Atoms Elements and Compounds ATOMS u Atoms are












- Slides: 19

Atoms, Elements, and Compounds

ATOMS u. Atoms are the basic building blocks of matter that make up everyday objects. u A desk, the air, even you are made up of atoms! u. Matter- Anything that takes up space

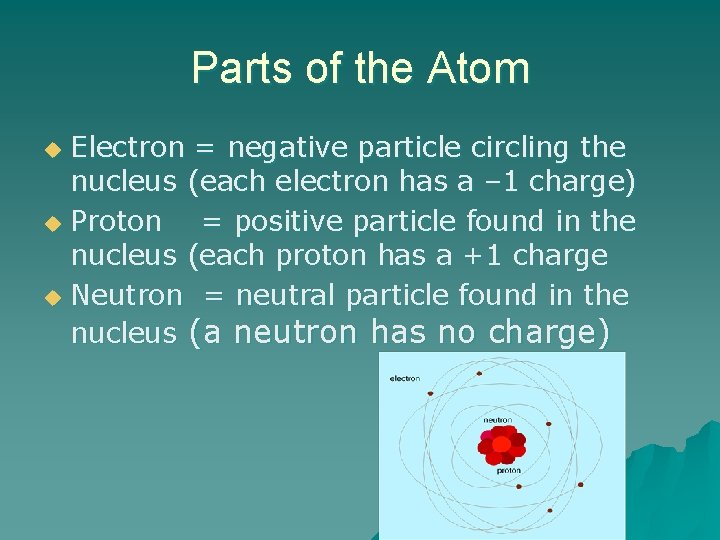
Parts of the Atom Electron = negative particle circling the nucleus (each electron has a – 1 charge) u Proton = positive particle found in the nucleus (each proton has a +1 charge u Neutron = neutral particle found in the nucleus (a neutron has no charge) u

Atomic Number u The number of protons in an atom defines what element it is. – For example carbon atoms have six protons, hydrogen atoms have one, and oxygen atoms have eight. u The number of protons in an atom also determines the chemical behavior of the element.

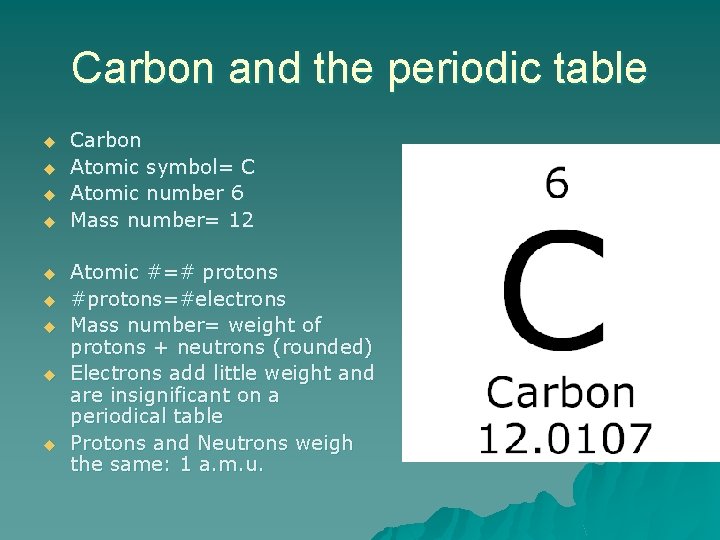
Carbon and the periodic table u u u u u Carbon Atomic symbol= C Atomic number 6 Mass number= 12 Atomic #=# protons #protons=#electrons Mass number= weight of protons + neutrons (rounded) Electrons add little weight and are insignificant on a periodical table Protons and Neutrons weigh the same: 1 a. m. u.

ELEMENTS u substances that cannot be decomposed or broken into simpler substances by ordinary chemical means. u Elements are made up of only one kind of atom [ex. Carbon(C), oxygen(O 2), helium(He)]

Element Name and Symbol u Atomic Symbol: The atomic symbol is one or two letters chosen to represent an element ("H" for "hydrogen, " etc. ). u Memorize C O H He Na Fe N S u u u u the symbols/ names of the following: Carbon Oxygen Hydrogen Helium Sodium Iron Nitrogen Sulfur

Compounds u Substance formed by a combination of two or more elements – (ex. Water (H 2 O) has hydrogen and oxygen

Mixtures/Solutions u Mixture- Substances that do not dissolve together but mix Solutions – Mixture that is evenly distributed throughout the solution

Mixtures u Solution u Solute- Na. Cl u Solvent- water Na. Cl Dissolving in Water When an ionic compound such as sodium chloride is placed in water, water molecules surround and separate the positive and negative ions.

