The Atomic Nucleus Reviewthe nucleus The nucleus is










- Slides: 45

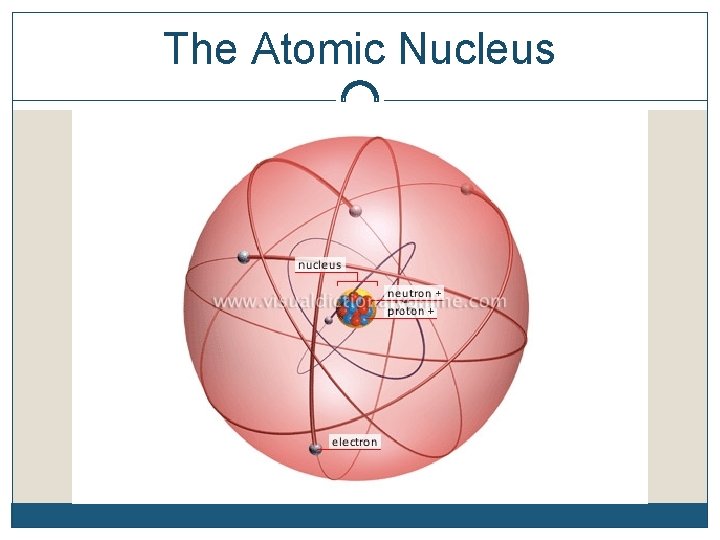
The Atomic Nucleus

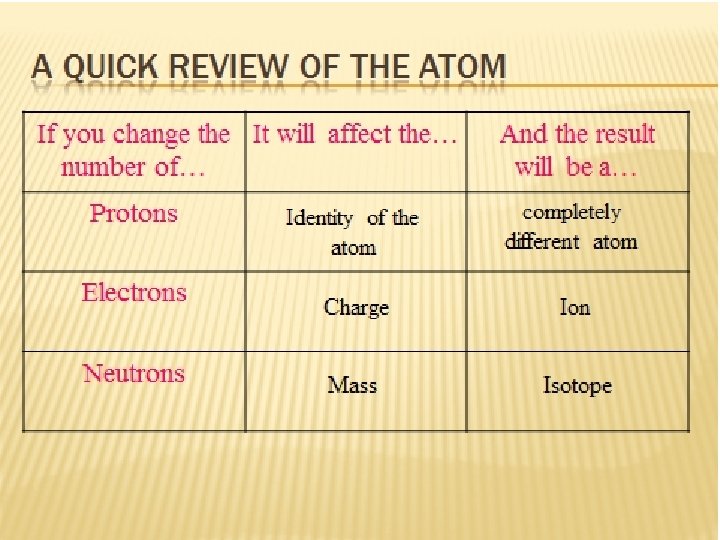
Review…the nucleus The nucleus is protons and neutrons composed of particles called nucleons. . __ & __ Neutrons and protons have the same mass, with ___ being slightly greater. Neutrons have nearly 2000 times the mass of _____. neutrons electrons

Review…the atom The mass of an atom is almost equal to the mass of the _____ alone nucleus Nucleons are bound together by an attractive nuclear force called the ____ force The positively charged protons in the nucleus hold the negatively charged electrons in their ____ strong orbits

Review…the atom The number of ___ in the nucleus determines the chemical properties of the atom The # of protons determines the # of ____ that orbit the atom The # of ___ has no direct effect on the # of electrons protons

Review…the atom The principal role of the neutrons in the nucleus is to act as a sort of ____ to hold the nucleus together cement The electrical force acts as a ____ force between protons The atom needs a certain balance of neutrons and protons for ___ repulsive stability

The symbol was created in 1946 UCBerkeley to represent “activity coming out of a atom”

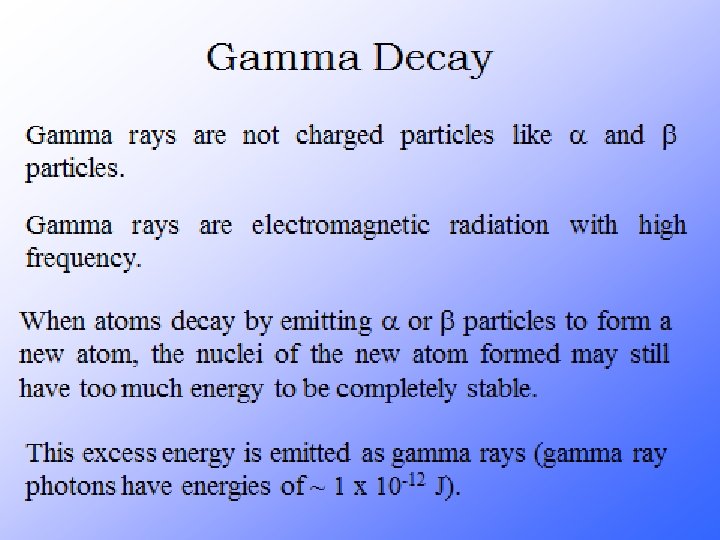
What is Radioactivity? Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The general idea: An unstable nucleus releases energy to become more stable

