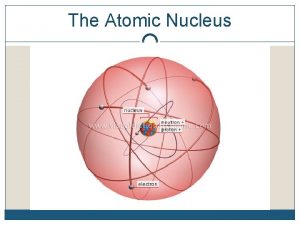
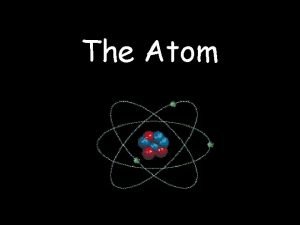
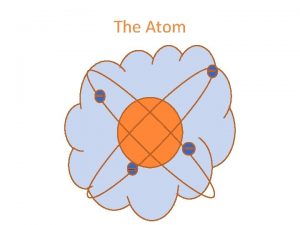
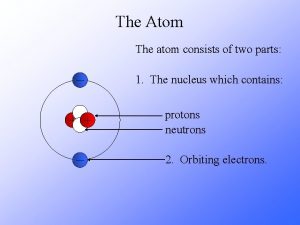
The Atom An atom consists of a Nucleus






- Slides: 21

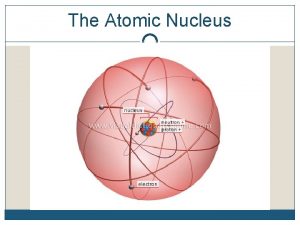
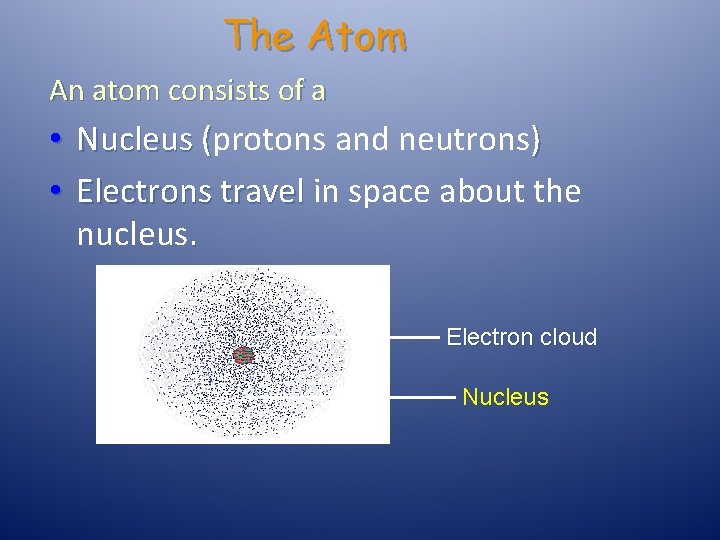
The Atom An atom consists of a • Nucleus (protons and neutrons) • Electrons travel in space about the nucleus. Electron cloud Nucleus

Atoms are extremely small. One teaspoon of water has 3 x as many atoms as the Atlantic Ocean has teaspoons of water!

