Chapter 2 Lipids 2 1 Lipids 1 LIPID



























- Slides: 79

Chapter 2 Lipids 2. 1 - Lipids 1

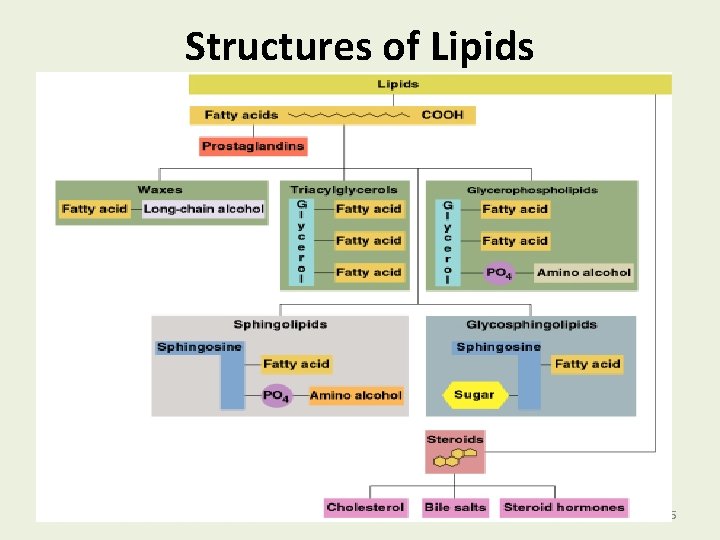
LIPID Lipids include: 1. Fatty acids 2. Neutral fats and oils 3. Waxes 4. Phospholipid 5. Sterols 6. Fat soluble vitamins 2

Lipids are • Biomolecules that contain fatty acids or a steroid nucleus. • Soluble in organic solvents but not in water. • Named for the Greek word lipos, which means “fat. ” • Extracted from cells using organic solvents. 3

Types of Lipids The types of lipids containing fatty acids are • Waxes. • Fats and oils (triacylglycerols). • Glycerophospholipids. • Prostaglandins. • Not steroids, as they do not contain fatty acids. 4

Structures of Lipids 5

Chapter 2 Lipids 2. 2 - Fatty Acids 6

Lipids are non-polar (hydrophobic) compounds, soluble in organic solvents. Most membrane lipids are amphipathic, having a non-polar end a polar end. Fatty acids consist of a hydrocarbon chain with a carboxylic acid at one end. - A 16 -C fatty acid: CH 3(CH 2)14 COO Non-polar - polar A 16 -C fatty acid with one cis double bond between C atoms 9 -10 may be represented as 16: 1 cis D 9. 7

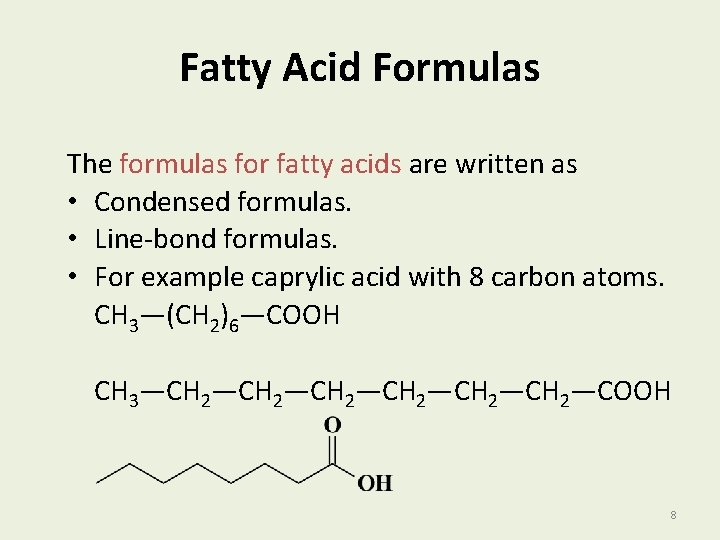
Fatty Acid Formulas The formulas for fatty acids are written as • Condensed formulas. • Line-bond formulas. • For example caprylic acid with 8 carbon atoms. CH 3—(CH 2)6—COOH CH 3—CH 2—CH 2—COOH 8

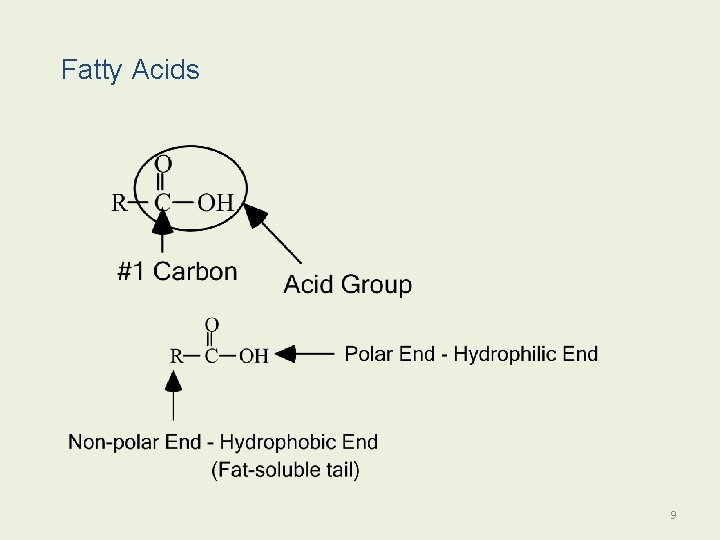
Fatty Acids 9

Fatty Acids Fatty acids • Are long-chain carboxylic acids. • Typically contain 1218 carbon atoms. • Are insoluble in water. • Can be saturated or unsaturated. 10

Saturated Fatty Acids Octanoic Acid 11

Unsaturated Fatty Acids 3 - Octenoic Acid 3, 6 - Octadienoic Acid Short hand: 8: 1 (D 3) 8: 2 (D 3, 6) 12

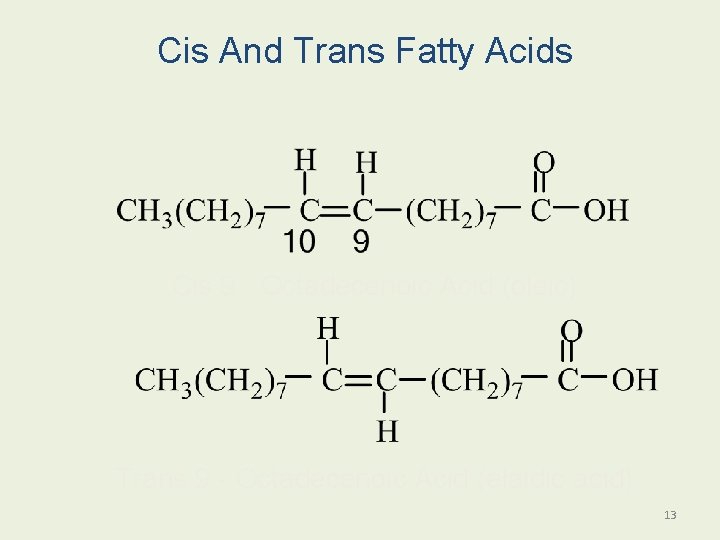
Cis And Trans Fatty Acids Cis 9 - Octadecenoic Acid (oleic) Trans 9 - Octadecenoic Acid (elaidic acid) 13

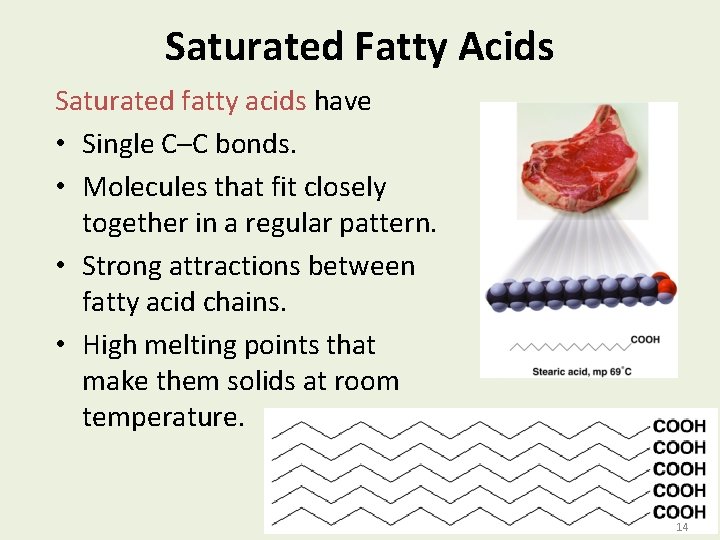
Saturated Fatty Acids Saturated fatty acids have • Single C–C bonds. • Molecules that fit closely together in a regular pattern. • Strong attractions between fatty acid chains. • High melting points that make them solids at room temperature. 14 14

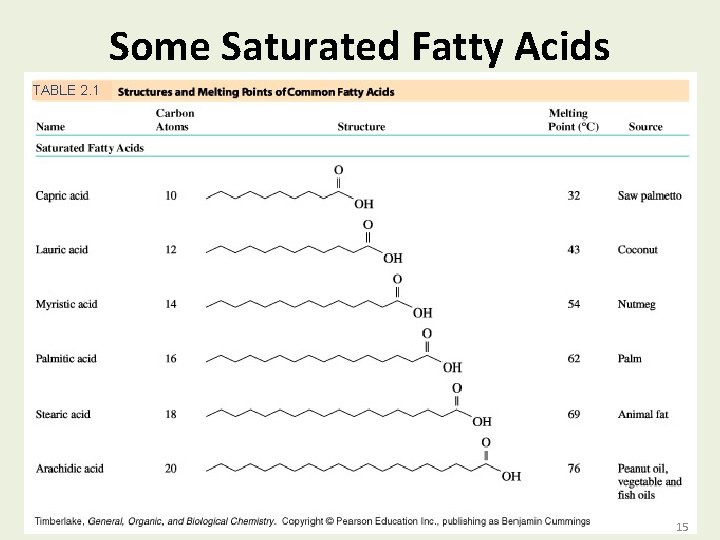
Some Saturated Fatty Acids TABLE 2. 1 Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 15 15