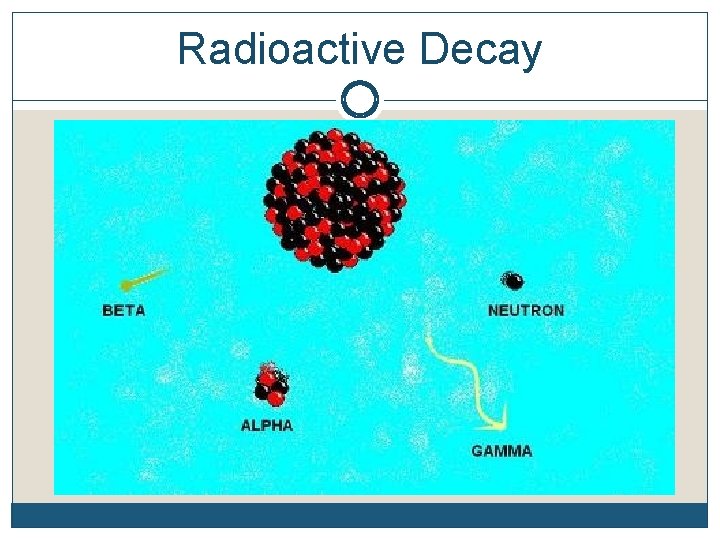
Radioactive Decay

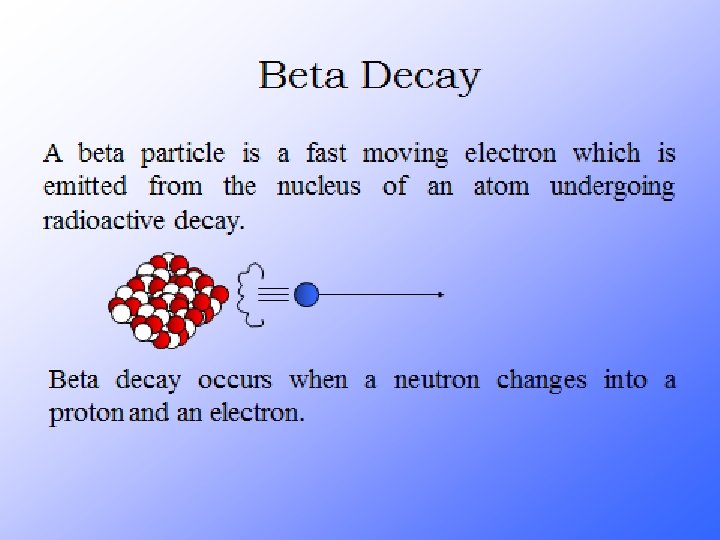
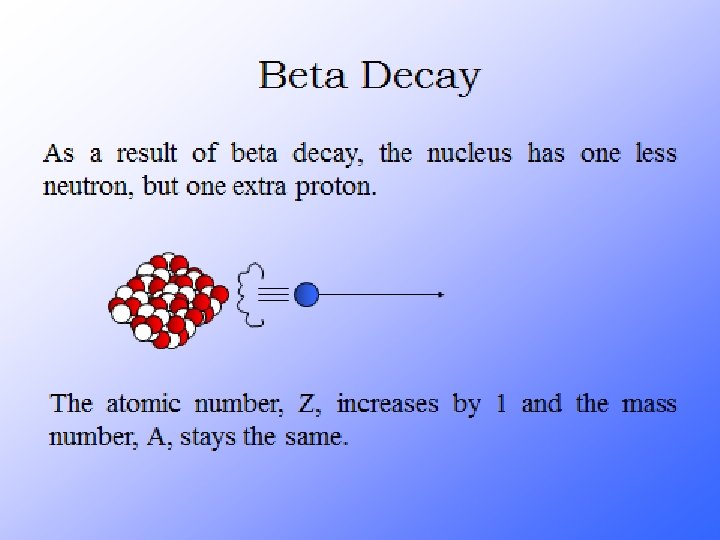
Radioactive Decay A neutron is very unstable. A lone neutron will spontaneously decay into a proton + an electron. If you have a lot of neutrons, within 11 minutes ½ of them will have decayed Particles that decay are said to be radioactive A lone neutron is radioactive

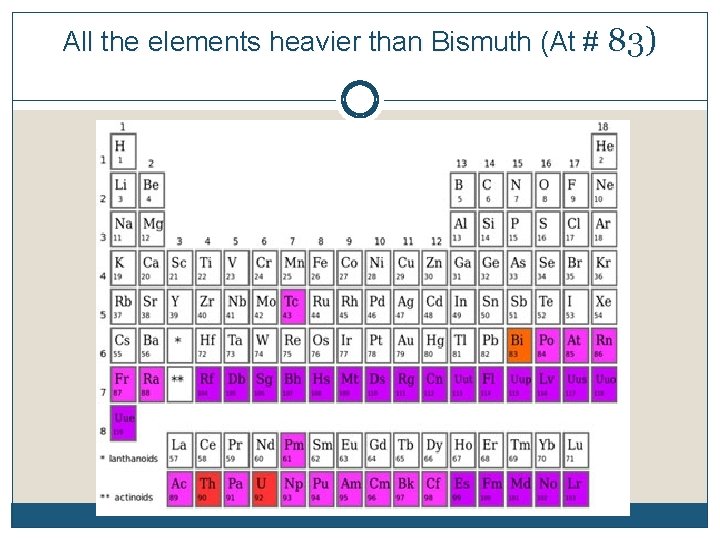
All the elements heavier than Bismuth (At # 83)