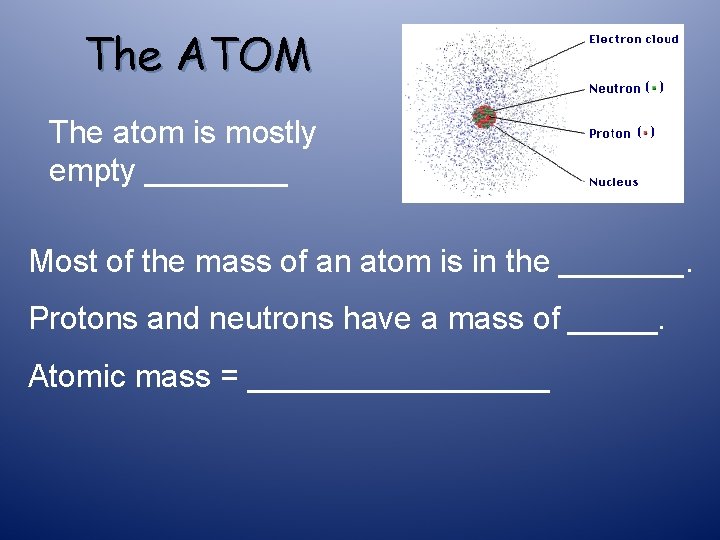
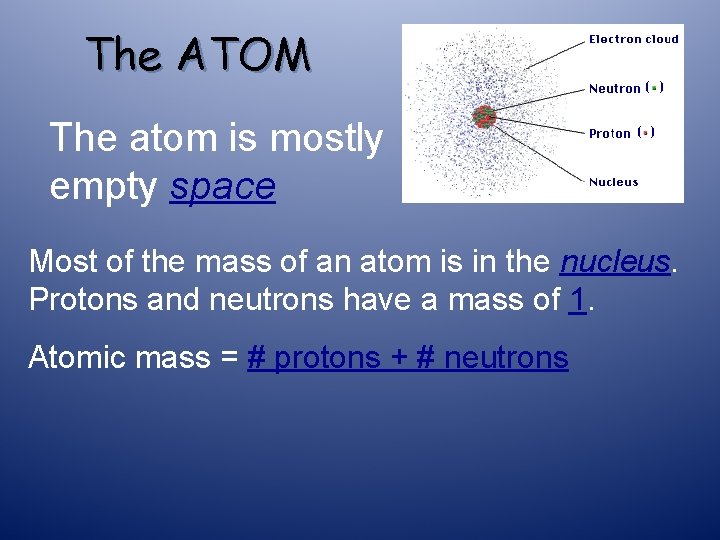
The ATOM The atom is mostly empty ____ Most of the mass of an atom is in the _______. Protons and neutrons have a mass of _____. Atomic mass = _________

The ATOM The atom is mostly empty space Most of the mass of an atom is in the nucleus. Protons and neutrons have a mass of 1. Atomic mass = # protons + # neutrons

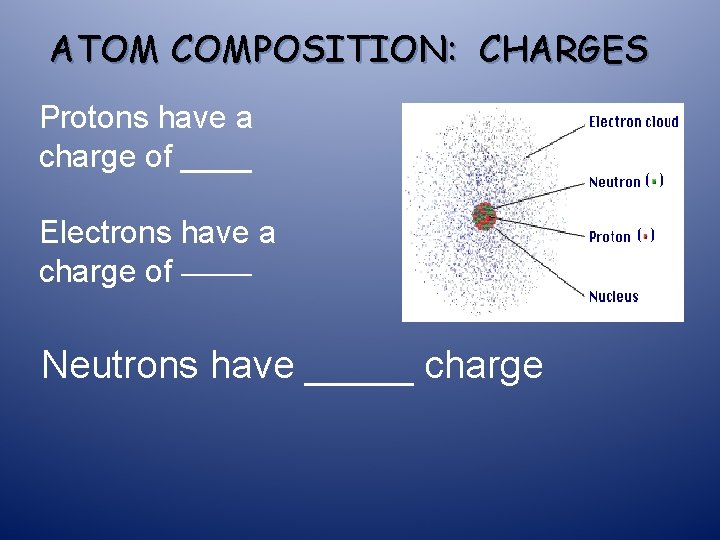
ATOM COMPOSITION: CHARGES Protons have a charge of ____ Electrons have a charge of ______ Neutrons have _____ charge

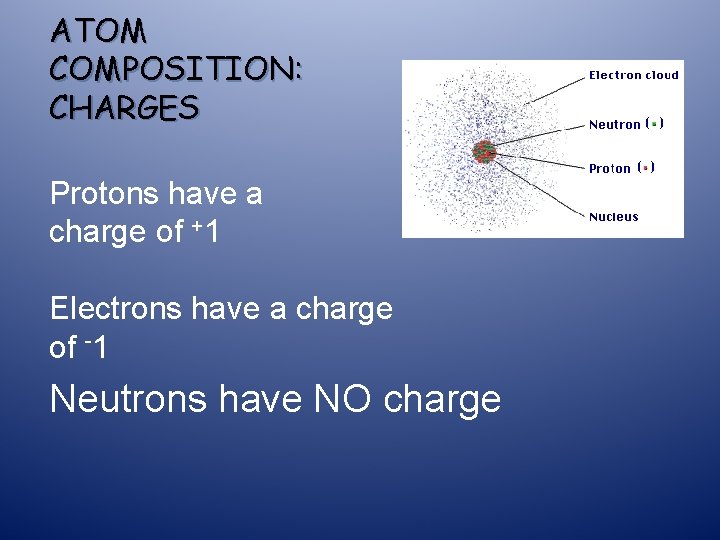
ATOM COMPOSITION: CHARGES Protons have a charge of +1 Electrons have a charge of -1 Neutrons have NO charge