Mixtures Mixture – Different particles not dissolved

Atom vs. Molecule u Atom – the smallest possible particle of a molecule that retains its chemical properties Example: A Hydrogen Atom u Molecule - two or more atoms in a definite arrangement held together by chemical bonds Water is always H O 2

What is in a Molecule u Formula’s – Subscript (Little number after an H 2 O element symbol in a molecule) means there are that number of the previous Atom u H 2 O Water – 2 Hydrogen 1 Oxygen u Ammonia NH 3 – 1 Nitrogen 3 Hydrogen u u u Sucrose C 12 H 22 O 11 Table Salt Na. Cl Sodium Bicarbonate Na. HCO 3

Definitions Bond - joins two atoms together The Octet Rule- Says that atoms tend to gain, lose or share electrons so as to have eight electrons in their outer electron shell. -To be stable atoms must have their outer orbit filled. -Usually the outer orbit holds 8 electrons -Hydrogen and Helium have an outer orbit that only holds 2 electrons. Chemical bond – a force that holds two atoms together (occurs because atoms want to achieve stability ) atoms want to have a complete outermost energy level

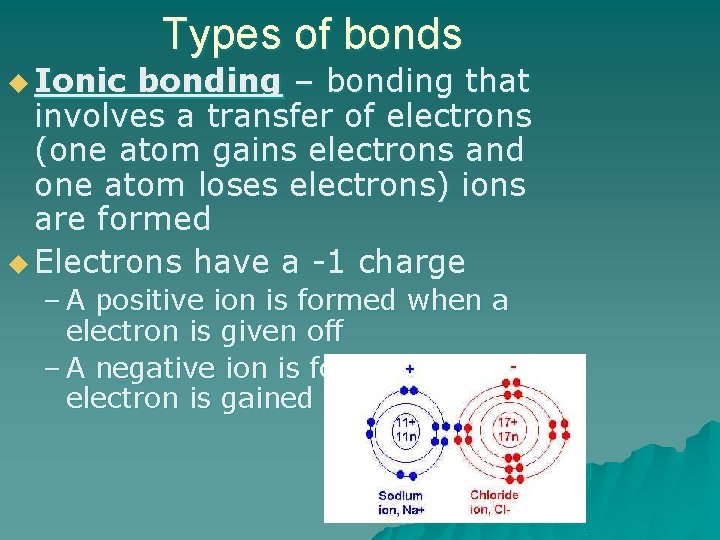
u Ionic Types of bonds bonding – bonding that involves a transfer of electrons (one atom gains electrons and one atom loses electrons) ions are formed u Electrons have a -1 charge – A positive ion is formed when a electron is given off – A negative ion is formed when a electron is gained

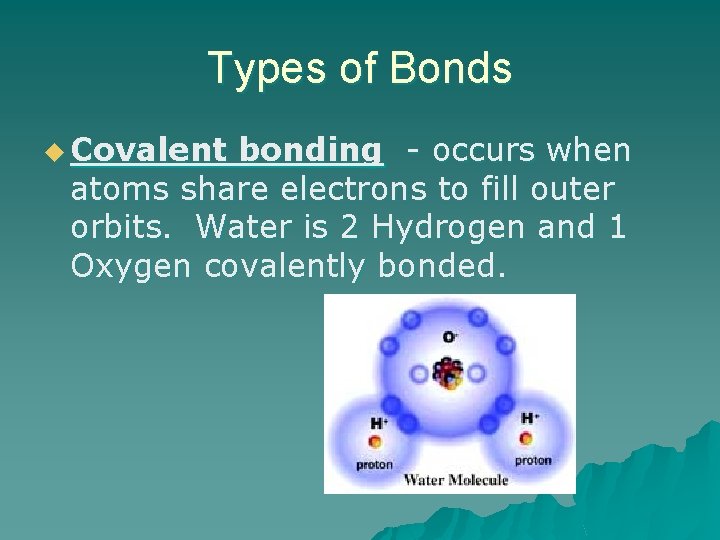
Types of Bonds u Covalent bonding - occurs when atoms share electrons to fill outer orbits. Water is 2 Hydrogen and 1 Oxygen covalently bonded.

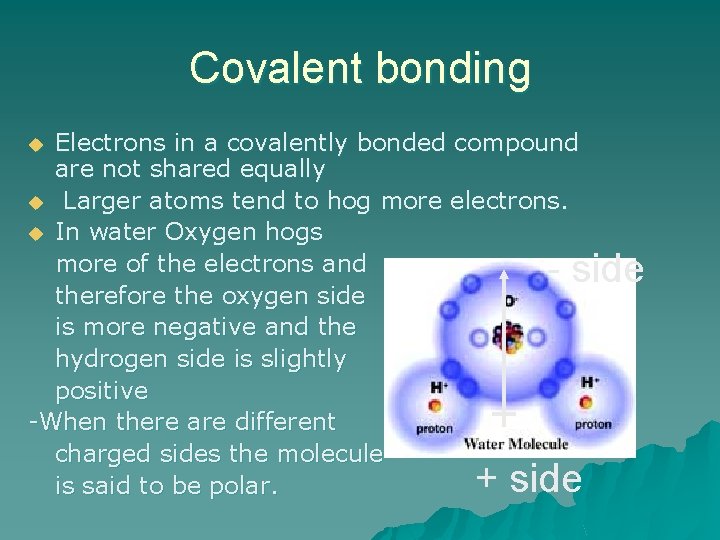
Covalent bonding Electrons in a covalently bonded compound are not shared equally u Larger atoms tend to hog more electrons. u In water Oxygen hogs more of the electrons and therefore the oxygen side is more negative and the hydrogen side is slightly positive -When there are different charged sides the molecule is said to be polar. u - side + side

Hydrogen Bonds u u Attraction that forms between two polar molecules (when hydrogen is present). The positive side of one polar molecule attracts and is “bonded” to the negative side of another polar molecule.

Compound u. A substance formed by the combination of two or more elements in definite proportions. u Exp: Na. Cl, Salt