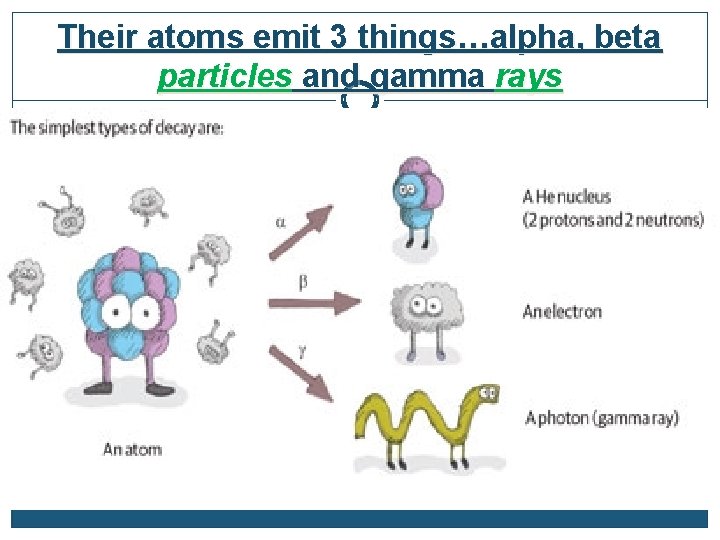
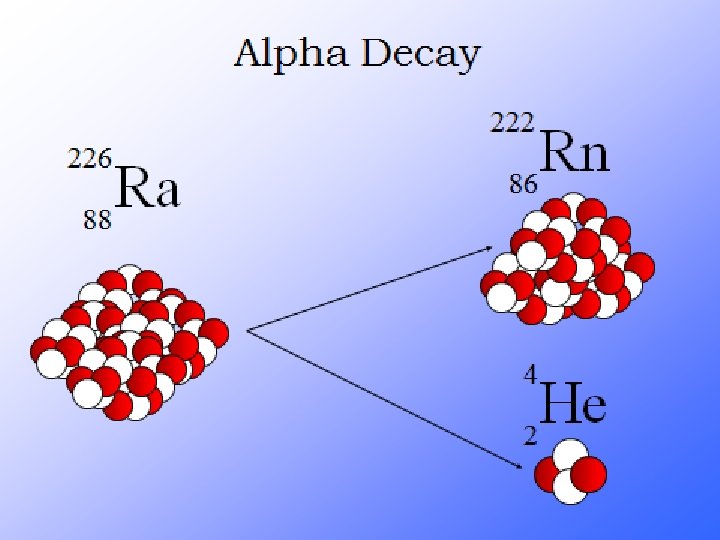
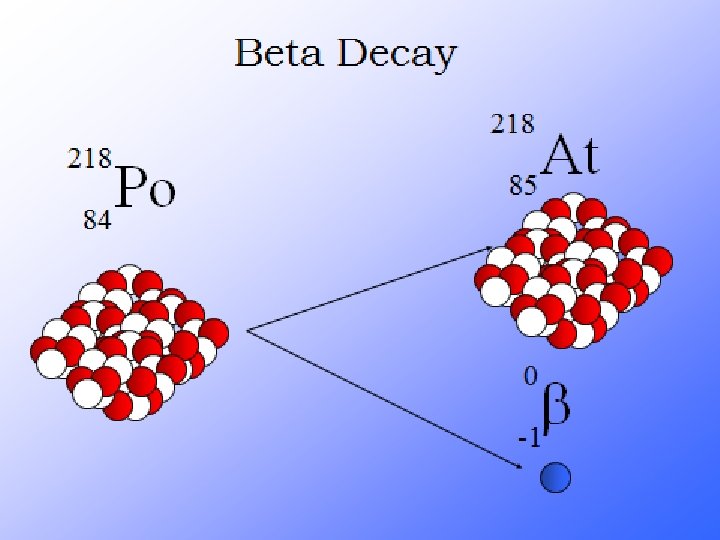
Their atoms emit 3 things…alpha, beta particles and gamma rays







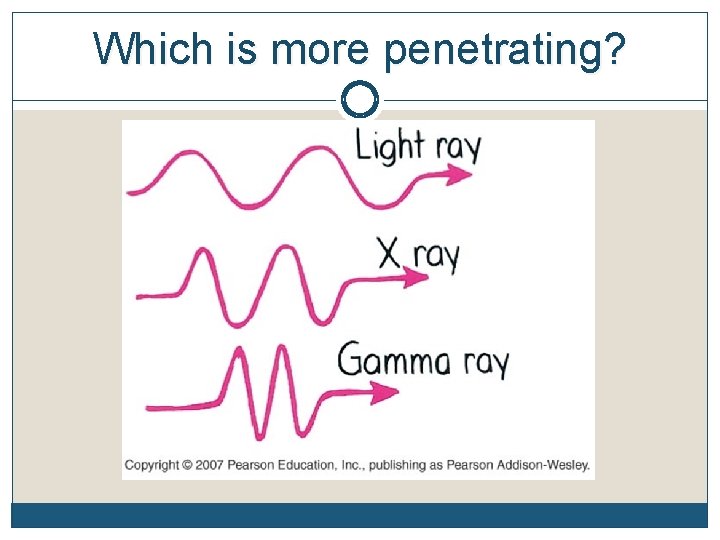
Which is more penetrating?