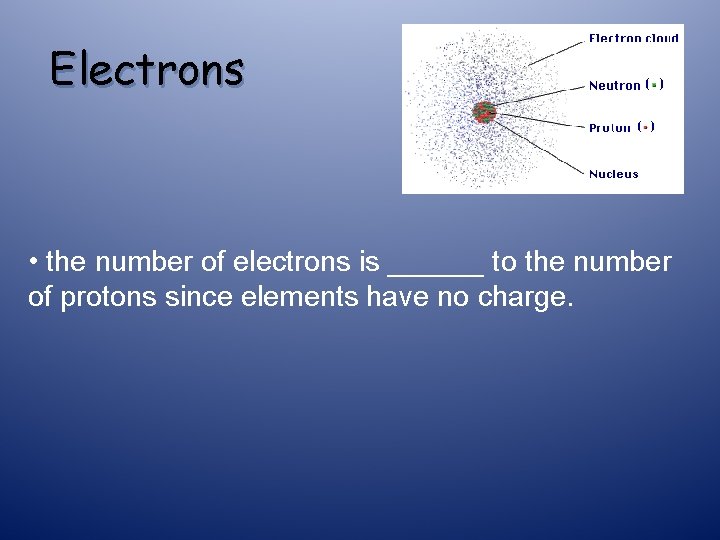
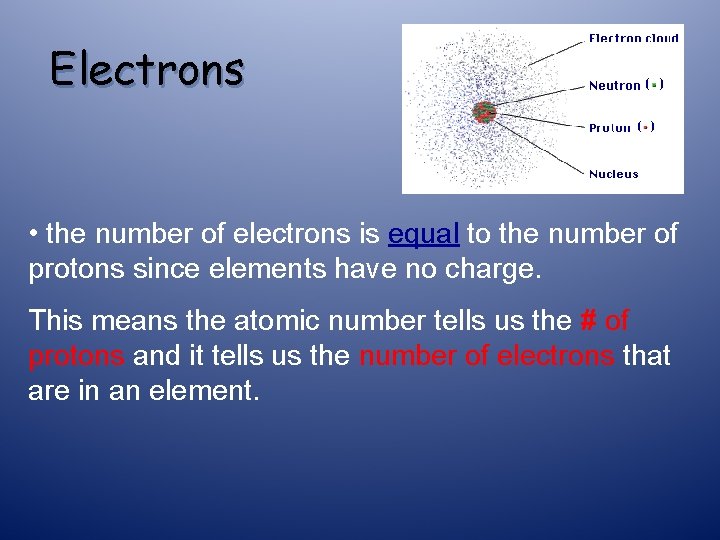
Electrons • the number of electrons is ______ to the number of protons since elements have no charge.

Electrons • the number of electrons is equal to the number of protons since elements have no charge. This means the atomic number tells us the # of protons and it tells us the number of electrons that are in an element.

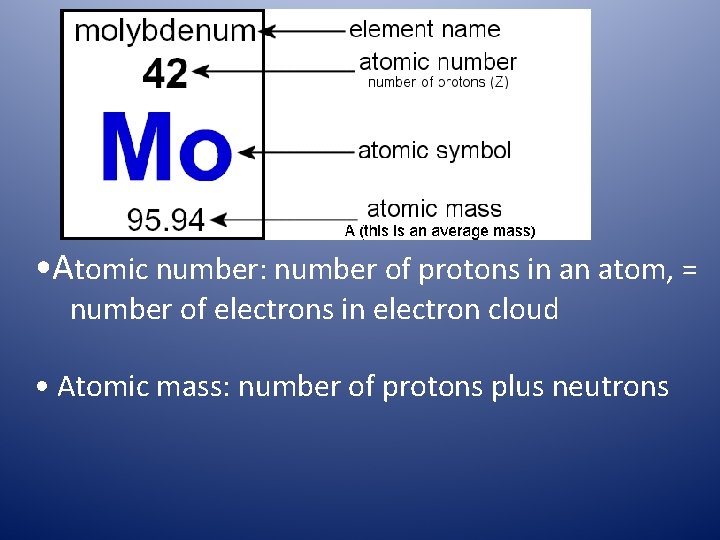
• Atomic number: number of protons in an atom, = number of electrons in electron cloud • Atomic mass: number of protons plus neutrons

