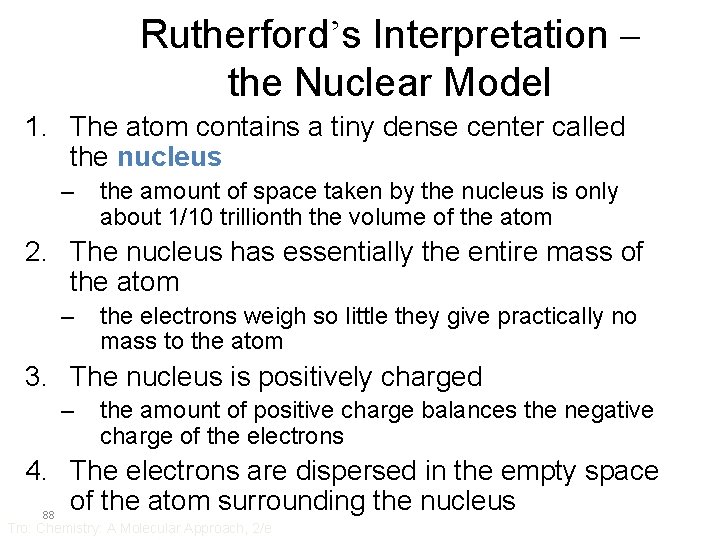
Rutherford’s Interpretation – the Nuclear Model 1. The atom contains a tiny dense center called the nucleus – the amount of space taken by the nucleus is only about 1/10 trillionth the volume of the atom 2. The nucleus has essentially the entire mass of the atom – the electrons weigh so little they give practically no mass to the atom 3. The nucleus is positively charged – the amount of positive charge balances the negative charge of the electrons 4. The electrons are dispersed in the empty space of the atom surrounding the nucleus 88 Tro: Chemistry: A Molecular Approach, 2/e

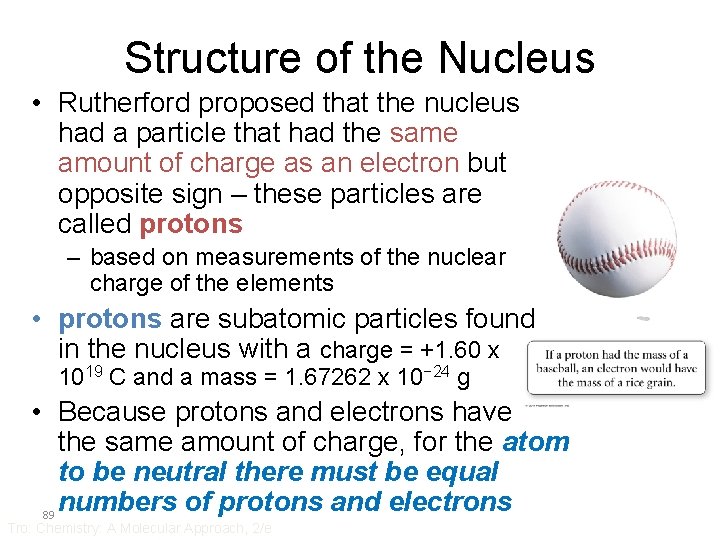
Structure of the Nucleus • Rutherford proposed that the nucleus had a particle that had the same amount of charge as an electron but opposite sign – these particles are called protons – based on measurements of the nuclear charge of the elements • protons are subatomic particles found in the nucleus with a charge = +1. 60 x 1019 C and a mass = 1. 67262 x 10− 24 g • Because protons and electrons have the same amount of charge, for the atom to be neutral there must be equal numbers of protons and electrons 89 Tro: Chemistry: A Molecular Approach, 2/e

Relative Mass and Charge • It is sometimes easier to compare things to each other rather than to an outside standard • When you do this, the scale of comparison is called a relative scale • We generally talk about the size of charge on atoms by comparing it to the amount of charge on an electron, which we call − 1 charge units – proton has a charge of +1 cu – protons and electrons have equal amounts of charge, but opposite signs • We generally talk about the mass of atoms by comparing it to 1/12 th the mass of a carbon atom with 6 protons and 6 neutrons, which we call 1 atomic mass unit – protons have a mass of 1 amu – electrons have a mass of 0. 00055 amu, which is generally too small to be relevant 90 Tro: Chemistry: A Molecular Approach, 2/e

Some Problems • How could beryllium have four protons stuck together in the nucleus? – shouldn’t they repel each other? • If a beryllium atom has four protons, then it should weigh 4 amu; but it actually weighs 9. 01 amu! Where is the extra mass coming from? – each proton weighs 1 amu – remember, the electron’s mass is only about 0. 00055 amu and Be has only four electrons – it can’t account for the extra 5 amu of mass 91 Tro: Chemistry: A Molecular Approach, 2/e

There Must Be Something Else! • To answer these questions, Rutherford and Chadwick proposed that there was another particle in the nucleus – it is called a neutron • Neutrons are subatomic particles with a mass = 1. 67493 x 10− 24 g and no charge, and are found in the nucleus ü 1 amu Øslightly heavier than a proton üno charge 92 Tro: Chemistry: A Molecular Approach, 2/e

93 Tro: Chemistry: A Molecular Approach, 2/e

Problems for Chapter 2 • 51 -72, 75 -78, 79 -90