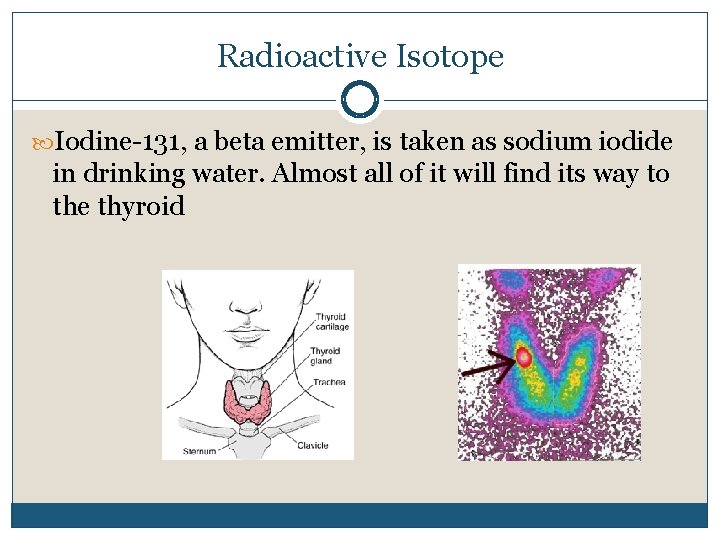
Radioactive Isotope Iodine-131, a beta emitter, is taken as sodium iodide in drinking water. Almost all of it will find its way to the thyroid