An element has 28 protons and 31 neutrons: What is it?

Nickel: 28 protons 31 neutrons • # neutrons = atomic mass – atomic number

An element has an atomic mass of 184. How many protons does it have? How many electrons does it have? How many neutrons does it have?

Atomic mass= 184 Atomic number = 74 74 protons, 74 electrons 110 neutrons (184 – 74 = 110)

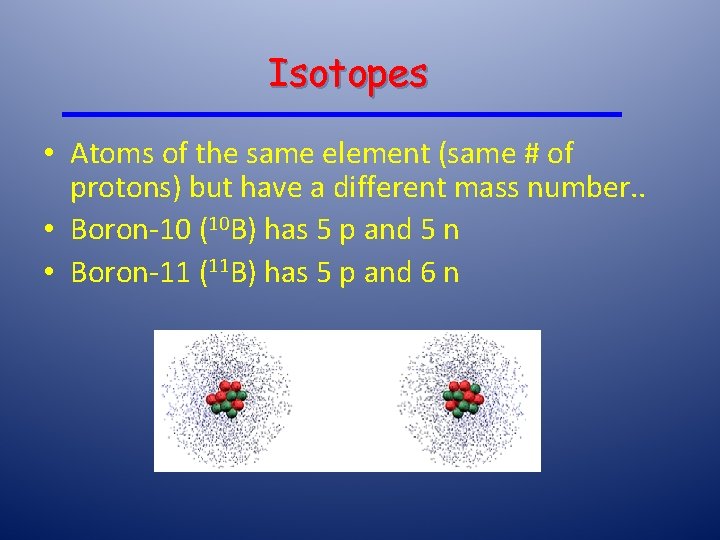
Isotopes • Atoms of the same element (same # of protons) but have a different mass number. . • Boron-10 (10 B) has 5 p and 5 n • Boron-11 (11 B) has 5 p and 6 n 11 B 10 B

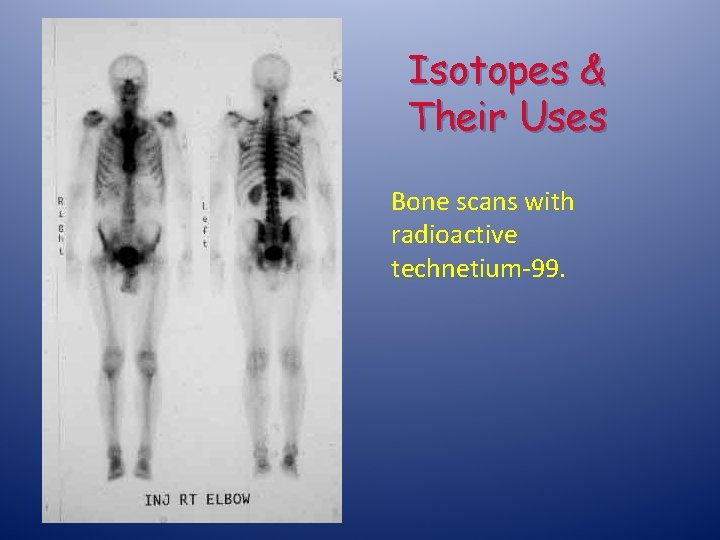
Isotopes & Their Uses Bone scans with radioactive technetium-99.