Unsaturated Fatty Acids Unsaturated fatty acids • Have one or more double C=C bond • Typically contain cis double bonds. 16

Properties of Unsaturated Fatty Acids Unsaturated fatty acids • Have “kinks” in the fatty acid chains. • Do not pack closely. • Have few attractions between chains. • Have low melting points. • Are liquids at room temperature. “kinks” in chain 17

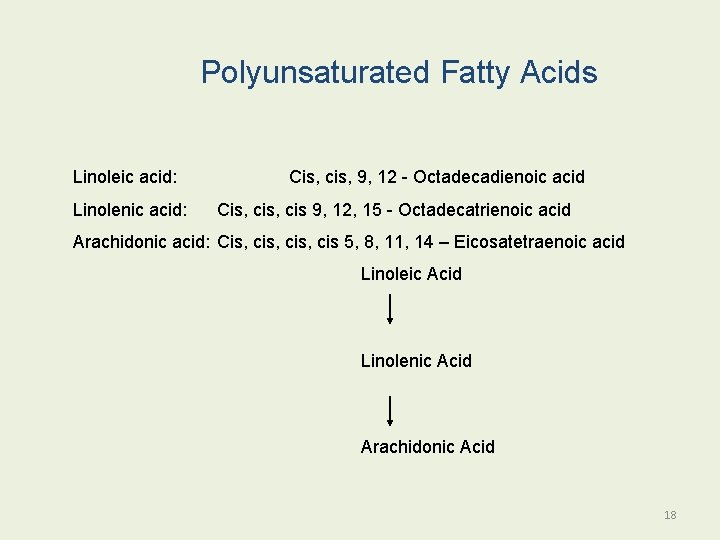
Polyunsaturated Fatty Acids Linoleic acid: Linolenic acid: Cis, cis, 9, 12 - Octadecadienoic acid Cis, cis 9, 12, 15 - Octadecatrienoic acid Arachidonic acid: Cis, cis, cis 5, 8, 11, 14 – Eicosatetraenoic acid Linoleic Acid Linolenic Acid Arachidonic Acid 18

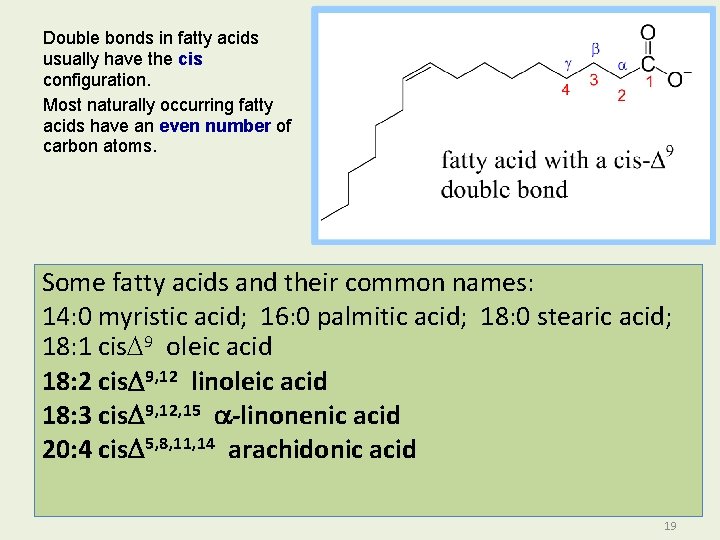
Double bonds in fatty acids usually have the cis configuration. Most naturally occurring fatty acids have an even number of carbon atoms. Some fatty acids and their common names: 14: 0 myristic acid; 16: 0 palmitic acid; 18: 0 stearic acid; 18: 1 cis. D 9 oleic acid 18: 2 cis. D 9, 12 linoleic acid 18: 3 cis. D 9, 12, 15 a-linonenic acid 20: 4 cis. D 5, 8, 11, 14 arachidonic acid 19

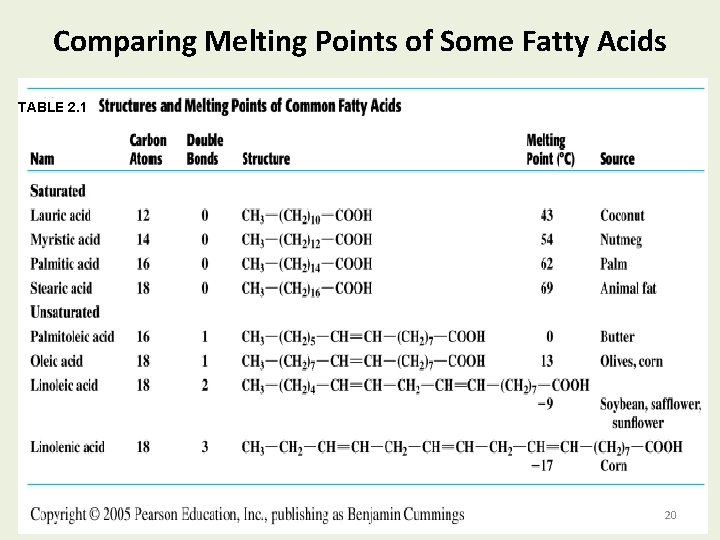
Comparing Melting Points of Some Fatty Acids TABLE 2. 1 20

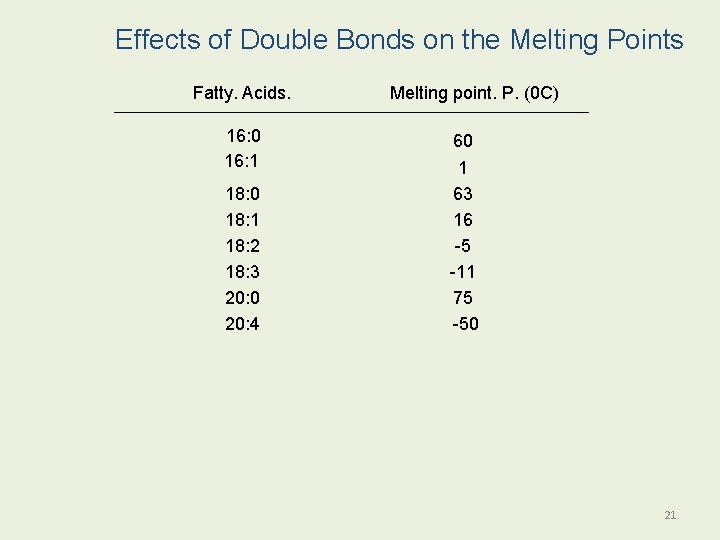
Effects of Double Bonds on the Melting Points Fatty. Acids. 16: 0 16: 1 18: 0 18: 1 18: 2 18: 3 20: 0 20: 4 Melting point. P. (0 C) 60 1 63 16 -5 -11 75 -50 21

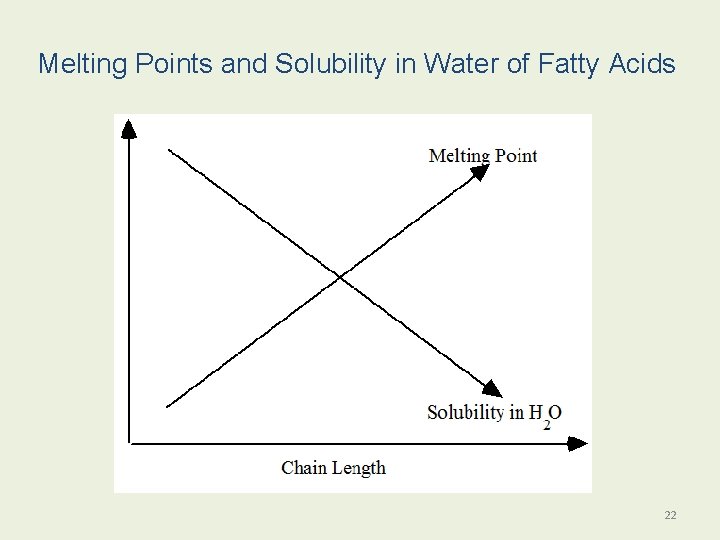
Melting Points and Solubility in Water of Fatty Acids 22