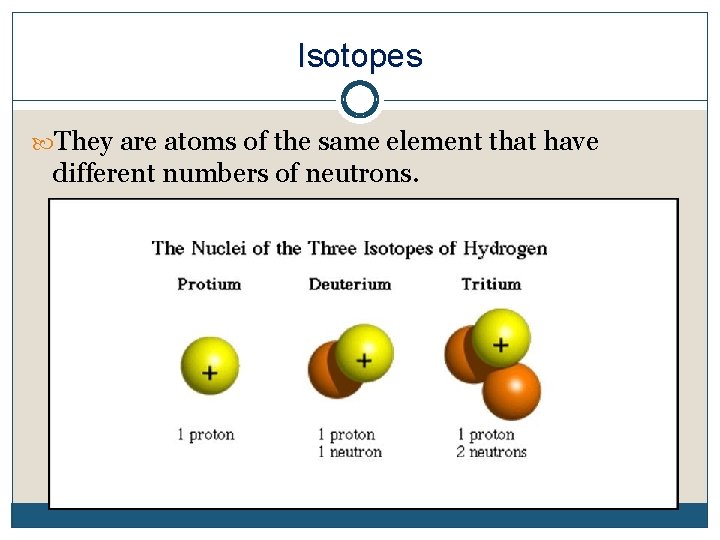
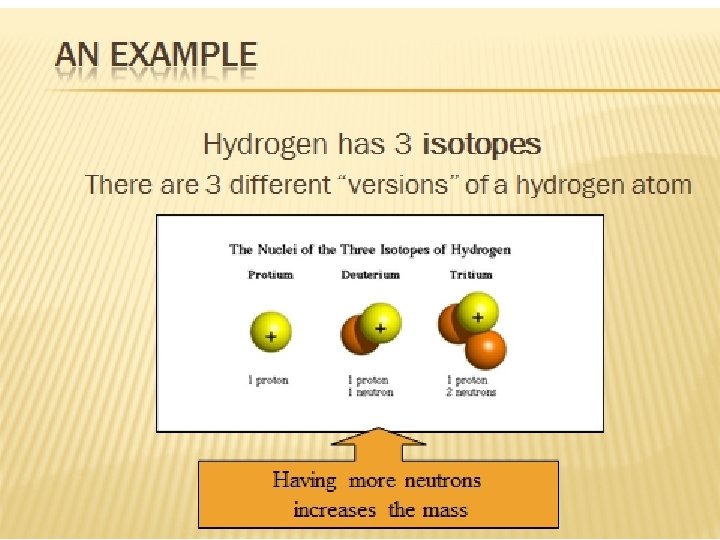
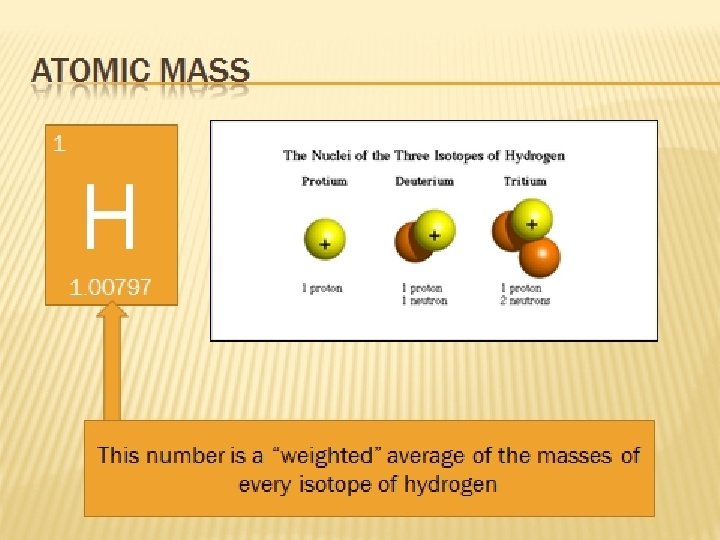
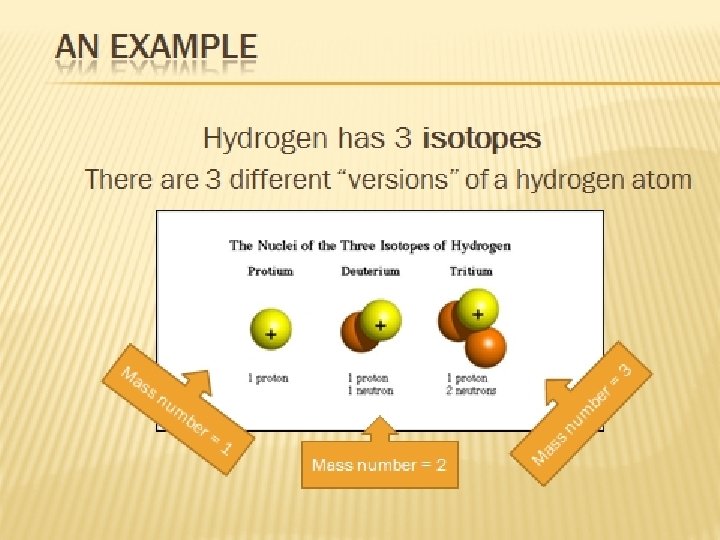
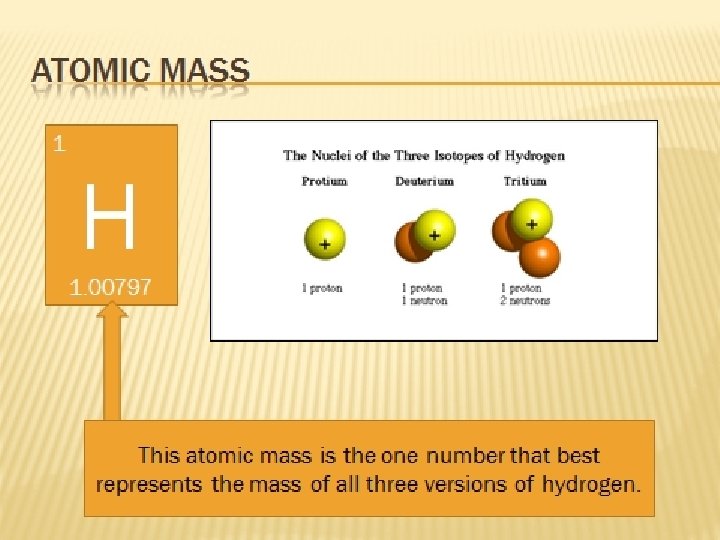
Isotopes They are atoms of the same element that have different numbers of neutrons.