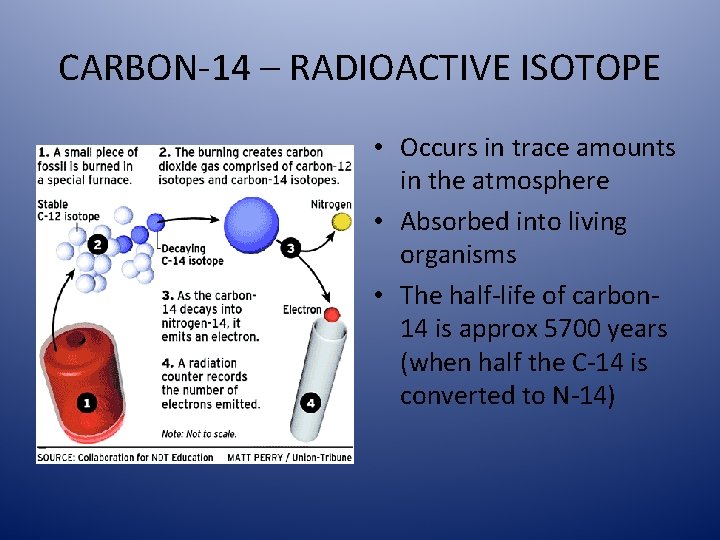
CARBON-14 – RADIOACTIVE ISOTOPE • Occurs in trace amounts in the atmosphere • Absorbed into living organisms • The half-life of carbon 14 is approx 5700 years (when half the C-14 is converted to N-14)

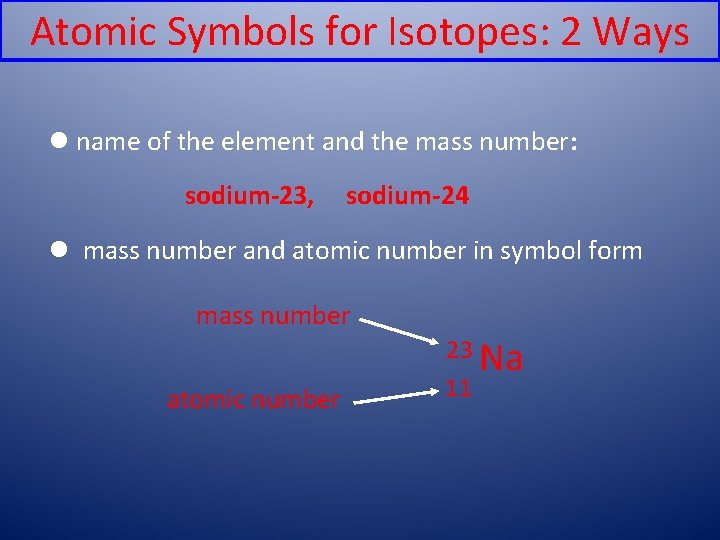
Atomic Symbols for Isotopes: 2 Ways l name of the element and the mass number: sodium-23, sodium-24 l mass number and atomic number in symbol form mass number atomic number 23 Na 11

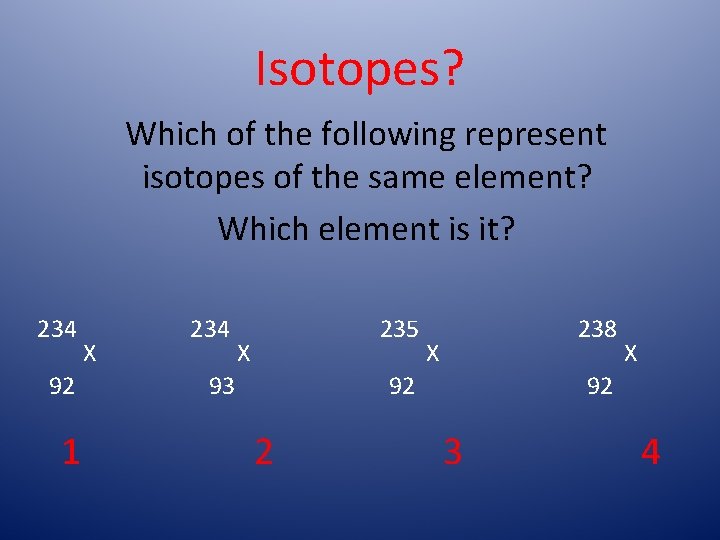
Isotopes? Which of the following represent isotopes of the same element? Which element is it? 234 92 1 X 234 235 X 93 238 X 92 2 X 92 3 4