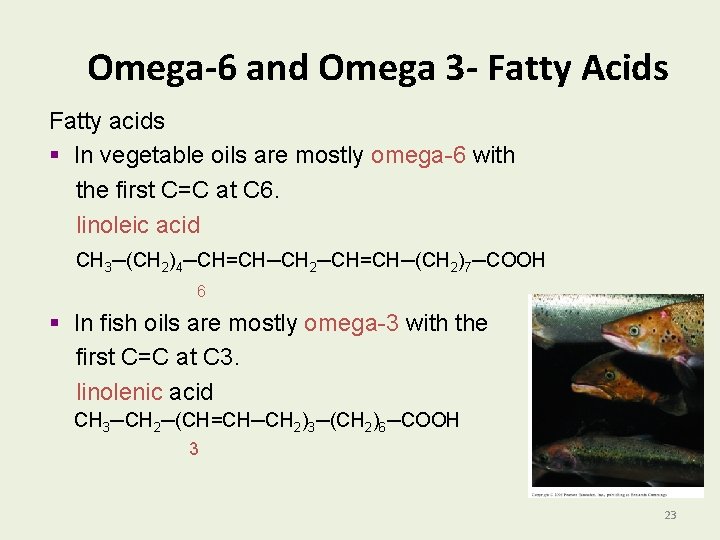
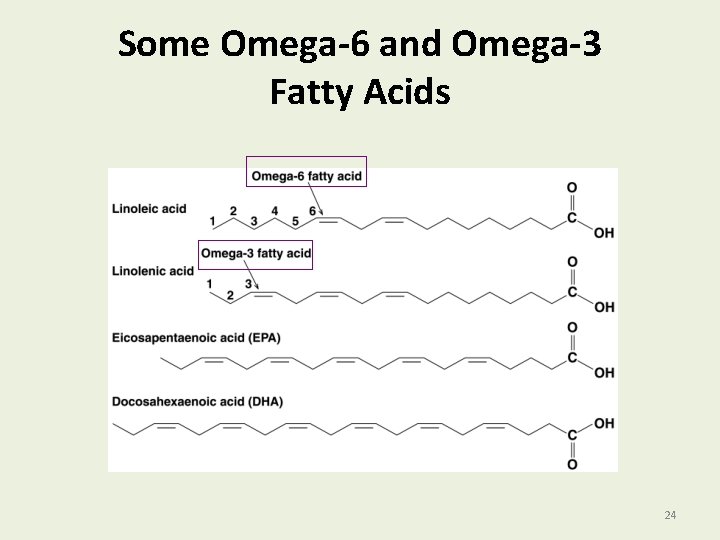
Omega-6 and Omega 3 - Fatty Acids Fatty acids § In vegetable oils are mostly omega-6 with the first C=C at C 6. linoleic acid CH 3─(CH 2)4─CH=CH─CH 2─CH=CH─(CH 2)7─COOH 6 § In fish oils are mostly omega-3 with the first C=C at C 3. linolenic acid CH 3─CH 2─(CH=CH─CH 2)3─(CH 2)6─COOH 3 23

Some Omega-6 and Omega-3 Fatty Acids 24

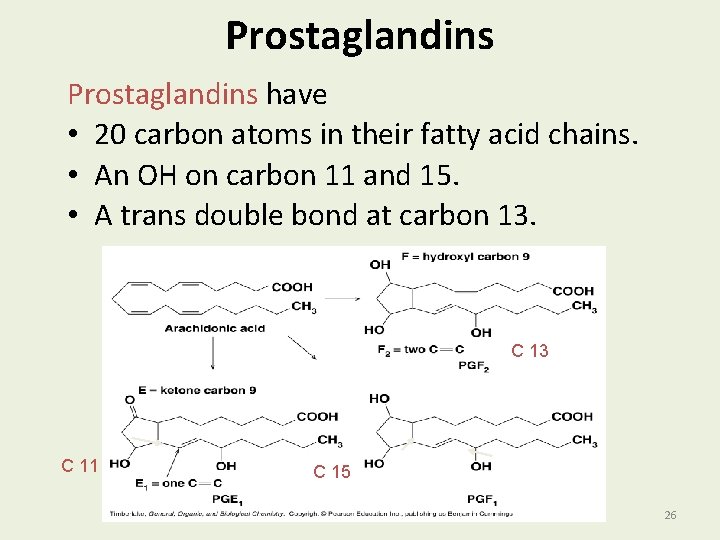
Prostaglandin: One of a number of hormone-like substances that participate in a wide range of body functions such as the contraction and relaxation of smooth muscle, the dilation and constriction of blood vessels, control of blood pressure, and modulation of inflammation. Prostaglandins are derived from a chemical called arachidonic acid 25

Prostaglandins have • 20 carbon atoms in their fatty acid chains. • An OH on carbon 11 and 15. • A trans double bond at carbon 13. C 13 C 11 C 15 26

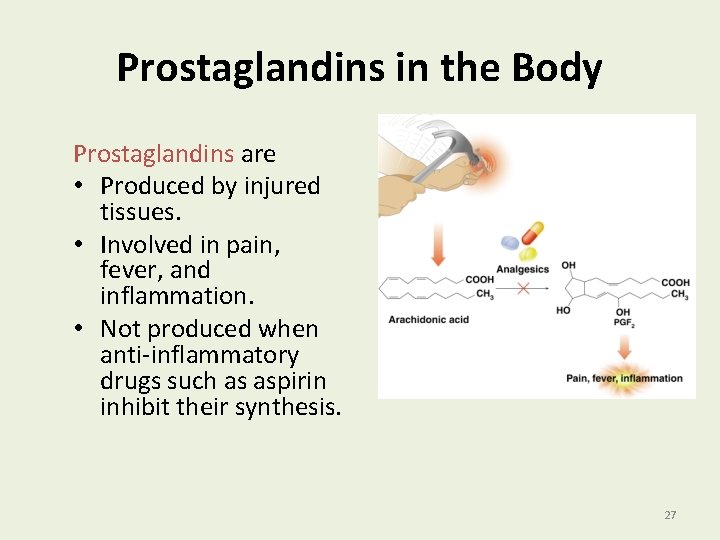
Prostaglandins in the Body Prostaglandins are • Produced by injured tissues. • Involved in pain, fever, and inflammation. • Not produced when anti-inflammatory drugs such as aspirin inhibit their synthesis. 27

Chapter 2 Lipids 2. 3 - Waxes, Fats, and Oils 28

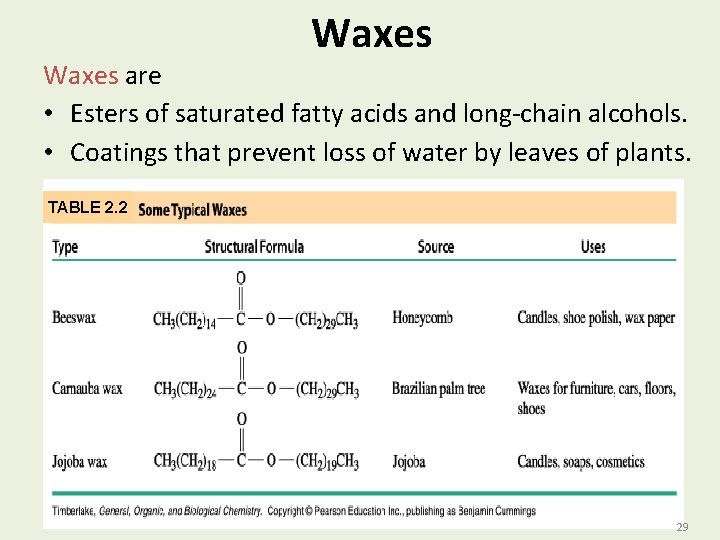
Waxes are • Esters of saturated fatty acids and long-chain alcohols. • Coatings that prevent loss of water by leaves of plants. TABLE 2. 2 29 29

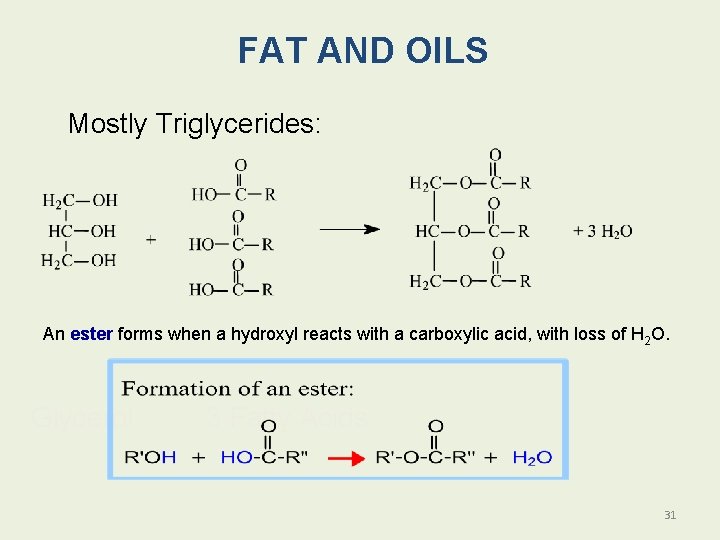
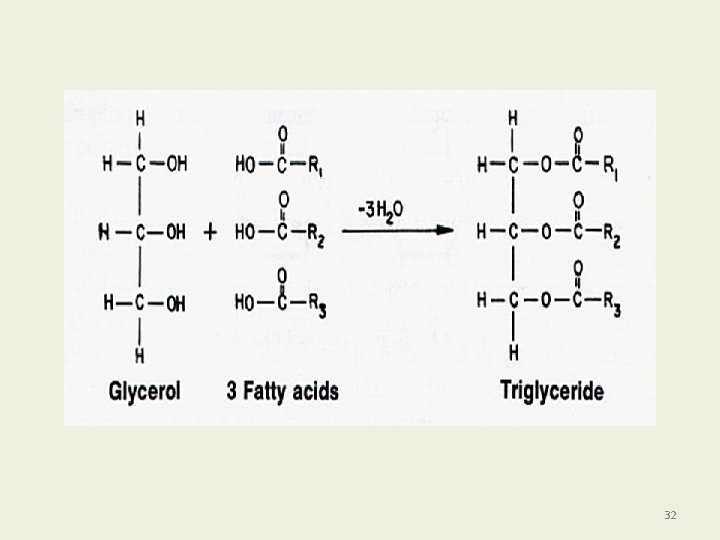
Fats and Oils: Triacylglycerols Fats and oils are • Also called triacylglycerols. • Esters of glycerol. • Produced by esterification. • Formed when the hydroxyl groups of glycerol react with the carboxyl groups of fatty acids. 30

FAT AND OILS Mostly Triglycerides: An ester forms when a hydroxyl reacts with a carboxylic acid, with loss of H 2 O. Glycerol 3 Fatty Acids 31

32

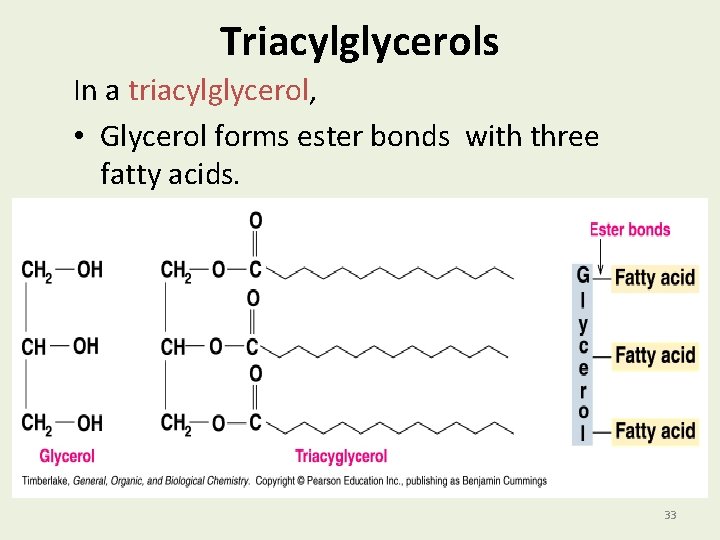
Triacylglycerols In a triacylglycerol, • Glycerol forms ester bonds with three fatty acids. 33

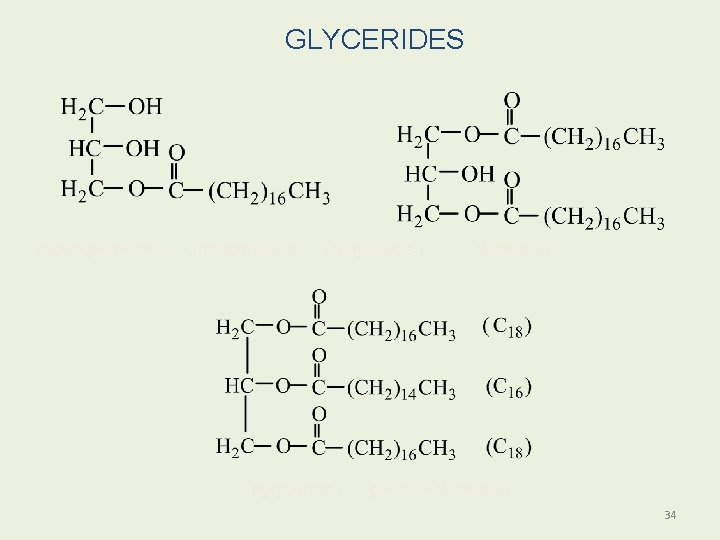
GLYCERIDES Monoglyceride (a - monostearin) Diglyceride (a, a' - distearin) Triglyceride (b - palmityl distearin) 34

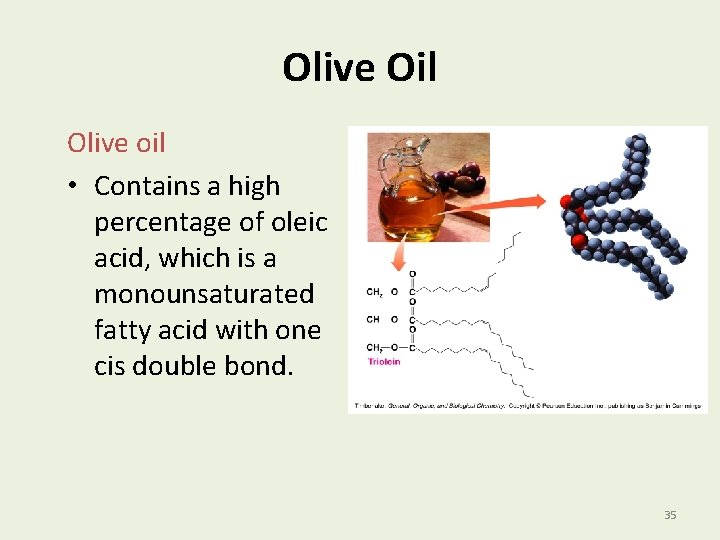
Olive Oil Olive oil • Contains a high percentage of oleic acid, which is a monounsaturated fatty acid with one cis double bond. 35

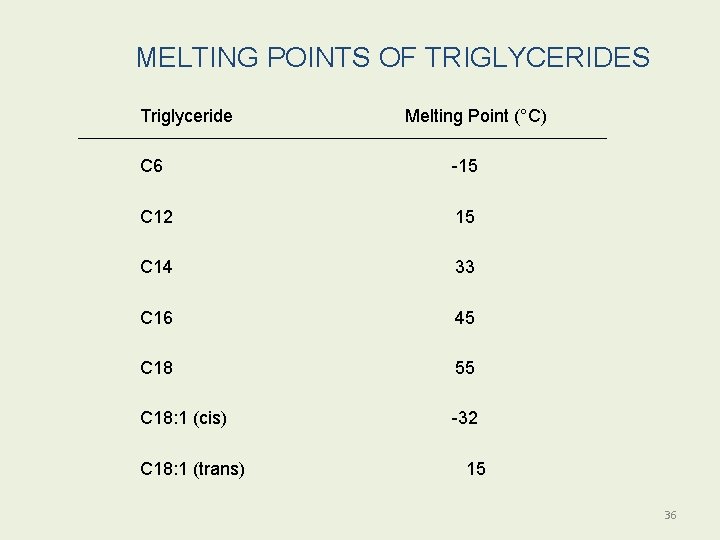
MELTING POINTS OF TRIGLYCERIDES Triglyceride Melting Point (°C) C 6 -15 C 12 15 C 14 33 C 16 45 C 18 55 C 18: 1 (cis) -32 C 18: 1 (trans) 15 36

Melting Points of Fats and Oils A triacylglycerol that is a fat • Is solid at room temperature. • Is prevalent in meats, whole milk, butter, and cheese. A triacylglycerol that is an oil • Is liquid at room temperature. • Is prevalent in plants such as olive and safflower. 37

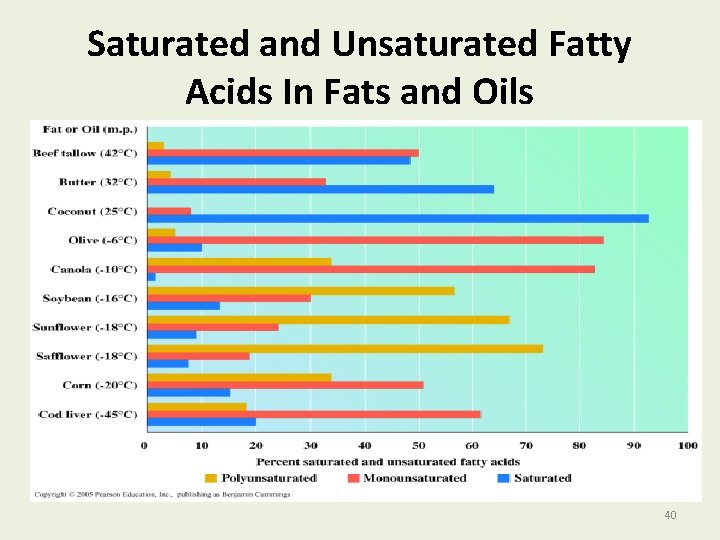
Oils with Unsaturated Fatty Acids Oils • Have more unsaturated fats. • Have cis double bonds that cause “kinks” in the fatty acid chains. • Cannot pack triacylglycerol molecules as close together as in fats. • Have lower melting points than saturated fats. • Are liquids at room temperature. 38

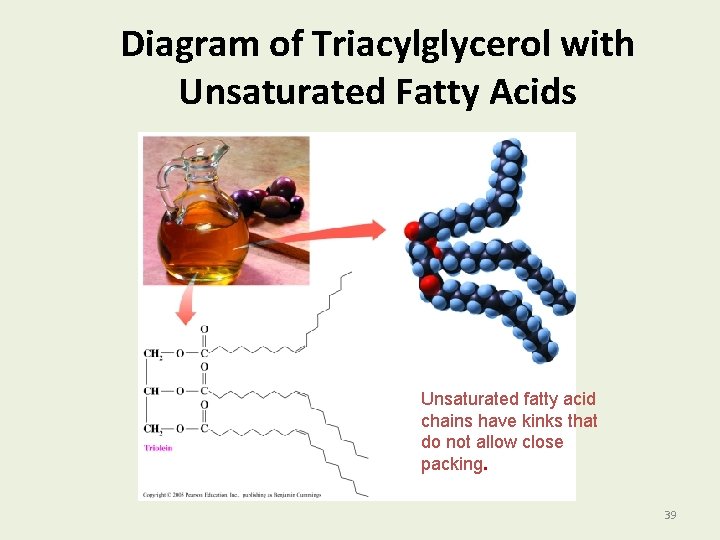
Diagram of Triacylglycerol with Unsaturated Fatty Acids Unsaturated fatty acid chains have kinks that do not allow close packing. 39

Saturated and Unsaturated Fatty Acids In Fats and Oils 40

Chapter 2 Lipids 2. 4 - Chemical Properties of Triacylglycerols 41

Chemical Properties of Triacylglycerols The chemical reactions of triacylglycerols are similar to those of alkenes and esters. • In hydrogenation, double bonds in unsaturated fatty acids react with H 2 in the presence of a Ni or Pt catalyst. • In hydrolysis, ester bonds are split by water in the presence of an acid, a base, or an enzyme. 42

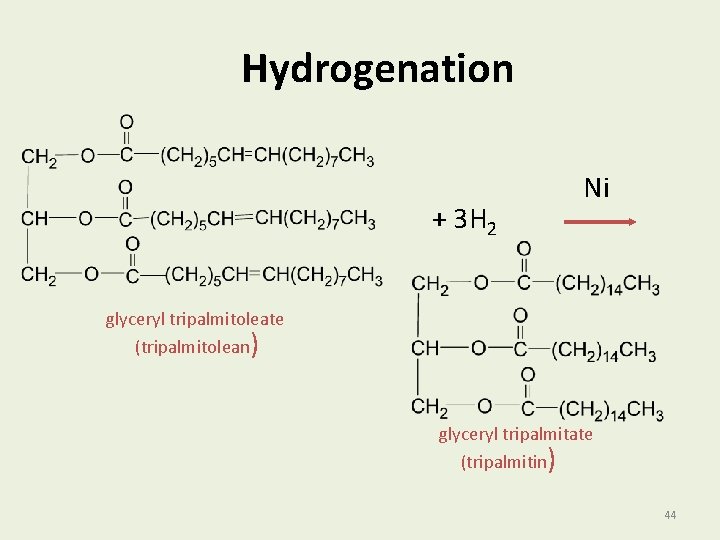
Hydrogenation of Oils The hydrogenation of oils • Adds hydrogen (H 2) to the carbon atoms of double bonds. • Converts double bonds to single bonds. • Increases the melting point. • Produces solids such as margarine and shortening. 43

Hydrogenation + 3 H 2 Ni glyceryl tripalmitoleate (tripalmitolean) glyceryl tripalmitate (tripalmitin) 44

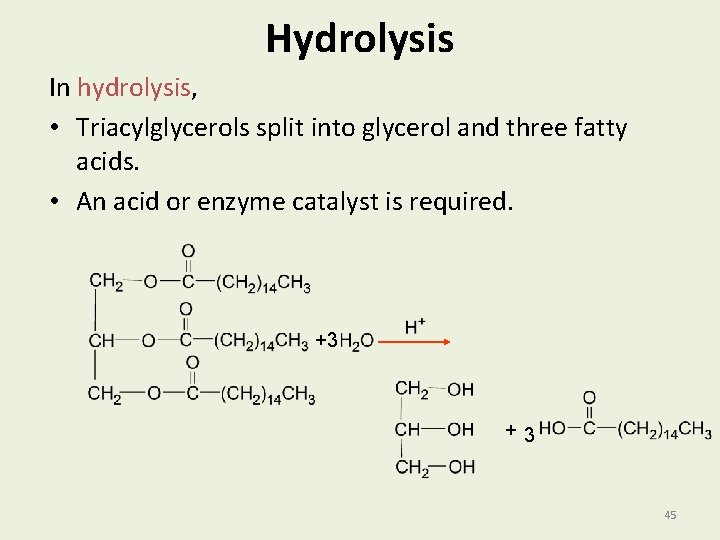
Hydrolysis In hydrolysis, • Triacylglycerols split into glycerol and three fatty acids. • An acid or enzyme catalyst is required. +3 +3 45

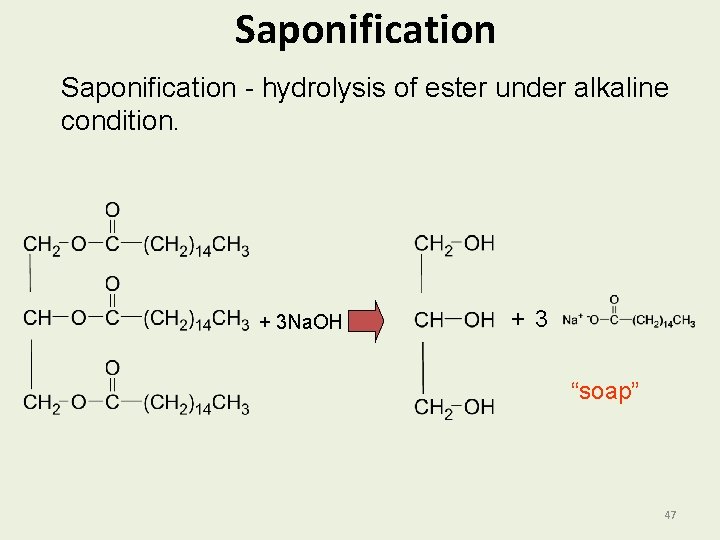
Saponification and Soap Saponification • Is the reaction of a fat with a strong base. • Splits triacylglycerols into glycerol and the salts of fatty acids. • Is the process of forming “soaps” (salts of fatty acids). • With KOH gives softer soaps. 46

Saponification - hydrolysis of ester under alkaline condition. + 3 Na. OH + 3 “soap” 47

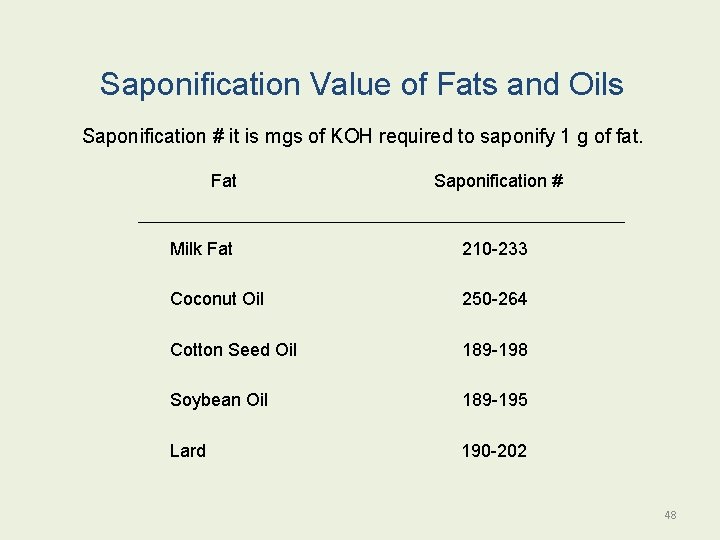
Saponification Value of Fats and Oils Saponification # it is mgs of KOH required to saponify 1 g of fat. Fat Saponification # Milk Fat 210 -233 Coconut Oil 250 -264 Cotton Seed Oil 189 -198 Soybean Oil 189 -195 Lard 190 -202 48

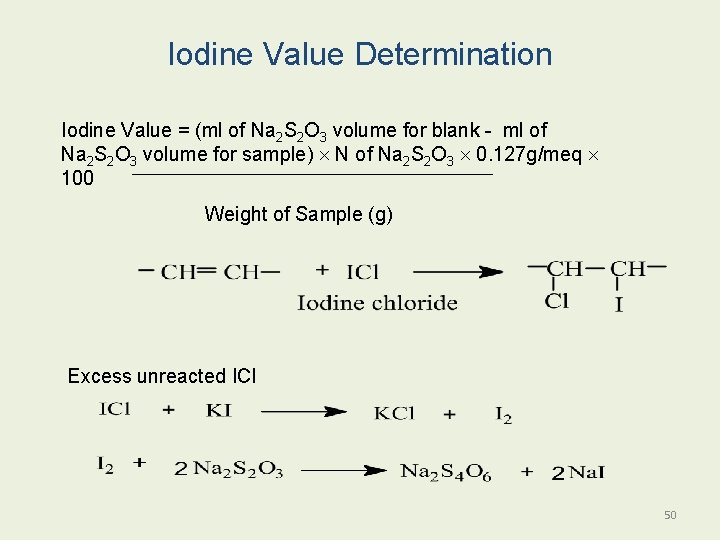
3. Iodine Number: it is grams of iodine absorbed by 100 g of oil. Molecular weight and iodine number can calculate the number of double bonds. 1 g of fat adsorbed 1. 5 g of iodine value = 150. 49

Iodine Value Determination Iodine Value = (ml of Na 2 S 2 O 3 volume for blank - ml of Na 2 S 2 O 3 volume for sample) N of Na 2 S 2 O 3 0. 127 g/meq 100 Weight of Sample (g) Excess unreacted ICl 50

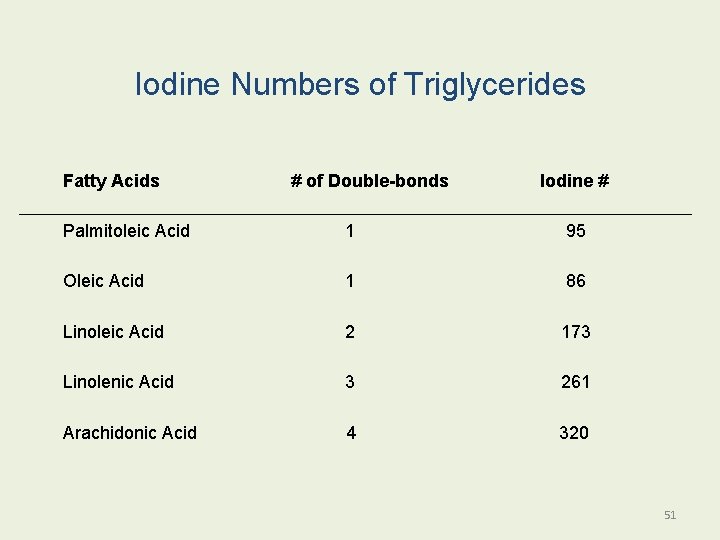
Iodine Numbers of Triglycerides Fatty Acids # of Double-bonds Iodine # Palmitoleic Acid 1 95 Oleic Acid 1 86 Linoleic Acid 2 173 Linolenic Acid 3 261 Arachidonic Acid 4 320 51

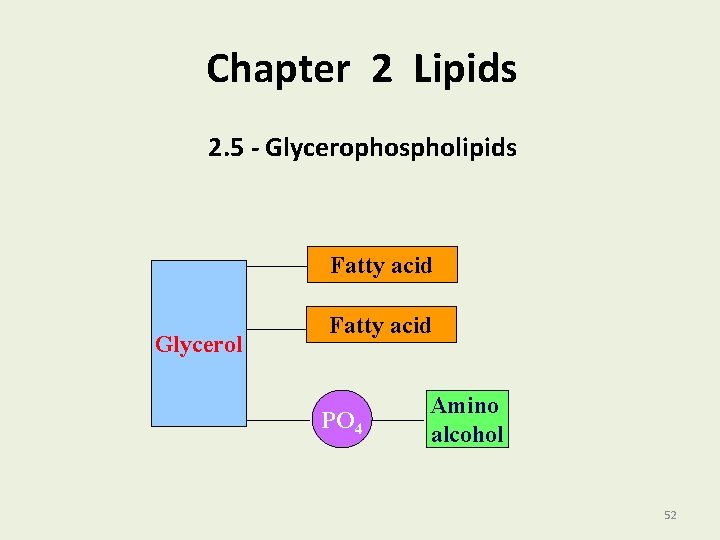
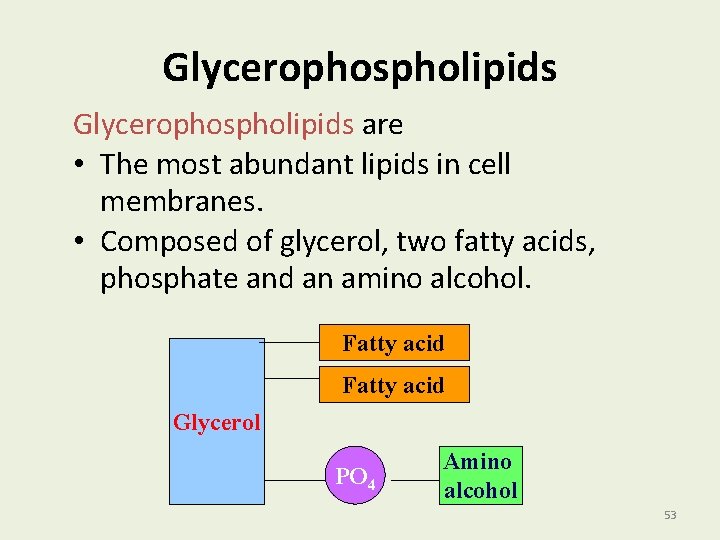
Chapter 2 Lipids 2. 5 - Glycerophospholipids Fatty acid Glycerol Fatty acid PO 4 Amino alcohol 52

Glycerophospholipids are • The most abundant lipids in cell membranes. • Composed of glycerol, two fatty acids, phosphate and an amino alcohol. Fatty acid Glycerol PO 4 Amino alcohol 53

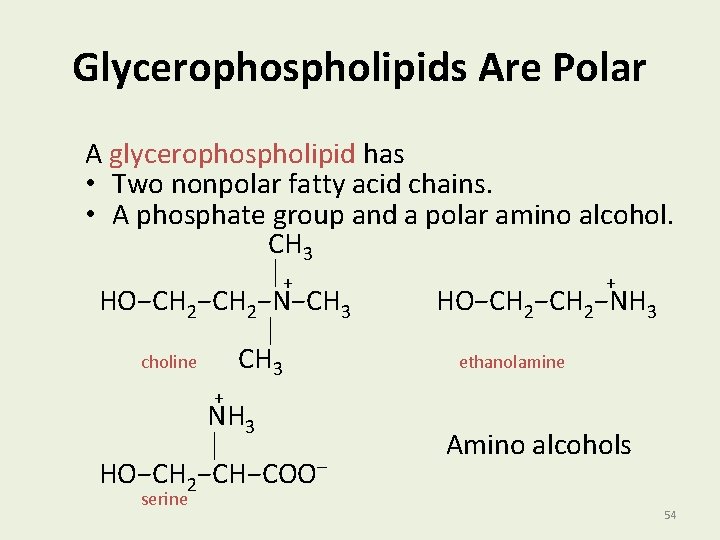
Glycerophospholipids Are Polar A glycerophospholipid has • Two nonpolar fatty acid chains. • A phosphate group and a polar amino alcohol. CH 3 │+ + HO−CH 2−N−CH 3 HO−CH 2−NH 3 │ choline CH 3 ethanolamine + NH 3 │ Amino alcohols HO−CH 2−CH−COO− serine 54

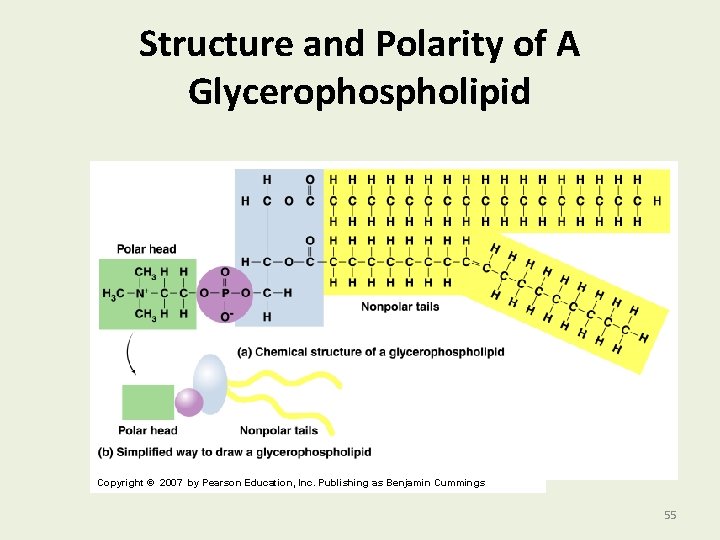
Structure and Polarity of A Glycerophospholipid Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 55

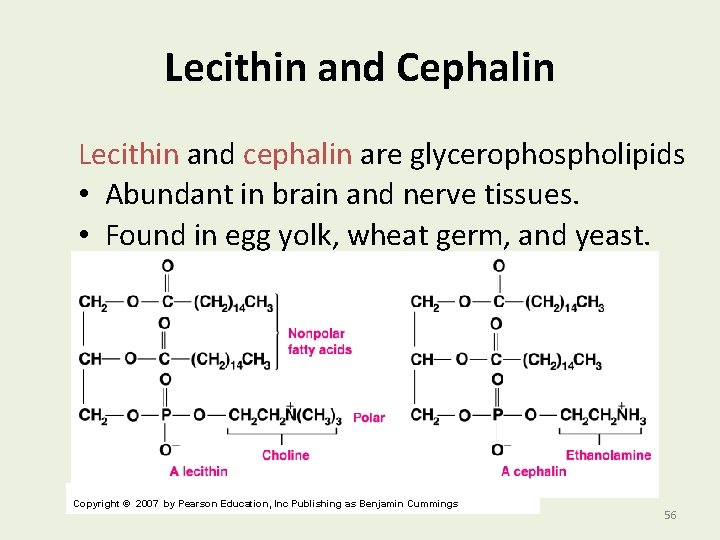
Lecithin and Cephalin Lecithin and cephalin are glycerophospholipids • Abundant in brain and nerve tissues. • Found in egg yolk, wheat germ, and yeast. Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 56

Chapter 2 Lipids 2. 6 - Sphingolipids 57

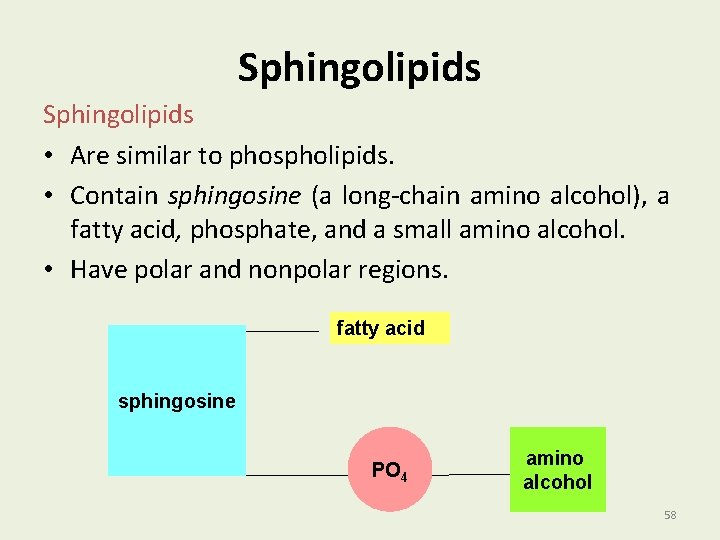
Sphingolipids • Are similar to phospholipids. • Contain sphingosine (a long-chain amino alcohol), a fatty acid, phosphate, and a small amino alcohol. • Have polar and nonpolar regions. fatty acid sphingosine PO 4 amino alcohol 58

Sphingosine is a long-chain unsaturated amino alcohol. CH 3−(CH 2)12 −CH=CH−CH−OH │ CH−NH 2 │ CH 2−OH sphingosine 59

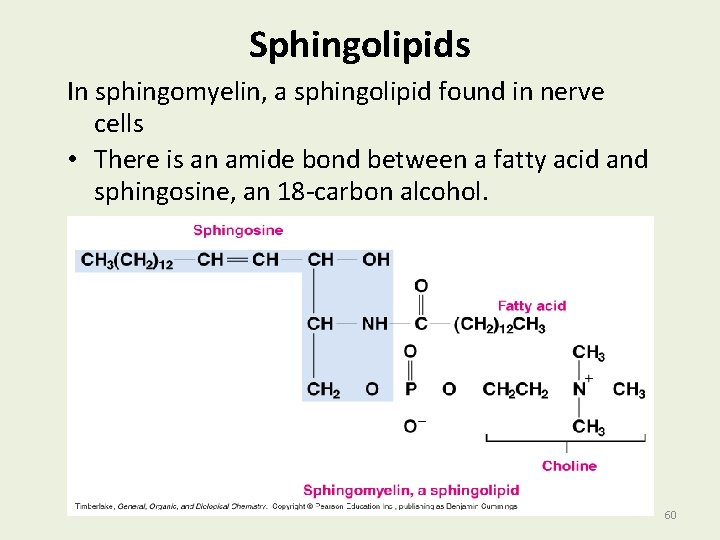
Sphingolipids In sphingomyelin, a sphingolipid found in nerve cells • There is an amide bond between a fatty acid and sphingosine, an 18 -carbon alcohol. 60

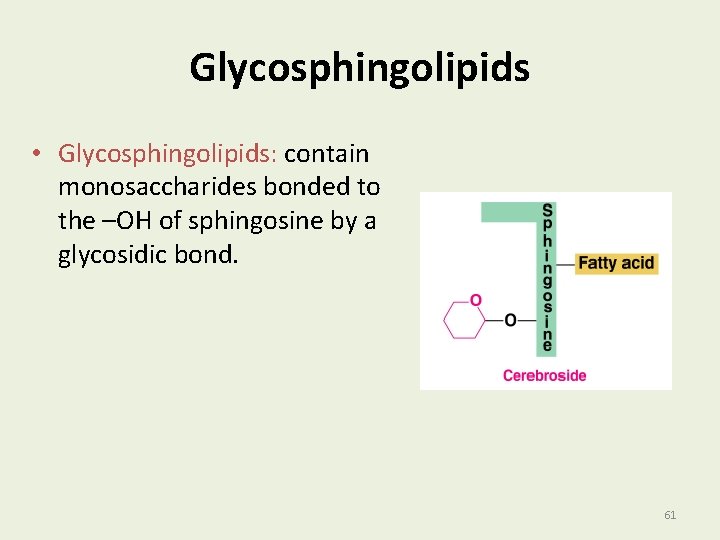
Glycosphingolipids • Glycosphingolipids: contain monosaccharides bonded to the –OH of sphingosine by a glycosidic bond. 61

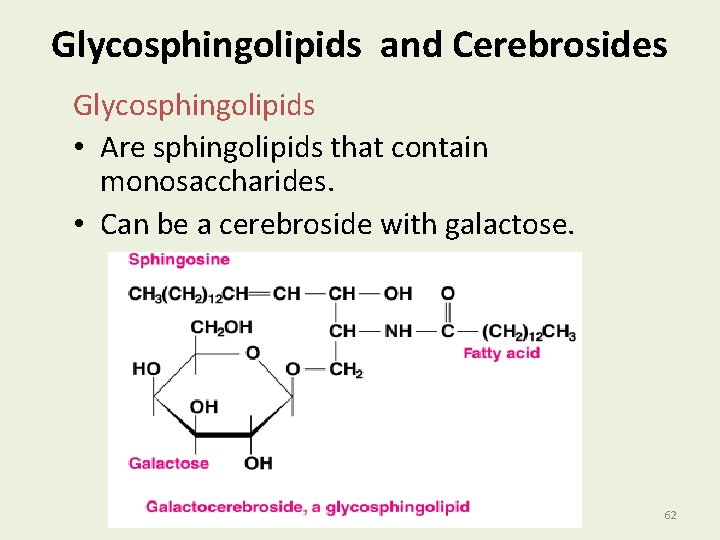
Glycosphingolipids and Cerebrosides Glycosphingolipids • Are sphingolipids that contain monosaccharides. • Can be a cerebroside with galactose. 62

Chapter 2 Lipids 2. 7 - Steroids: Cholesterol, Bile Salts, and Steroid Hormones 63

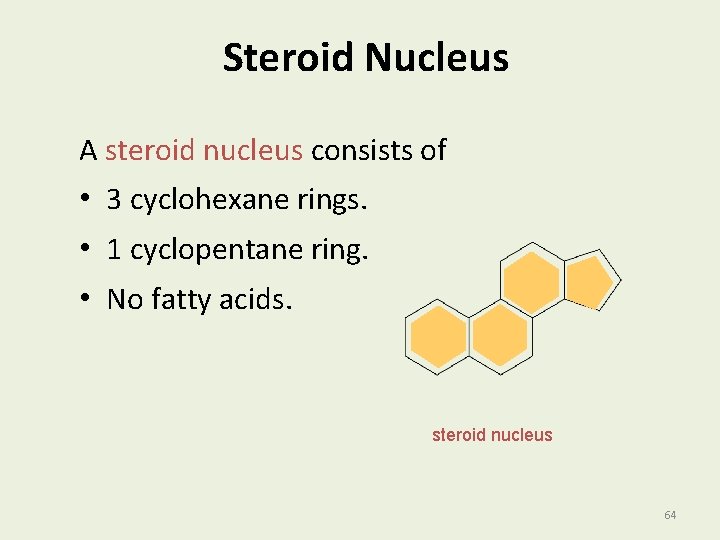
Steroid Nucleus A steroid nucleus consists of • 3 cyclohexane rings. • 1 cyclopentane ring. • No fatty acids. steroid nucleus 64

STEROLS Cholesterol Fat soluble vitamins Bile acids Male & female sex hormones Adrenal corticosteroids 65

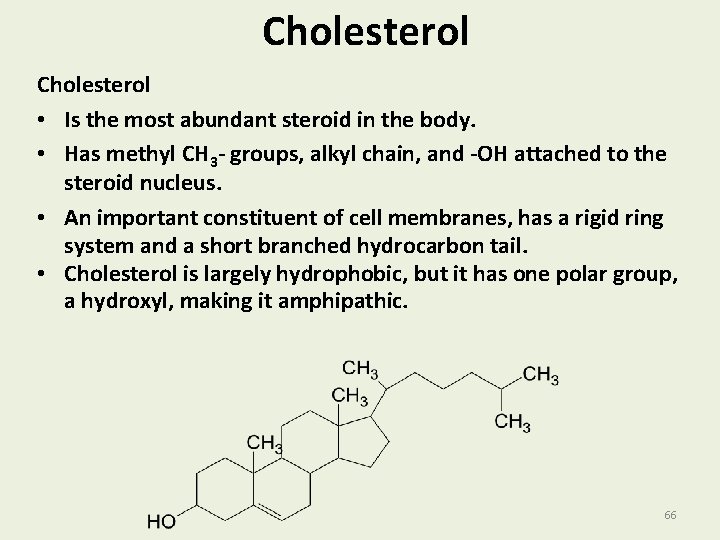
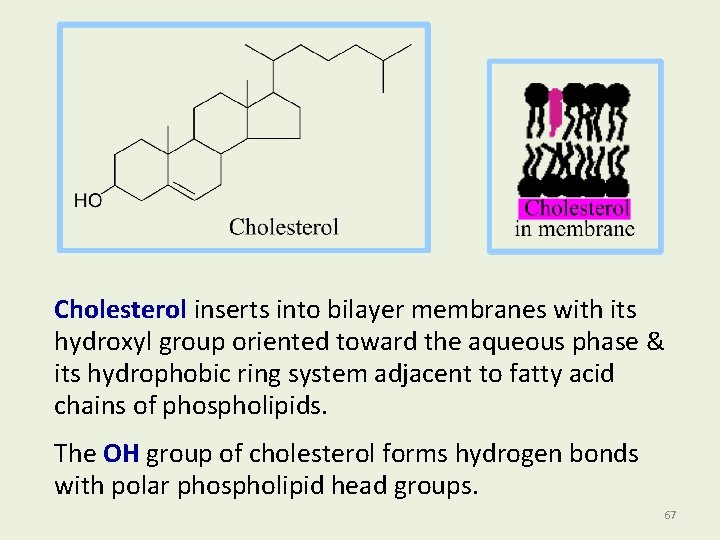
Cholesterol • Is the most abundant steroid in the body. • Has methyl CH 3 - groups, alkyl chain, and -OH attached to the steroid nucleus. • An important constituent of cell membranes, has a rigid ring system and a short branched hydrocarbon tail. • Cholesterol is largely hydrophobic, but it has one polar group, a hydroxyl, making it amphipathic. 66

Cholesterol inserts into bilayer membranes with its hydroxyl group oriented toward the aqueous phase & its hydrophobic ring system adjacent to fatty acid chains of phospholipids. The OH group of cholesterol forms hydrogen bonds with polar phospholipid head groups. 67

Cholesterol in the Body Cholesterol in the body • Is obtained from meats, milk, and eggs. • Is synthesized in the liver. • Is needed for cell membranes, brain and nerve tissue, steroid hormones, and Vitamin D. • Clogs arteries when high levels form plaque. • Considered elevated if plasma cholesterol exceeds 200 mg/d. L. A normal, open artery. An artery clogged by cholesterol plaque 68

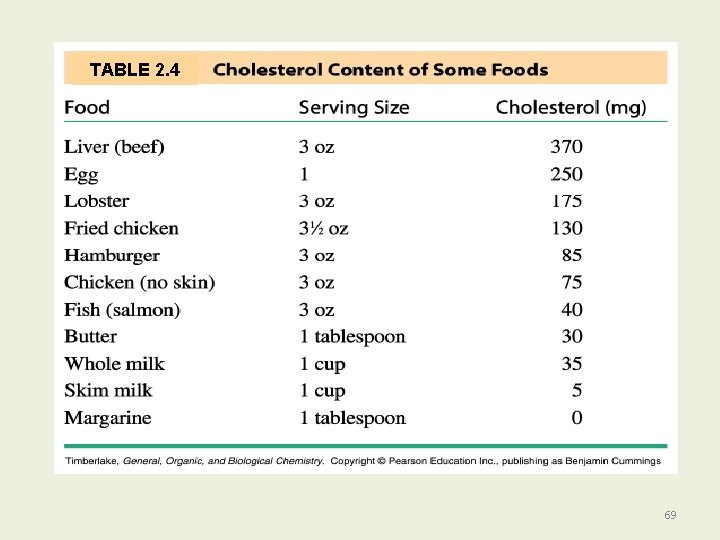
TABLE 2. 4 69

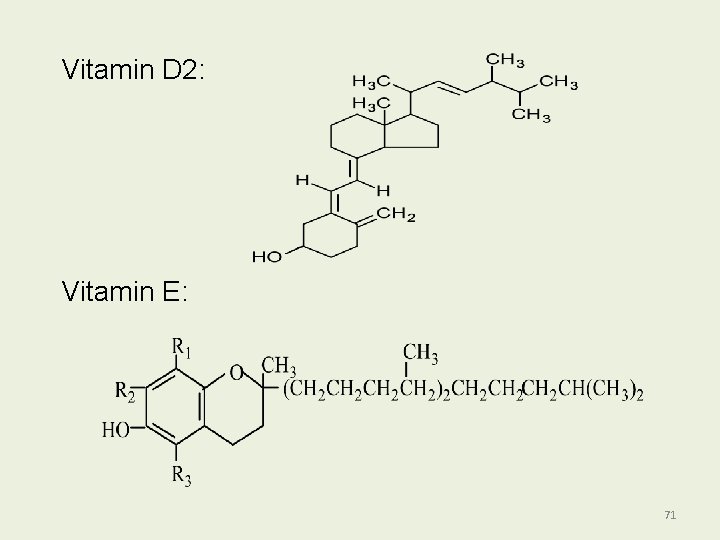
FAT SOLUBLE VITAMINS Vitamin A: 70

Vitamin D 2: Vitamin E: 71

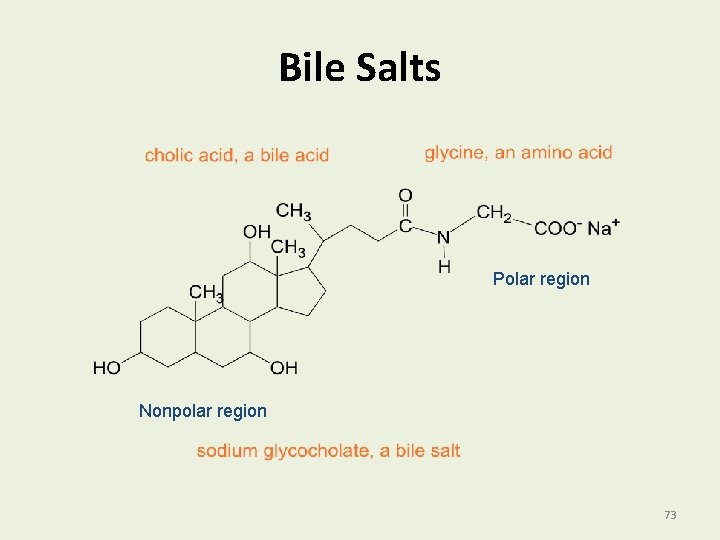
Bile Salts Bile salts • Are synthesized in the liver from cholesterol. • Are stored in the gallbladder. • Are secreted into the small intestine. • Have a polar and a nonpolar region • Mix with fats to break them part. • Emulsify fat particles to provide large surface area. 72

Bile Salts Polar region Nonpolar region 73

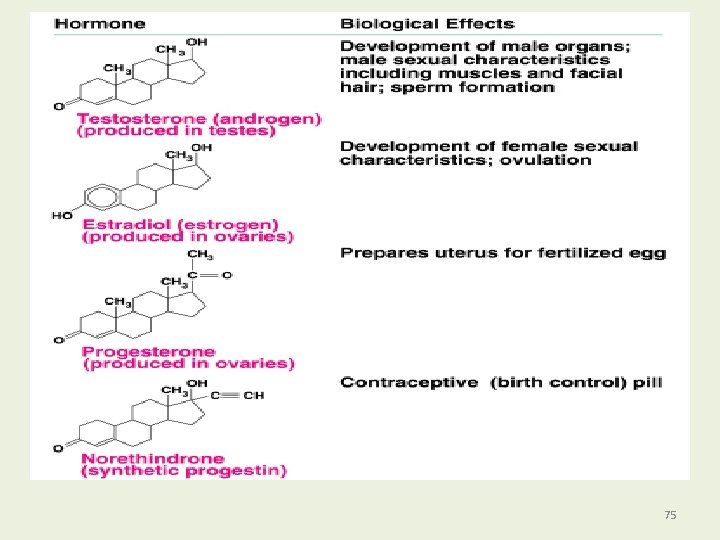
Steroid Hormones (Sex Hormones) Steroid hormones • Are chemical messengers in cells. • Are produced from cholesterol. • Include sex hormones such as androgens (testosterone) in males and estrogens (estradiol) in females. 74

75

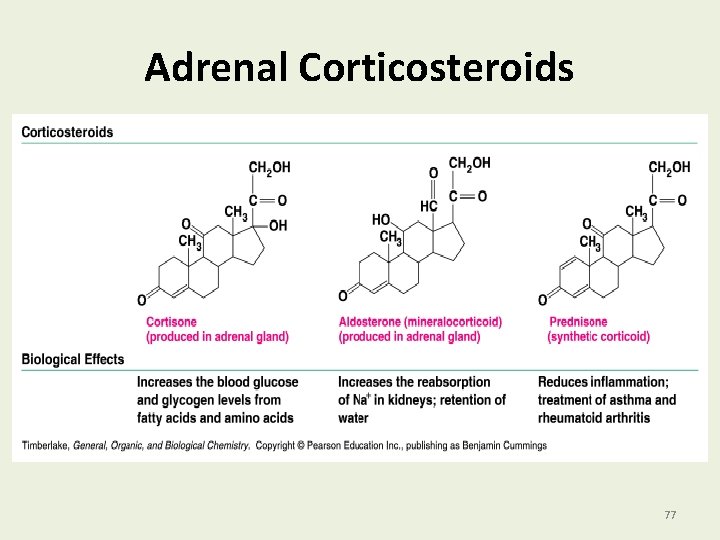
Adrenal Corticosteroids Adrenal corticosteroids are steroid hormones that • Are produced by the adrenal glands located on the top of each kidney. • Include aldosterone, which regulates electrolytes and water balance by the kidneys. • Include cortisone, a glucocorticoid, which increases blood glucose level and stimulates the synthesis of glycogen in the liver. 76

Adrenal Corticosteroids 77

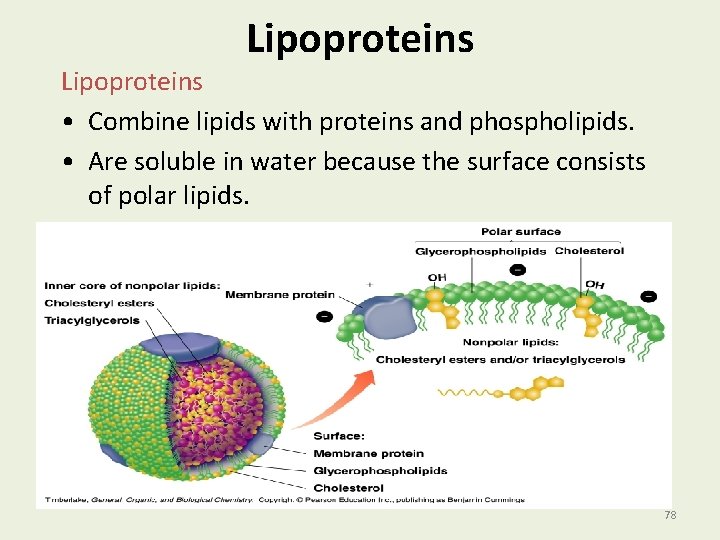
Lipoproteins • Combine lipids with proteins and phospholipids. • Are soluble in water because the surface consists of polar lipids. 78

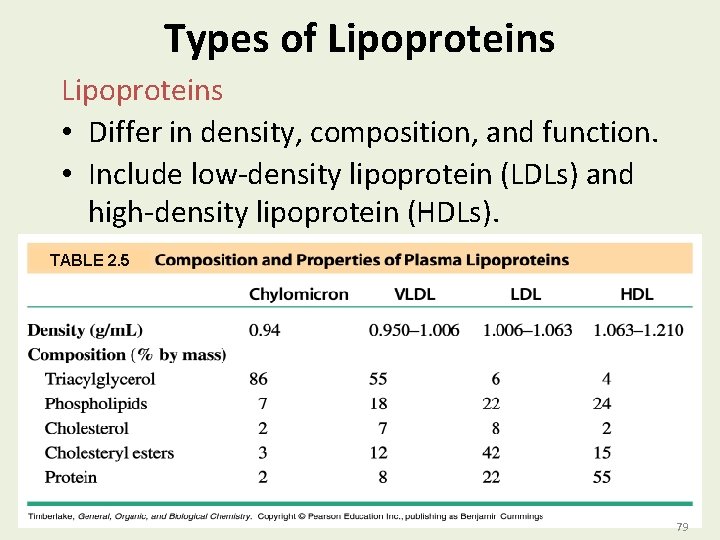
Types of Lipoproteins • Differ in density, composition, and function. • Include low-density lipoprotein (LDLs) and high-density lipoprotein (HDLs). TABLE 2. 5 79 79