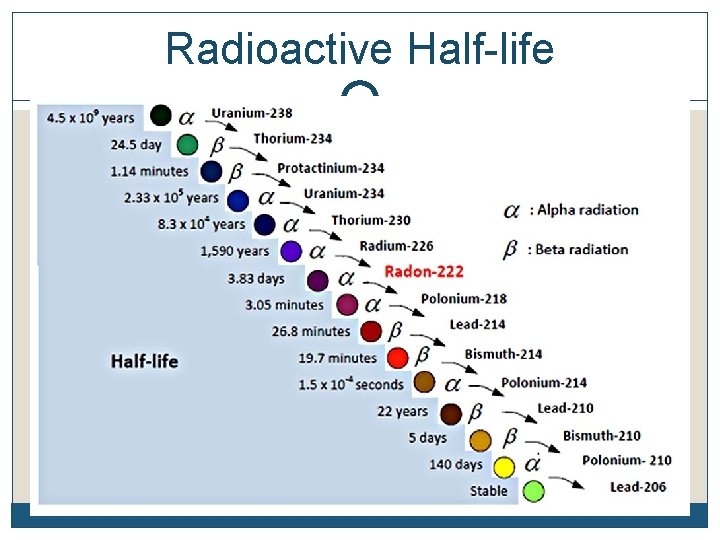
Radioactive Half-life

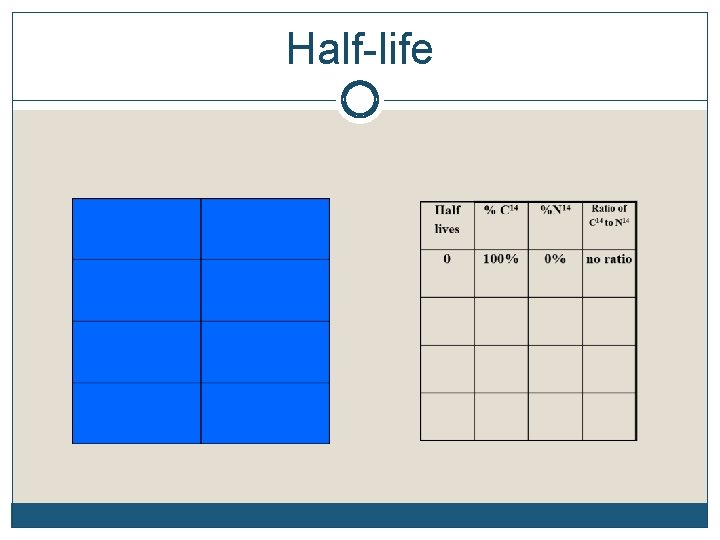
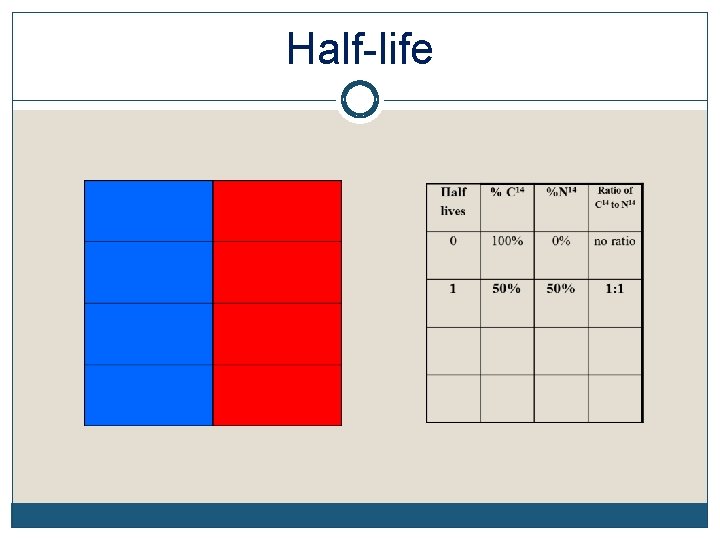
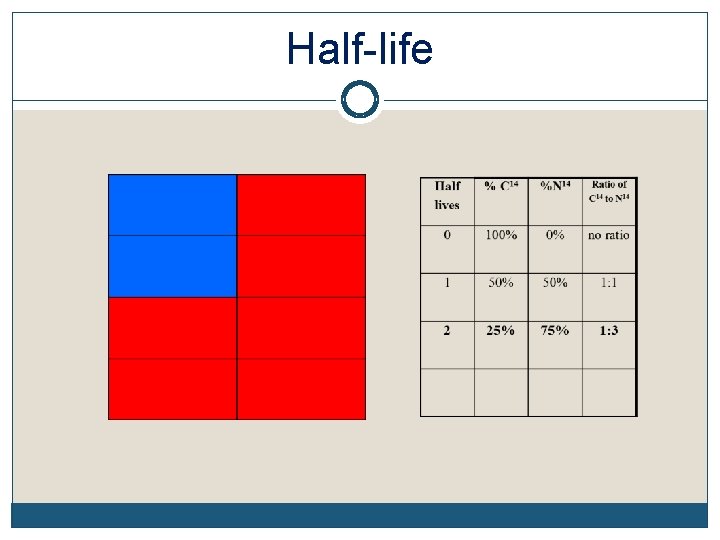
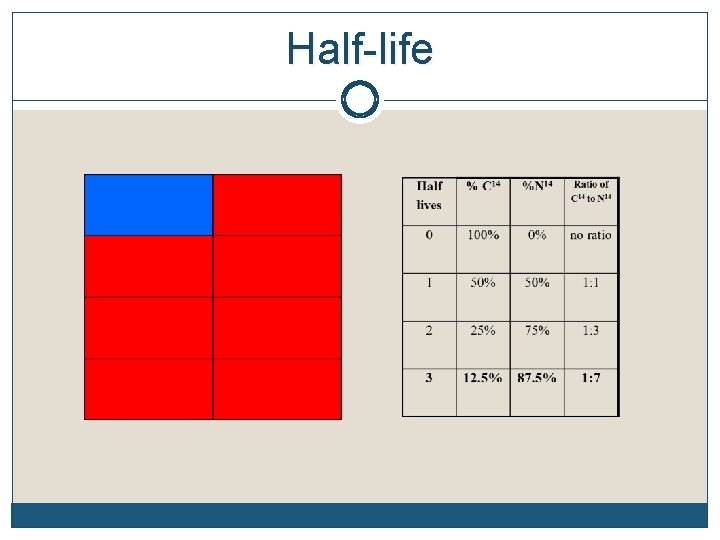
Radioactive Half-Life The half-life of an element is the time it takes for half of the material you started with to decay Remember, it doesn’t matter how much you start with. After 1 half-life, half of it will have decayed. Each element decays into a new element C 14 decays into N 14 while U 238 decays into Pb 206 (lead), etc The half-life of each element is constant. It’s like a clock keeping perfect time

Half-life

Half-life

Half-life

Half-life

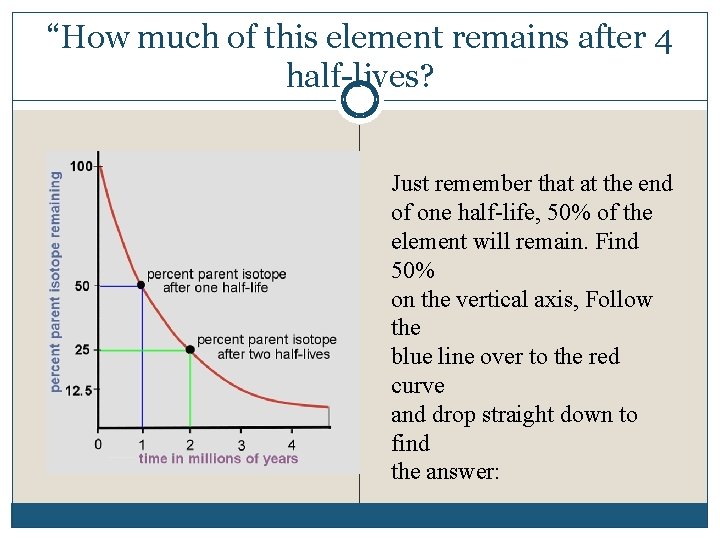
“How much of this element remains after 4 half-lives? Just remember that at the end of one half-life, 50% of the element will remain. Find 50% on the vertical axis, Follow the blue line over to the red curve and drop straight down to find the answer:

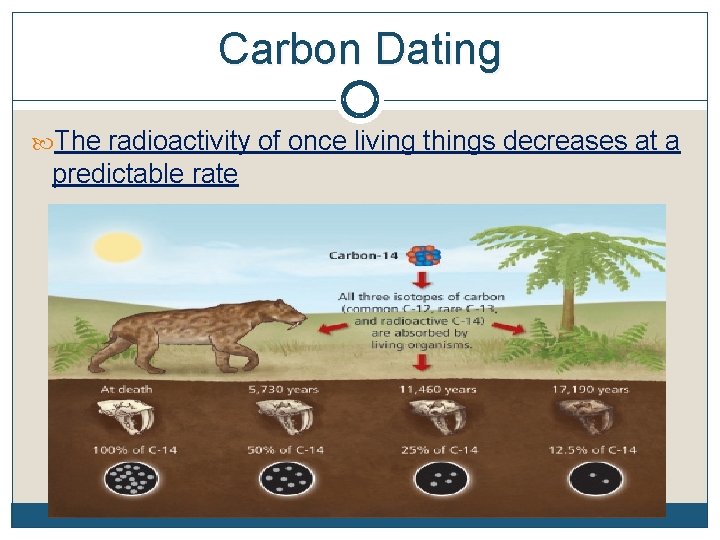
Carbon Dating The radioactivity of once living things decreases at a predictable rate

Uranium Dating The dating of older, non-living things (like rocks) is accomplished by radioactive minerals, such as Uranium decays very slowly. Rocks on Earth have been dated to 3. 7 bil yrs old Rocks on Moon dated to 4. 2 bil yrs old The Earth has been dated to 4. 6 bil yrs old

Review…whew! https: //archive. org/details/NASAReal. World. Math_ What. Is. Radioactive. Decay_HD http: //en. wikipedia. org/wiki/Barium_swallow

Radiation Penetrating Power

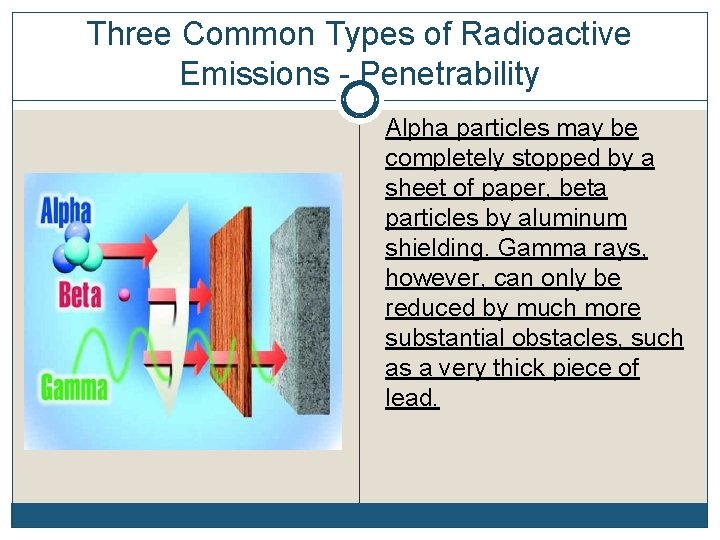
Three Common Types of Radioactive Emissions - Penetrability Alpha particles may be completely stopped by a sheet of paper, beta particles by aluminum shielding. Gamma rays, however, can only be reduced by much more substantial obstacles, such as a very thick piece of lead.

Radiation Penetrating Power http: //www. passmyexams. co. uk/GCSE/physics/pen etrating-properties-of-radiation. html

Radiation and You (and me)! http: //people. chem. duke. edu/~jds/cruise_chem/nu clear/exposure. html

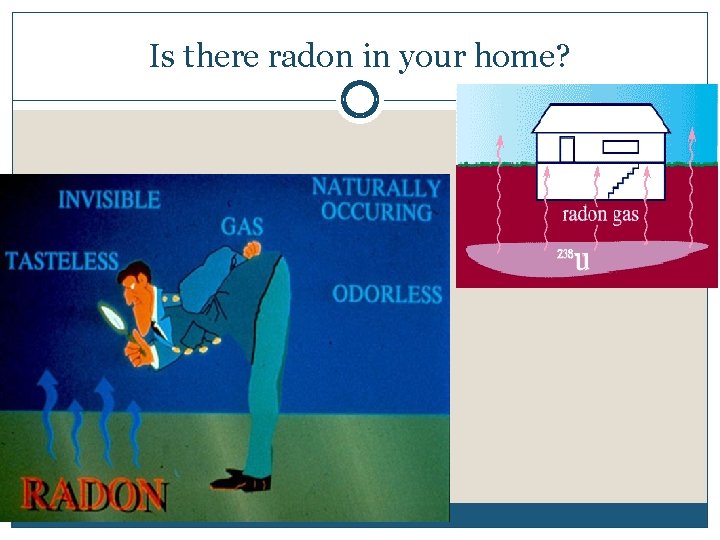
Is there radon in your home?

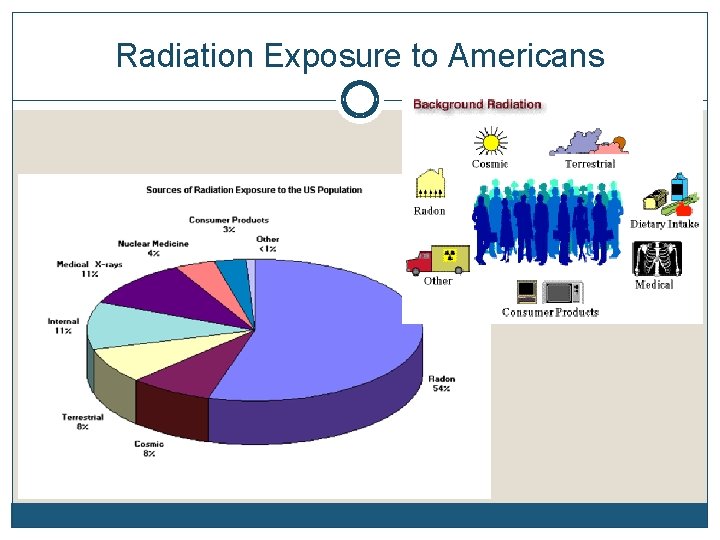
Radiation Exposure to Americans

Review Name three of the science pioneers in the study of Radioactivity. ? Why does a nucleus decay? Order these emissions from least to greatest penetrability: Gamma, Alpha, Beta. What is the greatest source of exposure to radioactivity in our everyday lives?

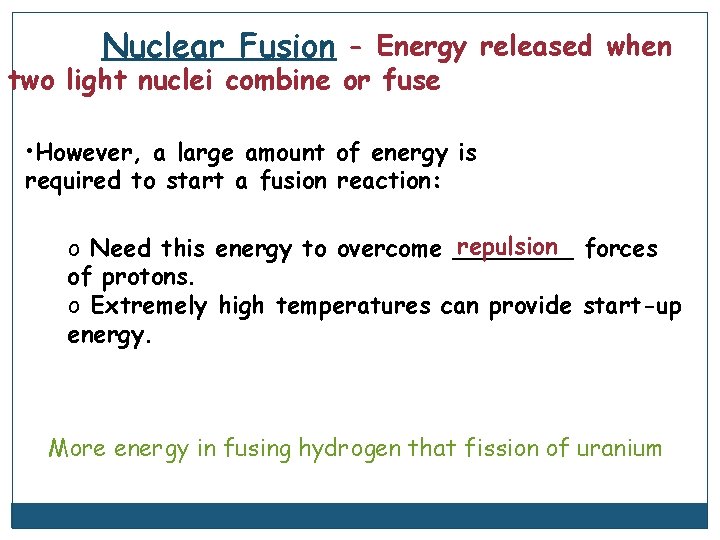
Nuclear Fusion - Energy released when two light nuclei combine or fuse • However, a large amount of energy is required to start a fusion reaction: repulsion forces o Need this energy to overcome ____ of protons. o Extremely high temperatures can provide start-up energy. More energy in fusing hydrogen that fission of uranium