Compounds • a pure substance composed of 2 or more elements in a fixed ratio that can be separated by heat and electricity • properties differ from those of individual elements • Ex : table salt (Na. Cl)

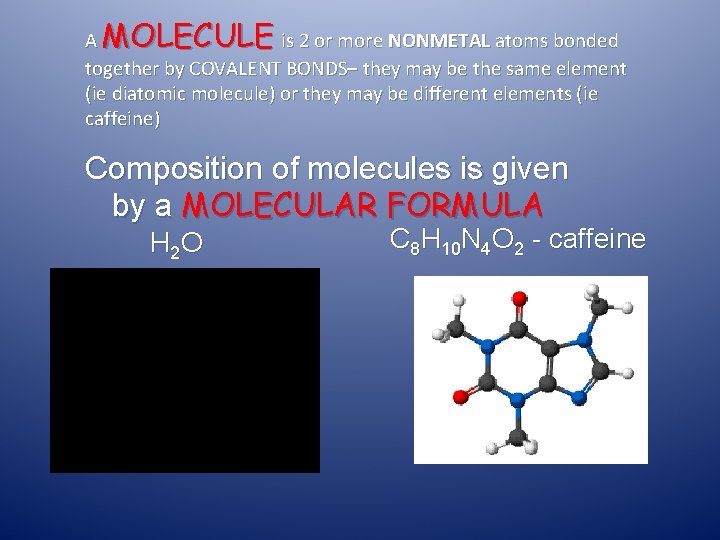
A MOLECULE is 2 or more NONMETAL atoms bonded together by COVALENT BONDS– they may be the same element (ie diatomic molecule) or they may be different elements (ie caffeine) Composition of molecules is given by a MOLECULAR FORMULA H 2 O C 8 H 10 N 4 O 2 - caffeine

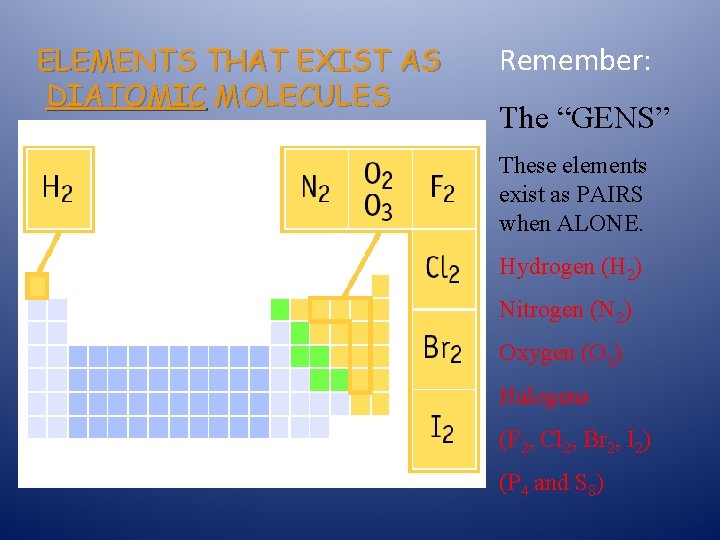
ELEMENTS THAT EXIST AS DIATOMIC MOLECULES Remember: The “GENS” These elements exist as PAIRS when ALONE. Hydrogen (H 2) Nitrogen (N 2) Oxygen (O 2) Halogens (F 2, Cl 2, Br 2, I 2) (P 4 and S 8)
 Whats found in the nucleus of an atom
Whats found in the nucleus of an atom Radioactive hula hoop
Radioactive hula hoop What surrounds the nucleus of an atom
What surrounds the nucleus of an atom What zooms around the nucleus of an atom
What zooms around the nucleus of an atom E=h x f
E=h x f The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Kelemahan dalton
Kelemahan dalton Số nguyên là gì
Số nguyên là gì đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Fecboak
Fecboak Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất ưu thế lai là gì
ưu thế lai là gì Sơ đồ cơ thể người
Sơ đồ cơ thể người Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Tư thế ngồi viết
Tư thế ngồi viết Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin